Yield Potential and Variability of Teff (Eragrostis tef (Zucc.) Trotter) Germplasms under Intensive and Conventional Management Conditions
Abstract
:1. Introduction
2. Materials and Methods
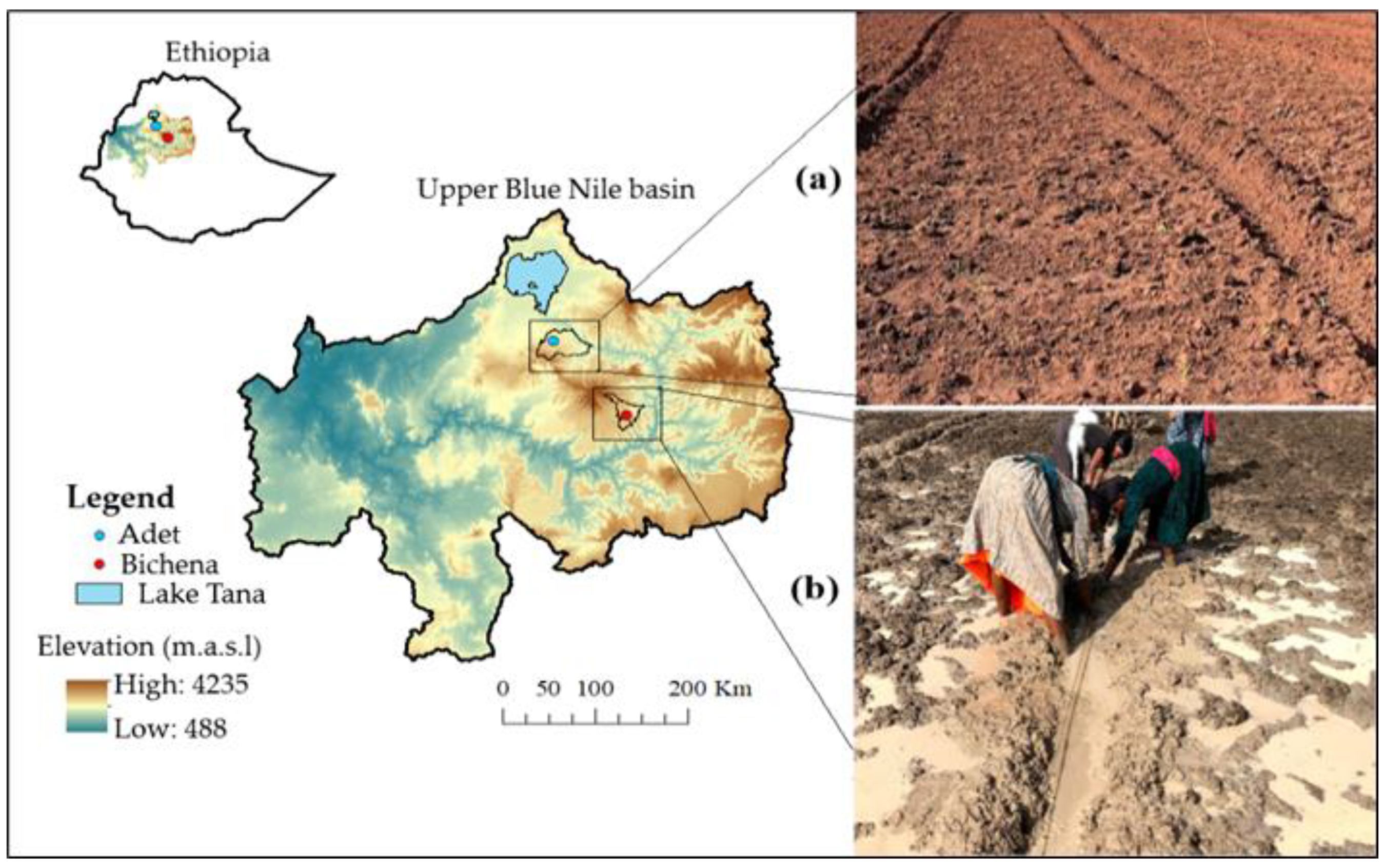
2.1. Description of Experimental Sites and Materials
2.2. Experimental Set Up and Management
2.2.1. Intensive Growing Condition
2.2.2. Field Growing Condition
2.3. Data Collection and Measurements
2.4. Statistical Data Analysis
3. Results
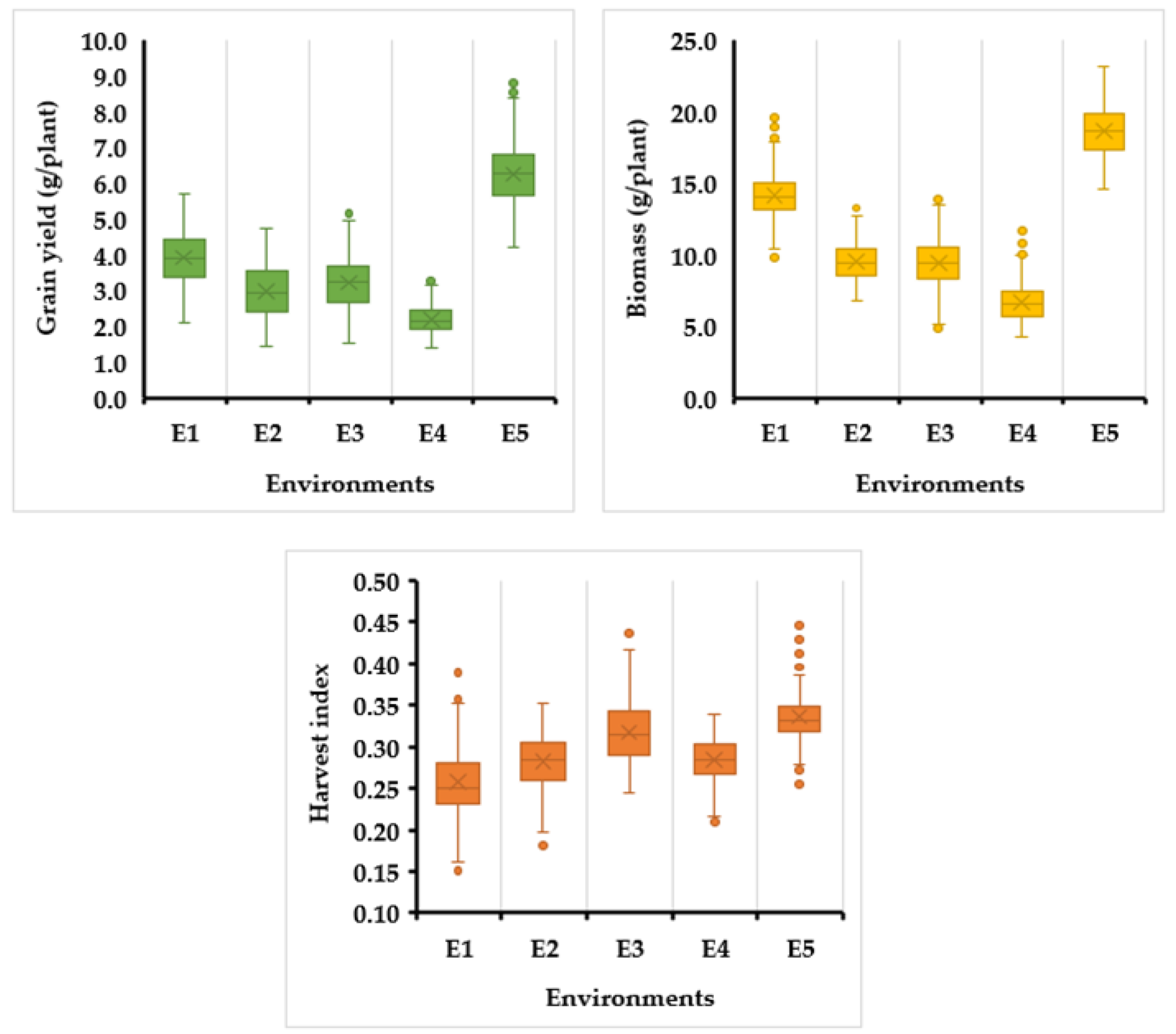
3.1. Grain Yield, Biomass, and Harvest Index
3.2. Phenology
3.3. Variance Components, Heritability, and Genetic Advance
3.4. Phenotypic and Genotypic Correlations
3.5. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurukulasuriya, P.; Rosenthal, S. Climate Change and Agriculture: A Review of Impacts and Adaptations; The World Bank: Washington, DC, USA, 2013. [Google Scholar]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Sun, H.; Taghizadeh-Hesary, F. How Does Climate Change Affect Rice Yield in China? Agriculture 2020, 10, 441. [Google Scholar] [CrossRef]
- Tadele, Z. Orphan crops: Their importance and the urgency of improvement. Planta 2019, 250, 677–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2011, 62, 469–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- Hawkesford, M.J.; Araus, J.-L.; Park, R.; Calderini, D.; Miralles, D.; Shen, T.; Zhang, J.; Parry, M.A.J. Prospects of doubling global wheat yields. Food Energy Secur. 2013, 2, 34–48. [Google Scholar] [CrossRef]
- Rose, T.; Kage, H. The Contribution of Functional Traits to the Breeding Progress of Central-European Winter Wheat Under Differing Crop Management Intensities. Front. Plant. Sci. 2019, 10, 1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Huybers, P. Reckoning wheat yield trends. Environ. Res. Lett. 2012, 7. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Austin, R.B.; Bingham, J.; Blackwell, R.D.; Evans, L.T.; Ford, M.A.; Morgan, C.L.; Taylor, M. Genetic improvements in winter wheat yields since 1900 and associated physiological changes. J. Agric. Sci. 1980, 94, 675–689. [Google Scholar] [CrossRef]
- Long, S.P. We need winners in the race to increase photosynthesis in rice, whether from conventional breeding, biotechnology or both. Plant Cell Environ. 2014, 37, 19–21. [Google Scholar] [CrossRef]
- Lee, H.; Teff, A. Rising Global Crop: Current Status of Teff Production and Value Chain. Open Agric. J. 2018, 12, 185–193. [Google Scholar] [CrossRef]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; Koning, F. The Ethiopian cereal tef in celiac disease. N. Engl. J. Med. 2005, 353, 1748–1749. [Google Scholar] [CrossRef] [PubMed]
- Cannarozzi, G.; Plaza-Wüthrich, S.; Esfeld, K.; Larti, S.; Wilson, Y.S.; Girma, D.; de Castro, E.; Chanyalew, S.; Blösch, R.; Farinelli, L. Genome and transcriptome sequencing identifies breeding targets in the orphan crop tef (Eragrostis tef). BMC Genom. 2014, 15, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F. Chemical composition and food uses of teff (Eragrostis tef). Food Chem. 2018, 239, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Chanyalew, S.; Ferede, S.; Damte, T.; Fikre, T.; Genet, Y.; Kebede, W.; Tolossa, K.; Tadele, Z.; Assefa, K. Significance and prospects of an orphan crop tef. Planta 2019, 250, 753–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assefa, K.; Cannarozzi, G.; Girma, D.; Kamies, R.; Chanyalew, S.; Plaza-Wuthrich, S.; Blosch, R.; Rindisbacher, A.; Rafudeen, S.; Tadele, Z. Genetic diversity in tef [Eragrostis tef (Zucc.) Trotter]. Front. Plant. Sci. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CSA. Agricultural Sample Survey: Report on Area and Production of Major Crops; Federal Democratic Republic of Ethiopia: Addis Ababa, Ethiopia, 2019. [Google Scholar]
- Abraha, M.T.; Shimelis, H.; Laing, M.; Assefa, K. Performance of Tef [Eragrostis tef (Zucc.) Trotter] Genotypes for Yield and Yield Components Under Drought-Stressed and Non-Stressed Conditions. Crop Sci. 2016, 56, 1799–1806. [Google Scholar] [CrossRef]
- Evans, L.; Fischer, R. Yield potential: Its definition, measurement, and significance. Crop Sci. 1999, 39, 1544–1551. [Google Scholar] [CrossRef]
- Senapati, N.; Semenov, M.A. Large genetic yield potential and genetic yield gap estimated for wheat in Europe. Glob. Food Sec. 2020, 24, 100340. [Google Scholar] [CrossRef]
- Abraha, M.T.; Hussein, S.; Laing, M.; Assefa, K. Genetic variation and trait association of tef (Eragrostis tef (Zucc.) Trotter) evaluated under optimal and moisture stressed environments. Aust. J. Crop Sci. 2017, 11, 241–247. [Google Scholar] [CrossRef]
- Assefa, K.; Tefera, H.; Merker, A.; Kefyalew, T.; Hundera, F. Variability, heritability and genetic advance in pheno-morphic and agronomic traits of tef [Eragrostis tef (Zucc.) Trotter] germplasm from eight regions of Ethiopia. Hereditas 2001, 134, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, H.; Genet, T.; Dessalegn, T.; Wondale, L. Multivariate diversity, heritability and genetic advance in tef landraces in Ethiopia. Afr. Crop Sci. J. 2011, 19, 201–212. [Google Scholar]
- Teklu, Y.; Tefera, H. Genetic improvement in grain yield potential and associated agronomic traits of tef (Eragrostis tef). Euphytica 2005, 141, 247–254. [Google Scholar] [CrossRef]
- Jifar, H.; Assefa, K.; Tadele, Z. Grain yield variation and association of major traits in brown-seeded genotypes of tef [Eragrostis tef (Zucc.) Trotter]. Agric. Food Secur. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- SAS. The SAS System for Windows, Ver. 9.0; SAS Institute Inc.: Cary, NC, USA, 2005. [Google Scholar]
- Alvarado, G.; Rodríguez, F.M.; Pacheco, A.; Burgueño, J.; Crossa, J.; Vargas, M.; Pérez-Rodríguez, P.; Lopez-Cruz, M.A. Meta-R: A software to analyze data from multi-environment plant breeding trials. Crop J. 2020, 8, 745–756. [Google Scholar] [CrossRef]
- Burton, G.W.; Devane, D.E. Estimating heritability in tall fescue (Festuca arundinacea) from replicated clonal material 1. Agron. J. 1953, 45, 478–481. [Google Scholar] [CrossRef]
- Johnson, H.W.; Robinson, H.; Comstock, R. Estimates of genetic and environmental variability in soybeans 1. Agron. J. 1955, 47, 314–318. [Google Scholar] [CrossRef]
- Singh, R.K.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalayani Publishers: New Delhi, India, 1985. [Google Scholar]
- Rajaram, S. Approaches for breaching yield stagnation in wheat. Genome 1999, 42, 629–634. [Google Scholar] [CrossRef]
- Assefa, K.; Ketema, S.; Tefera, H.; Nguyen, H.T.; Blum, A.; Ayele, M.; Bai, G.; Simane, B.; Kefyalew, T. Diversity among germplasm lines of the Ethiopian cereal tef [Eragrostis tef (Zucc.) Trotter]. Euphytica 1999, 106, 87–97. [Google Scholar] [CrossRef]
- George, T. Why crop yields in developing countries have not kept pace with advances in agronomy. Glob. Food Secur. 2014, 3, 49–58. [Google Scholar] [CrossRef]
- Hay, R. Harvest index: A review of its use in plant breeding and crop physiology. Ann. Appl. Biol. 1995, 126, 197–216. [Google Scholar] [CrossRef]
- Araya, A.; Keesstra, S.D.; Stroosnijder, L. Simulating yield response to water of Teff (Eragrostis tef) with FAO’s AquaCrop model. Field Crops Res. 2010, 116, 196–204. [Google Scholar] [CrossRef]
- Assefa, K.; Ketema, S.; Tefera, H.; Kefyalew, T.; Chundera, F. Trait diversity, heritability and genetic advance in selected germplasm lines of tef [Emgmstis tef (Zucc.) Trotter]. Hereditas 2000, 133, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Funte, S.; Negesse, T.; Legesse, G. Feed resources and their management systems in Ethiopian highlands: The case of Umbulo Whaco watershed in Southern Ethiopia. Trop. Subtrop. Agroecosyst. 2009, 12, 47–56. [Google Scholar]
- Duncan, A.J.; Bachewe, F.; Mekonnen, K.; Valbuena, D.; Rachier, G.; Lule, D.; Bahta, M.; Erenstein, O. Crop residue allocation to livestock feed, soil improvement and other uses along a productivity gradient in Eastern Africa. Agric. Ecosyst. Environ. 2016, 228, 101–110. [Google Scholar] [CrossRef]
- Mottaleb, K.A.; Rahut, D.B. Household production and consumption patterns ofTeffin Ethiopia. Agribusiness 2018, 34, 668–684. [Google Scholar] [CrossRef]
- Mengistu, A. Comparative Supplemental Value of Noug (Guizotia abyssinica) Meal for Fattening Draught Oxen Fed Teff(Eragrostis teff) Straw. J. Appl. Anim. Res. 2003, 24, 85–88. [Google Scholar] [CrossRef]
- Gebremariam, T.; Belay, S. Livestock feed resources utilization practices in Tanqua-Abergelle district of Tigray, Northern Ethiopia. Trop. Anim. Health Prod. 2016, 48, 1183–1190. [Google Scholar] [CrossRef]
- Bayable, M.; Tsunekawa, A.; Haregeweyn, N.; Ishii, T.; Alemayehu, G.; Tsubo, M.; Adgo, E.; Tassew, A.; Tsuji, W.; Asaregew, F.; et al. Biomechanical Properties and Agro-Morphological Traits for Improved Lodging Resistance in Ethiopian Teff (Eragrostis tef (Zucc.) Trottor) Accessions. Agronomy 2020, 10, 1012. [Google Scholar] [CrossRef]
- Jost, M.; Esfeld, K.; Burian, A.; Cannarozzi, G.; Chanyalew, S.; Kuhlemeier, C.; Assefa, K.; Tadele, Z. Semi-dwarfism and lodging tolerance in tef (Eragrostis tef) is linked to a mutation in the alpha-Tubulin 1 gene. J. Exp. Bot. 2015, 66, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Madić, M.; Knežević, D.; Paunović, A.; Đurović, D. Plant height and internode length as components of lodging resistance in barley. Acta Agric. Serb. 2016, 21, 99–106. [Google Scholar] [CrossRef]
- Woldeyohannes, A.B.; Accotto, C.; Desta, E.A.; Kidane, Y.G.; Fadda, C.; Pè, M.E.; Dell’Acqua, M. Current and projected eco-geographic adaptation and phenotypic diversity of Ethiopian teff (Eragrostis teff) across its cultivation range. Agric. Ecosyst. Environ. 2020, 300. [Google Scholar] [CrossRef]
- Atnaf, M.; Tesfaye, K.; Dagne, K.; Wegary, D. Genotype by trait biplot analysis to study associations and profiles of Ethiopian white lupin (Lupinus albus L.) landraces. Aust. J. Crop Sci. 2017, 11, 55–62. [Google Scholar] [CrossRef]
- Francia, E.; Tondelli, A.; Rizza, F.; Badeck, F.W.; Li Destri Nicosia, O.; Akar, T.; Grando, S.; Al-Yassin, A.; Benbelkacem, A.; Thomas, W.T.B.; et al. Determinants of barley grain yield in a wide range of Mediterranean environments. Field Crops Res. 2011, 120, 169–178. [Google Scholar] [CrossRef]
- Yang, C.; Fraga, H.; van Ieperen, W.; Trindade, H.; Santos, J.A. Effects of climate change and adaptation options on winter wheat yield under rainfed Mediterranean conditions in southern Portugal. Clim. Chang. 2019, 154, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Giunta, F.; Motzo, R.; Pruneddu, G. Trends since 1900 in the yield potential of Italian-bred durum wheat cultivars. Eur. J. Agron. 2007, 27, 12–24. [Google Scholar] [CrossRef]
- Molla, A.; Muhie, K. Tef (Eragrostis tef) based cropping systems in the hot to warm moist valleys of North Shewa, Ethiopia. Sci. Res. Essays 2011, 6, 1411–1416. [Google Scholar]
| Genotypes | Accession Number | GY | BM | HI | DH | DM | GFP | PH | PL | PDL | PW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ten high yielding genotypes | 242138-1 | 8.8 | 18.7 | 0.45 | 63 | 129 | 67 | 128.1 | 35.7 | 26.6 | 1.9 |
| 236756-2 | 8.7 | 19.0 | 0.44 | 59 | 131 | 73 | 128.9 | 33.3 | 25.6 | 2.2 | |
| 242200-1 | 8.5 | 21.6 | 0.38 | 65 | 130 | 66 | 136.0 | 30.7 | 28.2 | 1.8 | |
| 235671-1 | 8.3 | 19.4 | 0.41 | 63 | 123 | 60 | 141.1 | 31.1 | 29.8 | 1.8 | |
| 227786-4 | 8.2 | 17.8 | 0.45 | 66 | 130 | 65 | 130.9 | 32.9 | 25.3 | 1.9 | |
| 229101-1 | 8.2 | 19.3 | 0.41 | 60 | 125 | 65 | 134.5 | 35.8 | 26.9 | 2.3 | |
| Abishlemne | 8.1 | 21.1 | 0.38 | 63 | 125 | 63 | 115.4 | 35.1 | 24.0 | 1.7 | |
| 229971-3 | 8.1 | 20.3 | 0.40 | 57 | 123 | 66 | 139.5 | 31.0 | 24.4 | 1.8 | |
| 244783-3 | 8.1 | 17.9 | 0.44 | 58 | 130 | 73 | 134.4 | 33.3 | 25.8 | 2.3 | |
| 234430-1 | 8.1 | 23.1 | 0.34 | 59 | 130 | 72 | 128.3 | 32.4 | 24.7 | 2.2 | |
| Cultivars | Etsub | 6.7 | 22.4 | 0.30 | 60 | 130 | 71 | 138.5 | 36.8 | 25.6 | 2.1 |
| Quncho | 6.4 | 17.8 | 0.36 | 60 | 123 | 63 | 128.5 | 32.7 | 27.9 | 1.8 | |
| Abola | 6.4 | 19.6 | 0.33 | 63 | 121 | 58 | 144.2 | 42.1 | 21.9 | 2.3 | |
| Low yielding genotypes | 219850-1 | 4.3 | 16.7 | 0.27 | 62 | 108 | 46 | 102.9 | 34.5 | 25.6 | 1.7 |
| 239373-2 | 4.3 | 18.0 | 0.25 | 63 | 111 | 48 | 100.7 | 28.0 | 28.4 | 1.4 | |
| 55069-3 | 4.2 | 16.1 | 0.28 | 60 | 109 | 49 | 115.3 | 21.6 | 25.2 | 1.6 | |
| Grand mean | 6.2 | 18.4 | 0.34 | 60 | 121 | 60 | 126.9 | 32.2 | 26.4 | 1.8 | |
| LSD | 0.9 | 4.0 | 0.1 | 6.7 | 11 | 12 | 15.0 | 6.1 | 6.0 | 0.4 | |
| Genotypes | Accession Number | GY | BM | HI | DH | DM | GFP | PH | PL | PDL | PW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ten high yielding genotypes | 229971-3 | 4.3 | 10.9 | 0.33 | 55 | 127 | 71 | 107.7 | 31.5 | 22.7 | 1.5 |
| 236765-3 | 4.3 | 11.1 | 0.33 | 62 | 129 | 67 | 109.7 | 32.1 | 24.0 | 1.5 | |
| 234430-1 | 4.3 | 11.6 | 0.32 | 60 | 129 | 68 | 104.8 | 33.2 | 25.8 | 1.5 | |
| 236756-2 | 4.2 | 11.4 | 0.31 | 59 | 129 | 70 | 113.6 | 35.2 | 26.5 | 1.5 | |
| DZ-01-3502 | 4.2 | 11.2 | 0.31 | 58 | 130 | 71 | 94.1 | 31.0 | 21.8 | 1.3 | |
| RIL-260 | 4.1 | 12.6 | 0.30 | 61 | 131 | 69 | 128.6 | 42.2 | 25.1 | 1.8 | |
| 203010-4 | 4.1 | 13.5 | 0.27 | 61 | 131 | 69 | 125.0 | 38.7 | 26.2 | 1.5 | |
| 202978-2 | 4.1 | 11.0 | 0.32 | 62 | 129 | 67 | 108.7 | 33.8 | 24.5 | 1.3 | |
| 238223-2 | 4.0 | 11.9 | 0.29 | 58 | 128 | 69 | 104.1 | 30.1 | 25.1 | 1.0 | |
| 235659-3 | 4.0 | 12.1 | 0.30 | 59 | 127 | 67 | 110.1 | 32.3 | 25.9 | 1.2 | |
| Cultivars | Etsub | 3.9 | 12.8 | 0.30 | 60 | 128 | 68 | 110.2 | 34.8 | 23.4 | 1.4 |
| Quncho | 3.0 | 10.6 | 0.28 | 58 | 123 | 65 | 106.7 | 32.2 | 21.0 | 1.4 | |
| Abola | 3.4 | 12.8 | 0.27 | 64 | 130 | 66 | 113.4 | 37.4 | 23.3 | 1.5 | |
| Low yielding genotypes | 229101-3 | 1.8 | 8.3 | 0.22 | 62 | 124 | 62 | 93.0 | 31.4 | 25.2 | 0.9 |
| 234775-4 | 1.8 | 7.5 | 0.24 | 62 | 130 | 65 | 97.7 | 32.6 | 22.9 | 0.9 | |
| 219882-4 | 1.8 | 8.3 | 0.22 | 64 | 125 | 62 | 94.1 | 33.0 | 24.3 | 0.9 | |
| Grand mean | 3.1 | 10.7 | 0.29 | 60 | 126 | 65 | 99.3 | 31.2 | 24.5 | 1.1 | |
| LSD | 0.5 | 2.0 | 0.1 | 2.7 | 4.3 | 5.0 | 8.3 | 4.3 | 3.0 | 0.2 | |
| Traits | Mean Square | Means | Variance | GCV (%) | PCV (%) | H | GA (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| G | σ2e | σ2G | σ2P | ||||||
| DH | 40.46 ** | 6.69 | 60.39 | 16.88 | 20.23 | 6.80 | 7.45 | 0.83 | 12.73 |
| DM | 146.27 ** | 30.25 | 120.77 | 58.01 | 73.13 | 6.31 | 7.08 | 0.79 | 11.52 |
| GFP | 192.41 ** | 34.71 | 60.37 | 78.85 | 96.21 | 14.71 | 16.25 | 0.82 | 27.44 |
| PH | 307.11 ** | 59.43 | 126.90 | 123.84 | 153.56 | 8.77 | 9.77 | 0.81 | 16.29 |
| PL | 51.26 ** | 9.59 | 32.19 | 20.83 | 25.63 | 14.18 | 15.73 | 0.81 | 26.24 |
| PDL | 22.54 ** | 9.27 | 26.39 | 6.63 | 11.27 | 9.76 | 12.72 | 0.59 | 15.46 |
| PW | 0.12 ** | 0.02 | 1.77 | 0.05 | 0.06 | 12.88 | 14.01 | 0.84 | 24.24 |
| GY | 2.24 ** | 0.42 | 6.24 | 0.91 | 1.02 | 15.29 | 16.19 | 0.81 | 27.01 |
| BM | 10.54 ** | 2.40 | 18.62 | 4.07 | 4.70 | 10.83 | 11.64 | 0.77 | 18.47 |
| HI | 0.004 ** | 0.001 | 0.34 | 0.001 | 0.001 | 8.82 | 11.00 | 0.67 | 15.20 |
| Traits | Mean Square | Means | Variance | GCV (%) | PCV (%) | H | GA (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| G | E | G x E | σ2G | σ2P | ||||||
| DH | 69.82 ** | 23941 ** | 9.70 ** | 60.27 | 7.40 | 8.58 | 4.51 | 4.86 | 0.86 | 8.61 |
| DM | 165.7 ** | 27092 ** | 27.42 ** | 125.61 | 17.60 | 20.84 | 3.34 | 3.63 | 0.84 | 6.29 |
| GFP | 123.0 ** | 9017 ** | 29.6 ** | 65.34 | 12.20 | 15.68 | 5.35 | 6.06 | 0.78 | 9.74 |
| PH | 815 ** | 58858 ** | 27.4 ** | 99.26 | 93.62 | 98.72 | 9.75 | 10.01 | 0.95 | 19.59 |
| PL | 146.2 ** | 35059 ** | 39.5 ** | 28.20 | 13.05 | 17.87 | 11.58 | 13.55 | 0.73 | 22.50 |
| PDL | 63.0 ** | 3004.8 ** | 8.85 ** | 24.52 | 6.70 | 7.79 | 10.56 | 11.38 | 0.86 | 20.17 |
| PW | 0.44 ** | 32.28 ** | 0.08 ** | 1.08 | 0.05 | 0.05 | 19.64 | 21.67 | 0.82 | 36.60 |
| GY | 3.07 ** | 231.0 ** | 0.45 ** | 3.10 | 0.33 | 0.38 | 20.7 | 22.36 | 0.86 | 35.20 |
| BM | 18.3 ** | 6087.1 ** | 6.08 ** | 10.70 | 1.53 | 2.25 | 12.46 | 15.10 | 0.68 | 19.64 |
| HI | 0.009 ** | 0.385 ** | 0.004 ** | 0.29 | 0.0007 | 0.001 | 8.79 | 11.82 | 0.57 | 13.90 |
| DH | DM | GFP | PH | PL | PDL | PW | GY | BM | HI | |
|---|---|---|---|---|---|---|---|---|---|---|
| DH | −0.06 | −0.52 ** | −0.24 * | −0.13 ns | 0.35 * | −0.10 ns | −0.22 * | −0.15 * | −0.22 * | |
| DM | −0.04 ns | 0.89 *** | 0.61 ** | 0.49 ** | −0.07 ns | 0.51 ** | 0.77 *** | 0.71 *** | 0.54 ** | |
| GFP | −0.49 ** | 0.89 *** | 0.64 ** | 0.48 ** | −0.22 * | 0.48 ** | 0.76 *** | 0.68 *** | 0.56 ** | |
| PH | −0.19 * | 0.65 ** | 0.65 ** | 0.63 ** | 0.02 ns | 0.50 ** | 0.69 *** | 0.70 *** | 0.44 ** | |
| PL | −0.09 ns | 0.55 ** | 0.52 ** | 0.68 *** | −0.14 * | 0.45 ** | 0.52 ** | 0.63 ** | 0.23 * | |
| PDL | 0.20 * | −0.02 ns | −0.11 ns | 0.04 ns | −0.08 ns | 0.001 ns | −0.03 ns | −0.03 ns | 0.002 ns | |
| PW | −0.08 ns | 0.55 ** | 0.51 ** | 0.56 ** | 0.51 ** | 0.02 ns | 0.53 ** | 0.55 ** | 0.33 * | |
| GY | −0.17 * | 0.78 *** | 0.76 *** | 0.74 *** | 0.60 ** | 0.002 ns | 0.58 ** | 0.81 *** | 0.82 *** | |
| BM | −0.11 ns | 0.59 ** | 0.57 ** | 0.58 ** | 0.53 ** | −0.01 ns | 0.47 ** | 0.67 *** | 0.33 * | |
| HI | −0.14 * | 0.51 ** | 0.51 ** | 0.48 ** | 0.34 * | 0.02 ns | 0.37 * | 0.74 *** | −0.004 ns |
| DH | DM | GFP | PH | PL | PDL | PW | GY | BM | HI | |
|---|---|---|---|---|---|---|---|---|---|---|
| DH | 0.56 ** | −0.10 ns | 0.46 ** | 0.34 * | −0.18 * | 0.38 * | −0.23 * | −0.05 ns | −0.45 ** | |
| DM | 0.51 ** | 0.77 *** | 0.42 * | 0.31 * | 0.09 ns | 0.45 ** | 0.10 ns | 0.15 * | −0.03 ns | |
| GFP | −0.14 * | 0.78 *** | 0.16 * | 0.12 * | 0.25 * | 0.25 * | 0.30 * | 0.21 * | 0.31 * | |
| PH | 0.41 * | 0.36 * | 0.11 ns | 0.96 *** | −0.01 ns | 0.89 *** | 0.26 * | 0.60 ** | −0.25 * | |
| PL | 0.30 * | 0.24 * | 0.06 ns | 0.83 *** | −0.38 * | 0.89 *** | 0.36 * | 0.67 *** | −0.14 * | |
| PDL | −0.17 * | 0.07 ns | 0.21 * | −0.01 ns | −0.36 * | −0.06 ns | −0.10 ns | −0.16 * | 0.06 ns | |
| PW | 0.34 * | 0.39 * | 0.20 * | 0.80 *** | 0.72 *** | −0.04 ns | 0.46 ** | 0.69 *** | 0.05 ns | |
| GY | −0.22 * | 0.09 ns | 0.26 * | 0.23 * | 0.28 * | −0.07 ns | 0.40 * | 0.94 *** | 0.88 *** | |
| BM | −0.05 ns | 0.13 * | 0.18 * | 0.49 ** | 0.49 ** | −0.10 ns | 0.55 ** | 0.84 *** | 0.66 ** | |
| HI | −0.34 * | −0.03 ns | 0.21 * | −0.22 * | −0.13 * | 0.05 ns | 0.02 ns | 0.71 *** | 0.24 * |
| Cluster | DH | DM | GFP | PH | PL | PDL | PW | GY | BM | HI |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 58.60 | 121.35 | 62.73 | 128.21 | 33.75 | 24.14 | 1.87 | 6.38 | 19.32 | 0.33 |
| II | 60.22 | 128.32 | 68.35 | 136.57 | 35.60 | 26.50 | 1.91 | 6.98 | 20.28 | 0.34 |
| III | 61.34 | 126.90 | 65.78 | 133.60 | 33.04 | 26.96 | 1.96 | 7.92 | 18.71 | 0.41 |
| IV | 63.78 | 115.62 | 51.73 | 127.87 | 31.39 | 27.09 | 1.70 | 6.01 | 18.53 | 0.33 |
| V | 58.84 | 114.32 | 55.24 | 117.26 | 28.48 | 26.16 | 1.60 | 5.26 | 17.07 | 0.32 |
| VI | 58.91 | 119.86 | 60.90 | 126.18 | 32.12 | 27.24 | 1.76 | 6.19 | 18.17 | 0.34 |
| VII | 70.11 | 114.56 | 44.42 | 109.89 | 28.12 | 27.14 | 1.59 | 5.31 | 16.77 | 0.30 |
| Cluster | DH | DM | GFP | PH | PL | PDL | PW | GY | BM | HI |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 60.91 | 125.80 | 64.92 | 97.84 | 31.37 | 24.22 | 0.99 | 2.60 | 9.15 | 0.27 |
| II | 60.11 | 128.52 | 67.93 | 121.73 | 35.37 | 24.61 | 1.38 | 3.81 | 11.61 | 0.29 |
| III | 60.02 | 126.69 | 66.58 | 101.87 | 31.45 | 25.31 | 1.15 | 3.32 | 10.35 | 0.29 |
| IV | 58.74 | 118.18 | 59.92 | 95.67 | 30.79 | 23.34 | 1.01 | 2.98 | 9.97 | 0.29 |
| V | 63.13 | 128.61 | 65.65 | 106.26 | 34.06 | 21.60 | 1.21 | 2.89 | 10.11 | 0.27 |
| VI | 59.47 | 125.37 | 65.88 | 87.24 | 26.61 | 25.87 | 0.86 | 2.68 | 8.95 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayable, M.; Tsunekawa, A.; Haregeweyn, N.; Alemayehu, G.; Tsuji, W.; Tsubo, M.; Adgo, E.; Tassew, A.; Ishii, T.; Asaregew, F.; et al. Yield Potential and Variability of Teff (Eragrostis tef (Zucc.) Trotter) Germplasms under Intensive and Conventional Management Conditions. Agronomy 2021, 11, 220. https://doi.org/10.3390/agronomy11020220
Bayable M, Tsunekawa A, Haregeweyn N, Alemayehu G, Tsuji W, Tsubo M, Adgo E, Tassew A, Ishii T, Asaregew F, et al. Yield Potential and Variability of Teff (Eragrostis tef (Zucc.) Trotter) Germplasms under Intensive and Conventional Management Conditions. Agronomy. 2021; 11(2):220. https://doi.org/10.3390/agronomy11020220
Chicago/Turabian StyleBayable, Muluken, Atsushi Tsunekawa, Nigussie Haregeweyn, Getachew Alemayehu, Wataru Tsuji, Mitsuru Tsubo, Enyew Adgo, Asaminew Tassew, Takayoshi Ishii, Fekremariam Asaregew, and et al. 2021. "Yield Potential and Variability of Teff (Eragrostis tef (Zucc.) Trotter) Germplasms under Intensive and Conventional Management Conditions" Agronomy 11, no. 2: 220. https://doi.org/10.3390/agronomy11020220
APA StyleBayable, M., Tsunekawa, A., Haregeweyn, N., Alemayehu, G., Tsuji, W., Tsubo, M., Adgo, E., Tassew, A., Ishii, T., Asaregew, F., & Masunaga, T. (2021). Yield Potential and Variability of Teff (Eragrostis tef (Zucc.) Trotter) Germplasms under Intensive and Conventional Management Conditions. Agronomy, 11(2), 220. https://doi.org/10.3390/agronomy11020220








