Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate
Abstract
:1. Introduction
2. Adaptive Mechanisms during Abiotic Stress in Wheat
2.1. Biochemical Adaptation under Abiotic Oxidative Stress
2.2. Production of Antioxidants
2.3. Phytohormones
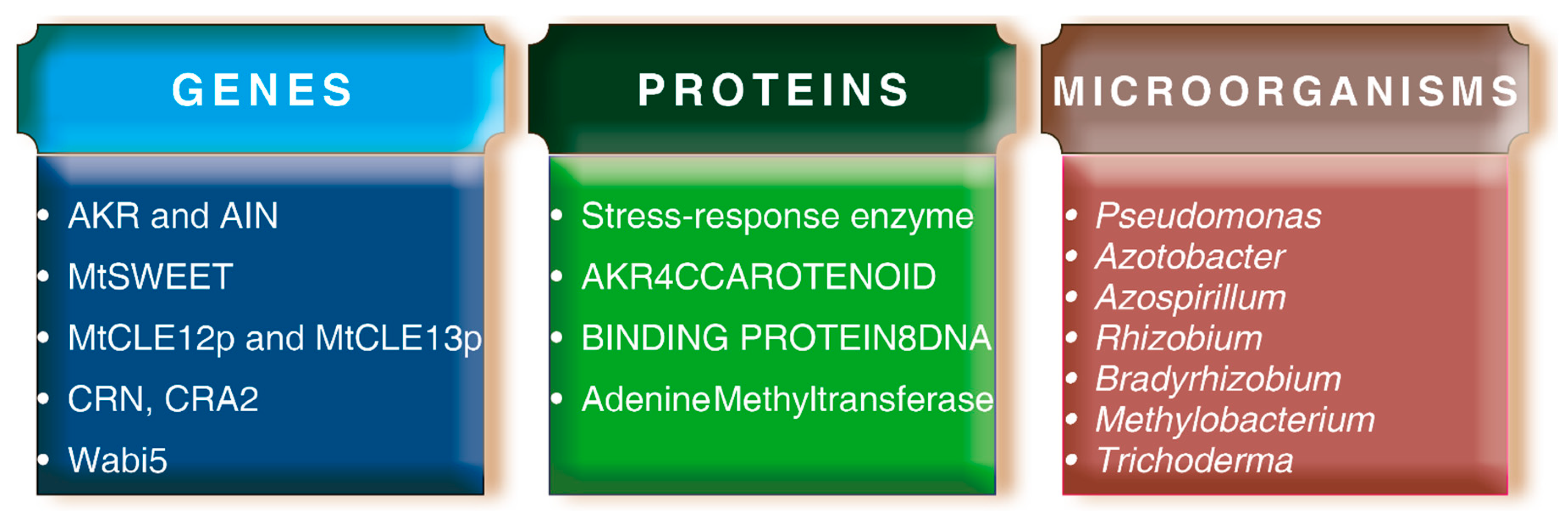
2.4. Role of Plant Growth-Promoting Microorganisms (PGPM) during Abiotic Stresses in Plants
2.5. Breeding, Biotechnology and Genetic Engineering Approaches for Stress Tolerance in Plants
2.5.1. Conventional Breeding
Germplasm Selection
Testing Environment
Target Traits
Heterosis Exploitation
2.5.2. Molecular Mechanisms
Genome-Wide Association (GWAS)
Quantitative Trait Loci (QTL) Mapping
Multi Parent Populations (MPP)
Backcross Populations (BC)
Homozygous Immortal Populations
2.5.3. Biotechnological Tools for Various Environmental Stress Tolerance in Plants
Tissue Culture
Somaclonal Variation
In Vitro Selection
2.6. Genetic Engineering
2.6.1. Genetic Modification Approach
2.6.2. Gene Expression and Functional Genomics
2.6.3. Molecular Marker-Assisted Breeding
2.6.4. Gene Pyramiding Assisted by MAS
2.7. Double Haploid Breeding
2.8. Wide Hybridization
3. Nano-Technology for Plants Tolerance to Abiotic Stress
4. Agronomic Approaches for Abiotic Stress Management
4.1. Adjustment of Sowing Time
4.2. Nutrients Management
4.2.1. Organic Nutrients Management
4.2.2. Inorganic Nutrients Management
4.3. Irrigation Management
4.4. Climate Smart Technology
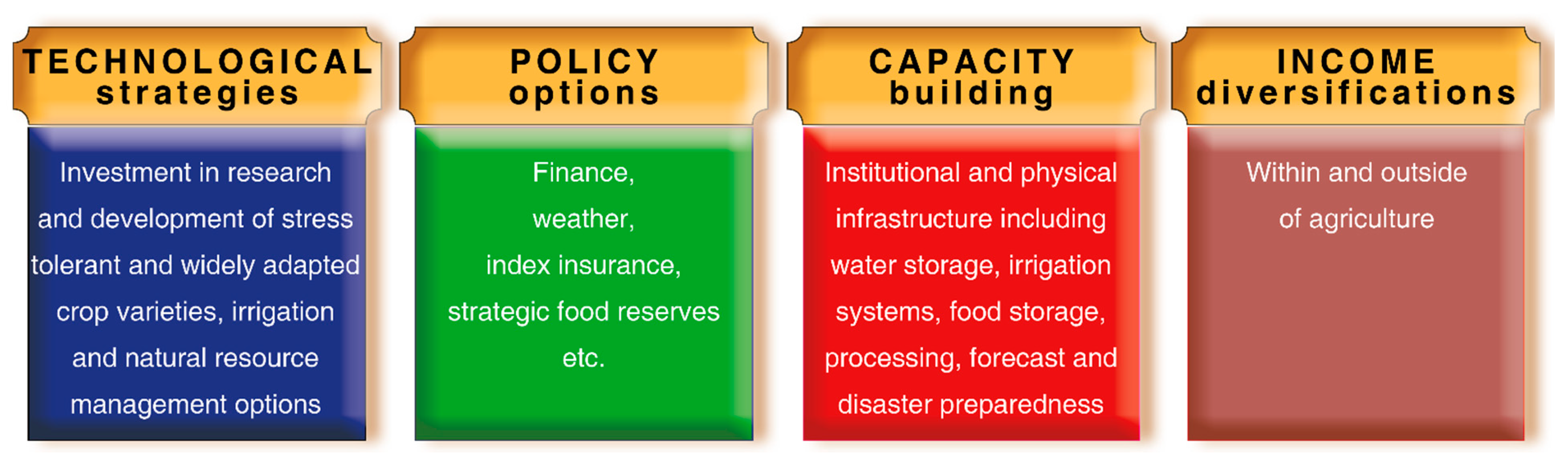
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ABA | Abscisic acid |
| APX | Ascorbate peroxidase |
| ASH | Ascorbate |
| ASH-GSH | Ascorbate–glutathione |
| BC | Backcross populations |
| CAT | Catalase GR glutathione reductase |
| CIMMYT | International Maize and Wheat Improvement Centre |
| CKs | Cytokinins |
| CRISPR-Cas | Clustered regularly interspaced short palindromic repeats |
| CSSLs | Chromosomal segment substitution lines |
| CWR | Crop Wild Relatives |
| DEPS | Degree of deep oxidation |
| DH | Doubled haploids |
| DHAR | Dehydro ascorbate reductase |
| DNA | Deoxyribonucleic acid |
| ET | Ethylene |
| FAO | Food and Agriculture Organization |
| GA | Gibberellic acid |
| GB | Glycine betaine |
| GR | Glutathione reductase |
| GS | Stomatal conductance |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| GST | Glutathione-s-transferase |
| GWAS | Genome-wide association studies |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| ICARDA | International Centre for Agricultural Research in Dry Areas |
| IPCC | Intergovernmental Panel on Climate Change |
| JA | Jasmonic acid |
| MDA | Malondialdehyde |
| MDHAR | Monodehydro ascorbate reductase |
| MPP | Multiparent populations |
| MTAs | Marker-trait associations |
| MWCNT | Multi-walled-carbon nanotube |
| NAM | Nested association mapping |
| NILs | Near-isogenic lines |
| NPQ | Non-photochemical quenching |
| NPs | Titanium dioxide nanoparticles |
| OECD | The Organisation for economic co-operation and development |
| PAL | Phenylalanine ammonialyase |
| PGPR | Plant growth-promoting rhizobacteria |
| PN | Net assimilation |
| POX | Peroxidase |
| PPO | Polyphenol oxidase |
| QTL | Quantitative trait loci |
| RILs | Recombinant inbred lines |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RWCS | Rice-wheat cropping systems |
| SA | Salicylic acid |
| SOD | Superoxide dismutases |
| VAM | Vesicular-arbuscular mycorrhiza |
| WUE | Water use efficiency |
References
- IPCC. Summary for policymakers. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Yohannes, H.A. Review on Relationship between Climate Change and Agriculture. J. Earth Sci. Clim. Chang. 2016, 7, 335. [Google Scholar] [CrossRef]
- Aryal, J.P.; Sapkota, T.B.; Khurana, R.; Khatri-Chhetri, A.; Rahut, D.B.; Jat, M.L. Climate change and agriculture in South Asia: Adaptation options in smallholder production systems. Environ. Dev. Sustain. 2020, 22, 5045–5075. [Google Scholar] [CrossRef] [Green Version]
- Oo, A.T.; Van Huylenbroeck, G.; Speelman, S. Measuring the Economic Impact of Climate Change on Crop Production in the Dry Zone of Myanmar: A Ricardian Approach. Climate 2020, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agricultural Organization). FAO Statistical Pocket Book; Food and Agricultural Organization: Rome, Italy, 2015; p. 28. [Google Scholar]
- FAOSTAT. Wheat Production Statistics. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 January 2020).
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, M.H.; Sarwar, M.F.; Sarwar, M.; Qadri, N.A.; Moghal, S. The importance of cereals (Poaceae: Gramineae) nutrition in human health: A review. J. Cereals Oilseeds 2013, 4, 32–35. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production; Hasanuzzaman, M., Fujita, M., Teixeira Filho, M.C.M., Nogueira, T.A.R., Galindo, F.S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Wheat Initiative. In Annual Report: Coordinating Global Wheat Research; Federal Research Centre for Cultivated Plants: Berlin, Germany, 2018; p. 60. Available online: https://www.wheatinitiative.org/ (accessed on 28 January 2021).
- Islam, T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; Hossain, M.S.; Gupta, D.R.; Rahman, M.; Mahboob, M.G.; et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 2016, 14, 84. [Google Scholar] [CrossRef] [Green Version]
- Islam, T.; Gupta, D.R.; Hossain, A.; Roy, K.K.; He, X.; Kabir, M.R.; Singh, P.K.; Khan, A.R.; Rahman, M.; Wang, G.-L. Wheat blast: A new threat to food security. Phytopathol. Res. 2020, 2, 28. [Google Scholar] [CrossRef]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.N.; Jamshed, M.; Samuel, M.A. Abiotic Stress Signaling in Wheat—An Inclusive Overview of Hormonal Interactions during Abiotic Stress Responses in Wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rotter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of Drought Stress and its Mechanism in Plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, R.; Sheoran, S.; Rane, J. Wheat improvement for drought and heat tolerance. In Recent Trends on Production Strategies of Wheat in India; Shukla, R.S., Mishra, P.C., Chatrath, R., Gupta, R.K., Tomar, S.S., Sharma, I., Eds.; Directorate of Wheat Research: Karnal, Haryana, India, 2015; pp. 39–58. [Google Scholar]
- Singh, C. Modern Techniques of Raising Field Crops, 4th ed.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 1988; p. 46. [Google Scholar]
- Porter, J.R.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1988, 10, 23–26. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Islam, M.T.; Islam, M.T. Wheat (Triticum aestivum L.) in the rice-wheat systems of South Asia is influenced by terminal heat stress at late sown condition: A case in Bangladesh. In Plant Stress Physiology; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Rane, J.; Pannu, R.K.; Sohu, V.S.; Saini, R.S.; Mishra, B.; Shoran, J.; Crossa, J.; Vargas, M.; Joshi, A.K. Performance of yield and stability of advanced wheat genotypes under heat stress environments of the Indo-Gangetic Plains. Crop Sci. 2007, 47, 1561–1573. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Dawson, I.A.; Munibi, P.; Fewster, R. The tolerance of wheat to high temperatures during reproductive growth. I. Survey procedures and general response patterns. Aust. J. Agric. Res. 1989, 40, 1–13. [Google Scholar] [CrossRef]
- Mukherjee, A.; Wang, S.-Y.S.; Promchote, P. Examination of the Climate Factors That Reduced Wheat Yield in Northwest India during the 2000s. Water 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Mamrutha, H.M.; Sareen, S.; Sheoran, S.; Tiwari, R.; Sharma, P.; Singh, C.; Singh, G.; Rane, J. Managing abiotic stresses in wheat. In Abiotic Stress Management for Resilient Agriculture; Minhas, P.S., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2017; pp. 313–337. [Google Scholar] [CrossRef]
- Loutfy, N.; Sakuma, Y.; Gupta, D.K.; Inouhe, M. Modifications of water status, growth rate and antioxidant system in two wheat cultivars as affected by salinity stress and salicylic acid. J. Plant Res. 2020, 133, 549–570. [Google Scholar] [CrossRef]
- IPCC. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. In A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.K., Allen, S.K., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; 582p. [Google Scholar]
- Newberry, M.; Zwart, A.B.; Whan, A.; Mieog, J.C.; Sun, M.; Leyne, E.; Pritchard, J.; Daneri-Castro, S.N.; Ibrahim, K.; Diepeveen, D.; et al. Does late maturity alpha-amylase impact wheat baking quality? Front. Plant Sci. 2018, 9, 1356. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M. Agricultural Productivity Current Scenario, Constraints and Future Prospects in Pakistan. Sarhad J. Agric. 2016, 32, 289–303. [Google Scholar] [CrossRef]
- Singh, S.K.; Saxena, R.; Porwal, A.N.; Ray, S.S. Assessment of Hailstorm Damage in Wheat Crop Using Remote Sensing. Curr. Sci. 2017, 112, 2095–2100. [Google Scholar] [CrossRef]
- Bhowmik, P.; Hassan, M.M.; Molla, K.; Rahman, M.; Islam, M.T. Application of CRISPR-Cas genome editing tools for the improvement of plant abiotic stress tolerance. In Approaches for Enhancing Abiotic Stress Tolerance in Plants; Hasanuzzaman, M., Nahar, K., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 459–472. [Google Scholar]
- Rychter, A.M. Antioxidants and reactive oxygen species in plants. Ann. Bot. 2006, 98, 1114. [Google Scholar] [CrossRef] [Green Version]
- Mattos, L.M.; Moretti, C.L. Oxidative stress in plants under drought conditions and the role of different enzymes. Enz. Eng. 2015, 5, 136. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Rustamova, S.M.; Suleymanov, S.Y.; Aliyeva, D.R.; Mam-madov, A.C.; Aliyev, J.A. Drought-induced changes in pho-tosynthetic apparatus and antioxidant components of wheat (Triticum durum Desf.) varieties. Photosynth. Res. 2016, 130, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef] [Green Version]
- Tambussi, E.A.; Bartoli, C.G.; Beltrano, J.; Guiamet, J.J.; Araus, J.L. Oxidative damage to thylakoid proteins in water-stressed leaves of wheat (Triticum aestivum). Physiol. Plant. 2000, 108, 398–404. [Google Scholar] [CrossRef]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, U.; Pradhan, B. Drought stress-induced oxidative stress and antioxidative responses in four wheat (Triticum aestivum L.) varieties. Arch. Agron. Soil Sci. 2012, 58, 617–630. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Sartawe, F.A.B.; Qaryouti, M.M. Characterization of γ-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J. Plant Physiol. 2013, 170, 1003–1009. [Google Scholar] [CrossRef]
- Masood, S.; Saleh, L.; Witzel, K.; Plieth, C.; Mühling, K.H. Determination of oxidative stress in wheat leaves as influenced by boron toxicity and NaCl stress. Plant Physiol. Biochem. 2012, 56, 56–61. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ashraf, M.A.; Ashraf, M.; Ali, Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents. Pak. J. Bot. 2010, 42, 559–565. [Google Scholar]
- Savicka, M.; Škute, N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 2010, 56, 26–33. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop. Sci. 2012, 6, 1314–1323. [Google Scholar]
- Boscaiu, M.; Fita, A. Physiological and Molecular Characterization of Crop Resistance to Abiotic Stresses. Agronomy 2020, 10, 1308. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Yoshimasa, N.; Yasuaki, S.; Yoshiyuki, M. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 65, 813–824. [Google Scholar] [CrossRef]
- Swamy, B.P.; Kaladhar, K.; Ashok, R.G.; Viraktamath, B.C.; Sarla, N. Mapping and introgression QTLs for yield and related traits in two backcross populations derived from O. sativa cv Swarna and two accessions of O. nivara. J. Genet. 2014, 93, 643–654. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 155–166. [Google Scholar]
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plant 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Hong-Bo, S.; Chen, X.Y.; Chu, L.Y.; Zhao, X.N.; Gang, W.; Yuan, Y.B.; Hu, Z.M. Investigation on the relationship of proline with wheat anti-drought under soil water deficits. Biointerfaces 2006, 53, 113–119. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Hussein, S.; Tsilo, T.J. Variance components and heritability of yield and yield components of wheat under drought-stressed and non-stressed conditions. Aust. J. Crop. Sci. 2017, 11, 1425–1430. [Google Scholar] [CrossRef]
- Kamran, M.; Shahbaz, M.; Ashraf, M.; Akram, N.A. Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot. 2019, 41, 621–632. [Google Scholar]
- Maleki, A.; Jalal, S.; Farid, S. Inheritance of proline content in bread wheat (Triticum aestivum L.) under rainfed conditions. J. Food Agric. Environ. 2010, 8, 155–157. [Google Scholar]
- Farooq, M.; Nawaz, A.; Chaudhry, M.A.M.; Indrasti, R.; Rehman, A. Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma-aminobutyric acid. J. Agron. Crop. Sci. 2017, 203, 464–472. [Google Scholar] [CrossRef]
- Talat, A.; Nawaz, K.; Hussian, K.; Bhatti, K.H.; Siddiqi, E.H.; Khalid, A.; Anwer, S.; Sharif, M.U. Foliar application of proline for salt tolerance of two wheat (Triticum aestivum L.) cultivars. World Appl. Sci. J. 2013, 22, 547–554. [Google Scholar]
- Mahboob, W.; Khan, M.A.; Shirazi, M.U. Induction of salt tolerance in wheat (Triticum aestivum L.) seedlings through exogenous application of proline. Pak. J. Bot. 2016, 48, 861–867. [Google Scholar]
- Goyal, M.; Asthir, B. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul. 2010, 60, 13–25. [Google Scholar] [CrossRef]
- Yokoi, S.; Quintero, F.J.; Cubero, B.; Ruiz, M.T.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002, 30, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Fujita, M. Roles of osmolytes in plant adaptation to drought and salinity. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 37–68. [Google Scholar]
- Fariduddin, Q.; Varshney, P.; Yusuf, M.; Ali, A.; Ahmad, A. Dissecting the role of glycine betaine in plants under abiotic stress. Plant Stress 2013, 7, 8–18. [Google Scholar]
- Khan, M.S.; Shah, S.J.; Ullah, M. Assessment of salinity stress and the protective effects of glycine betaine on local wheat varieties. ARPN J. Agric. Biol. Sci. 2006, 9, 360–365. [Google Scholar]
- Raza, S.H.; Athar, H.U.R.; Ashraf, M. Influence of exogenously applied glycinebetaine on the photosynthetic capacity of two differently adapted wheat cultivars under salt stress. Pak. J. Bot. 2006, 38, 341–351. [Google Scholar]
- Raza, S.H.; Athar, H.R.; Ashraf, M.; Hameed, A. Glycinebetaine-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ. Exp. Bot. 2007, 60, 368–376. [Google Scholar] [CrossRef]
- Salama, K.H.; Mansour, M.M.F.; Hassan, N.S. Choline priming improves salt tolerance in wheat (Triticum aestivum L.). Aust. J. Basic Appl. Sci. 2011, 5, 126–132. [Google Scholar]
- Salama, K.H.; Mansour, M.M.F. Choline priming-induced plasma membrane lipid alterations contributed to improved wheat salt tolerance. Acta Physiol. Plant 2015, 37, 170. [Google Scholar] [CrossRef]
- Gupta, N.; Sanjeev, K.T.; Navtej, S.B. Glycine betaine application modifies biochemical attributes of osmotic adjustment in drought stressed wheat. Plant Growth Regul. 2014, 72, 221–228. [Google Scholar] [CrossRef]
- Kusano, T.; Yamaguchi, K.; Berberich, T.; Takahashi, Y. Advances in polyamine research in 2007. J. Plant Res. 2007, 120, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, R.; Cuevas, J.C.; Planas, J.; Zarza, X.; Bortolotti, C.; Carrasco, P.; Salinas, J.; Tiburcio, A.F.; Altabella, T. Integration of polyamines in the cold acclimation response. Plant Sci. 2011, 180, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Fu, H.; Bei, Q.; Luan, S. Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant. Physiol. 2000, 124, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- El-Shintinawy, F. Photosynthesis in two wheat cultivars differing in salt susceptibility. Photosynthetica 2000, 38, 615–620. [Google Scholar] [CrossRef]
- Liu, J.H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotech. 2007, 24, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Nishibori, N.; Imai, I.; Hollibaugh, J.T. Response of polyamine pools in marine phytoplankton to nutrient limitation and variation in temperature and salinity. Mar. Ecol. Prog. Ser. 2016, 544, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liang, H.; Lv, X.; Liu, D.; Wen, X.; Liao, Y. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Biochem. 2016, 100, 113–129. [Google Scholar]
- Mansour, M.M.F.; Salama, K.H.A.; Al-Mutawa, M.M.; Abou Hadid, A.F. Effect of NaCl and polyamines on plasma membrane lipids of wheat roots. Biol. Plant. 2002, 45, 235–239. [Google Scholar] [CrossRef]
- Sakr, M.T.; El-Metwally, M.A. Alleviation of the harmful effects of soil salt stress on growth, yield and endogenous antioxidant content of wheat plant by application of antioxidants. Pak. J. Biol. Sci. 2009, 12, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Asthir, B.; Koundal, A.; Bains, N.S. Putrescine modulates antioxidant defense response in wheat under high temperature stress. Biol. Plant 2012, 56, 757–761. [Google Scholar] [CrossRef]
- Jing, J.; Guo, S.; Li, Y.; Li, W. The alleviating effect of exogenous polyamines on heat stress susceptibility of different heat resistant wheat (Triticum aestivum L.) varieties. Sci. Rep. 2020, 10, 7467. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.L.S.; Darin, P.; Jaco, V.; Van Den Ende, W.I.M.; Cuypers, A.N.N. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Hilal, M.; Juan, A.G.; Fernando, E.P. Low-temperature effect on enzyme activities involved in sucrose–starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiol. Biochem. 2009, 47, 300–307. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U.; Alia Saradhi, P.; Mohanty, P. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Farshadfar, E.; Ghasempour, H.; Vaezi, H. Molecular aspects of drought tolerance in bread wheat (T. aestivum). PJBS 2008, 11, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics 2014, 7, 271–283. [Google Scholar]
- Aldesuquy, H.; Ghanem, H. Exogenous salicylic acid and trehalose ameliorate short term drought stress in wheat cultivars by up-regulating membrane characteristics and antioxidant defense system. J. Hortic. 2015, 2, 139. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Gao, Y.M.; Wang, W.; Zou, C.J. Application of trehalose ameliorates heat stress and promotes recovery of winter wheat seedlings. Biol. Plant 2014, 58, 395–398. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Llanes, A.; Bertazza, G.; Palacio, G.; Luna, V. Different sodium salts cause different solute accumulation in the halophyte Prosopis strombulifera. Plant Biol. 2013, 1, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Iqbal, N. Chemo-priming with mannose, mannitol and H2O2 mitigate drought stress in wheat. Cereal Res. Commun. 2014, 42, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Seckin, B.; Sekmen, A.H.; Türkan, İ. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regul. 2009, 28, 12–20. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Kaur, R.; Sharma, A.; Kumar, V.; Thukral, A.K.; Arora, S.; Bhardwaj, R. Role of Compatible Solutes in Enhancing Antioxidative Defense in Plants Exposed to Metal Toxicity. In Plants under Metal and Metalloid Stress; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 207–228. [Google Scholar]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. Myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Srivalli, B.; Chinnusami, V.; Renu, K.C. Antioxidant defense in response to abiotic stresses in plants. J. Plant. Biol. 2003, 30, 121–139. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant. Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Dudziak, K.; Zapalska, M.; Börner, A.; Szczerba, H.; Kowalczyk, K.; Nowak, M. Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Sci. Rep. 2019, 9, 2743. [Google Scholar] [CrossRef] [Green Version]
- Budak, H.; Kantar, M.; Kurtoglu, K.Y. Drought tolerance in modern and wild wheat. Sci. World J. 2013, 2013, 548246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant 2013, 49, 85–91. [Google Scholar] [CrossRef]
- Hameed, A.; Goher, M.; Iqbal, N. Heat stress-induced cell death, changes in antioxidants, lipid peroxidation, and protease activity in wheat leaves. J. Plant Growth Regul. 2012, 31, 283–291. [Google Scholar] [CrossRef]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Ratnayaka, H.H.; Molin, W.T.; Sterling, T.M. Physiological and antioxidant responses of cotton and spurred anoda under interference and mild drought. J. Exp. Bot. 2003, 54, 2293–2305. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hollander-Czytko, H.; Grabowski, J.; Sandorf, I.; Weckermann, K.; Weiler, E.W. Tocopherol content and activities of tyrosine aminotransferase and cystine lyase in Arabidopsis under stress conditions. J. Plant Physiol. 2005, 162, 767–770. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Agriculture, Ecosystems and Environment Stressed food-The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Farouk, S. Ascorbic acid and α-tocopherol minimize salt-induced wheat leaf senescence. J. Stress Physiol. Biochem. 2011, 7, 58–79. [Google Scholar]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Balouchi, H.R. Screening wheat parents of mapping population for heat and drought tolerance, detection of wheat genetic variation. Int. J. Biol. Life Sci. 2010, 4, 63–73. [Google Scholar]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.T.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef]
- Morgan, P.W. Effects of abiotic stresses on plant hormone systems. In Stress Responses in Plants: Adaptation and Acclimation Mechanisms; Wiley-Liss Inc.: New York, NY, USA, 1990; pp. 113–146. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2006. [Google Scholar]
- Nilsen, E.T.; Orcutte, D.M. Phytohormones and plant responses to stress. In Physiology of Plant under Stress: Abiotic Factors; Nilsen, E.T., Orcutte, D.M., Eds.; John Wiley and Sons: New York, NY, USA, 1996; pp. 183–198. [Google Scholar]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef]
- Gulnaz, A.J.; Iqbal, J.; Azam, F. Seed treatment with growth regulators and crop productivity. II. Response of critical growth stages of wheat (Triticum aestivum L.) under salinity stress. Cereal Res. 1999, 27, 419–426. [Google Scholar] [CrossRef]
- Sakhabutdinova, A.R.; Fatkhutdinova, D.R.; Bezrukova, M.; Shakirova, F.M. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant. Physiol. 2003, 21, 314–319. [Google Scholar]
- Iqbal, M.; Ashraf, M. Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. J. Integr. Plant Biol. 2007, 49, 1003–1015. [Google Scholar] [CrossRef]
- Akbari, G.; Sanavy, S.A.; Yousefzadeh, S. Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars (Triticum aestivum L.). Pak. J. Biol. Sci. 2007, 10, 2557–2561. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P. Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Arch. Agron. Soil Sci. 2010, 56, 575–588. [Google Scholar] [CrossRef]
- Choudhuri, M.A. Free radicals and leaf senescence-a review. Plant Physiol. Biochem. 1988, 15, 18–29. [Google Scholar]
- Nayyar, H.; Walia, D.P.; Kaistha, B.L. Performance of bread wheat (Triticum aestivum L.) seed primed with growth regulators and inorganic salts. Indian J. Agric. Sci. 1995, 65, 116–122. [Google Scholar]
- Kumar, B.; Singh, B. Effect of plant hormones on growth and yield of wheat irrigated with saline water. Annu. Agric. Res. 1996, 17, 209–212. [Google Scholar]
- Manjili, F.A.; Sedghi, M.; Pessarakli, M. Effects of phytohormones on proline content and antioxidant enzymes of various wheat cultivars under salinity stress. J. Plant Nutr. 2012, 35, 1098–1111. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2013, 86, 76–85. [Google Scholar] [CrossRef]
- Tabatabei, S.A. The effect of salicylic acid and gibberellin on enzyme activity and germination characteristics of wheat seeds under salinity stress conditions. Int. J. Agric. Crop. Sci. 2013, 6, 236–240. [Google Scholar]
- Shaddad, M.A.K.; Abd El-Samad, H.M.; Mostafa, D. Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. Int. J. Plant Physiol. Biochem. 2013, 5, 50–57. [Google Scholar]
- Davies, P.J. Plant Hormones: Biosynthesis, Signal Transduction, Action; Kluwer Academic Publishers and Science Press: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Keskin, B.C.; Sarikaya, A.T.; Yuksel, B.; Memon, A.R. Abscisic acid regulated gene expression in bread wheat. Aust. J. Crop. Sci. 2010, 4, 617–625. [Google Scholar]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Bohmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachtman, D.P.; Goodger, J.Q. Chemical root to shoot signaling under drought. Trends Plant Sci. 2018, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Ullah, F.; Nosheen, A. Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant Soil Environ. 2012, 58, 181–185. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Bano, A.; Najeeb, U.; Zhang, J.; Khan, S.U.; Flowers, T.J. Exogenously applied silicate and abscisic acid ameliorates the growth of salinity stressed wheat (Triticum aestivum L.) seedlings through Na+ exclusion. Aust. J. Crop Sci. 2013, 7, 1123. [Google Scholar]
- Khan, N.A.; Khan, M.I.R. The Ethylene: From senescence hormone to key player in plant metabolism. J. Plant Biochem. Physiol. 2014, 2, e124. [Google Scholar] [CrossRef]
- Narayana, Y.; Lalonde, S.; Saini, H.S. Water-stress induced ethylene production in wheat. A fact or artifact? Plant Physiol. 1991, 96, 406–410. [Google Scholar] [CrossRef]
- Young, T.E.; Meeley, R.B.; Gallie, D.R. ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J. 2004, 40, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Ludwig, Y.; Slamet-Loedin, I.H. Genetic biofortification to enrich rice and wheat grain iron: From genes to product. Front. Plant Sci. 2019, 10, 833. [Google Scholar] [CrossRef]
- Watson, D. Adaption to climate change: Climate adaptive breeding of maize, wheat and rice. In Sustainable Solutions for Food Security; Springer: Cham, Switzerland, 2019; pp. 67–89. [Google Scholar]
- Islam, M.T.; Rahman, M.; Pandey, P.; Boehme, M.H.; Haesaert, G. Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Springer Nature Switzerland AG. Part of Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Finnegan, P.M.; Mahmood, S.; Anwar, Y.; Al-Garni, S.M.S.; Bahieldin, A.; Islam, M.T. Enhancement of abiotic stress tolerance in plants by probiotic bacteria. In Plant Tolerance to Environmental Stress; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 381–402. [Google Scholar]
- Cominelli, E.; Pilu, R.; Sparvoli, F. Phytic acid and mineral biofortification strategies: From plant science to breeding and biotechnological approaches. Plants. 2020, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Shankar, A.; Singh, S.; Kesari, V.; Rai, R.; Patel, A.K.; Rai, L.C. Beneficial microorganisms and abiotic stress tolerance in plants. In Approaches for Enhancing Abiotic Stress Tolerance in Plants; Hasanuzzaman, M., Nahar, K., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 473–502. [Google Scholar]
- Loskutov, I.G.; Khlestkina, E.K. Wheat, Barley, and Oat Breeding for Health Benefit Components in Grain. Plants 2021, 10, 86. [Google Scholar] [CrossRef]
- Rey, M.D.; Ramírez, C.; Martín, A.C. Wheat, Rye, and Barley Genomes Can Associate during Meiosis in Newly Synthesized Trigeneric Hybrids. Plants 2021, 10, 113. [Google Scholar] [CrossRef]
- Bansal, M.; Jindal, S.; Wani, S.H.; Ganie, S.A.; Singh, R. Genome Editing and Trait Improvement in Wheat. In Physiological, Molecular, and Genetic Perspectives of Wheat Improvement; Wani, S.H., Mohan, A., Singh, G.P., Eds.; Springer: Cham, Switzerland, 2021; pp. 263–283. [Google Scholar] [CrossRef]
- Rasmussen, S.K. Molecular genetics, genomics, and biotechnology in crop plant breeding. Agronomy 2020, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Michel, S.; Löschenberger, F.; Ametz, C.; Pachler, B.; Sparry, E.; Bürstmayr, H. Simultaneous selection for grain yield and protein content in genomics-assisted wheat breeding. Theor. Appl. Genet. 2019, 132, 1745–1760. [Google Scholar] [CrossRef]
- Alam, M.J.; Humphreys, E.; Sarkar, M.A.R.; Yadav, S. Intensification and diversification increase land and water productivity and profitability of rice-based cropping systems on the High Ganges River Floodplain of Bangladesh. Field Crop. Res. 2017, 209, 10–26. [Google Scholar] [CrossRef]
- Cormier, F.; Faure, S.; Dubreuil, P.; Heumez, E.; Beauchene, K.; Lafarge, S.; Praud, S.; Le Gouis, J. A multi-environmental study of recent breeding progress on nitrogen use efficiency in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2013, 126, 3035–3048. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, B.M. Maize in Asia-trends, challenges and opportunites. In Proceedings of the Addressing Climate Change Effects and Meeting Maize Demand for Asia- Book of Extended Summaries of the 11th Asian Maize Conference, Nanning, China, 7–11 November 2011; CIMMYT: Texcoco, Mexico, 2011. [Google Scholar]
- Chen, J.; Xu, W.; Velten, J.; Xin, Z.; Stout, J. Characterization of maize inbred lines for drought and heat tolerance. J. Soil Water Conser. 2012, 67, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Babić, V.; Babić, M.; Ivanović, M.; Kraljević-Balalić, M.; Dimitrijević, M. Understanding and utilization of genotype-byenvironment interaction in maize breeding. Genetika 2010, 42, 79–90. [Google Scholar] [CrossRef]
- Babić, V.; Prodanović, S.; Babić, M.; Deletić, N.; Anđelković, V. The identification of bands related to yields and stability in maize hybrids and their parental components. Genetika 2013, 45, 589–599. [Google Scholar] [CrossRef]
- Fikere, M.; Tadesse, T.; Letta, T. Genotype-environment interactions and stability parameters for grain yield of faba beans (Vacia faba L.) genotypes grown in south eastern Ethiopia. Int. J. Sustain. Crop. Prod. 2008, 3, 80–87. [Google Scholar]
- Edmeades, G.O.; Bolanos, J.; Hernandez, M.; Bello, S. Causes for silk delay in delay in a lowland tropical maize population. Crop Sci. 1993, 33, 1029–1035. [Google Scholar] [CrossRef]
- Ribaut, J.M.; Fracheboud, Y.; Monneveux, P.; Banziger, M.; Vargas, M.; Jiang, C. Quantitative trait loci for yield and correlated traits under high and low soil nitrogen conditions in tropical maize. Mol. Breed. 2007, 20, 15–29. [Google Scholar] [CrossRef]
- Betrán, F.J.; Beck, D.; Bänziger, M.; Edmeades, G.O. Secondary traits in parental inbreds and hybrids under stress and non-stress environments in tropical maize. Field Crop. Res. 2003, 83, 51–65. [Google Scholar] [CrossRef]
- Monneveux, P.; Jing, R.; Misra, S.C. Phenotyping for drought adaptation in wheat using physiological traits. Front. Physio. 2012, 3, 429. [Google Scholar] [CrossRef] [Green Version]
- Khodarahmpour, Z.; Choukan, R.; Bihamta, M.R.; Majidi, H.E. Determination of the best heat stress tolerance indices in maize (Zea mays L.) inbred lines and hybrids under Khuzestan Province conditions. J. Agric. Sci. Technol. 2011, 13, 111–121. [Google Scholar]
- Morgan, C.L.; Austin, R.B.; Ford, M.A.; Bingham, J.; Angus, W.J.; Chowdhury, S. An evaluation of F1 hybrid winter wheat genotypes produced a chemical hybridizing agent. J. Agric. Sci. 1989, 112, 143–149. [Google Scholar] [CrossRef]
- Le Gouis, J.; Beghin, D.; Heumez, E.; Pluchard, P. Diallel analysis of winter wheat at two nitrogen levels. Crop Sci. 2002, 42, 1129–1134. [Google Scholar] [CrossRef]
- Ushakumari, R.; Muthukamatchi, R.; Thamodharan, G. Heterosis analysis in relation to drought tolerance in rice land races and their genotypes. J. Appl. Nat. Sci. 2014, 16, 804–811. [Google Scholar] [CrossRef] [Green Version]
- John Kingsly, N.B.; Gomathinayagam, P.; Jebaraj, S. Genetic Analysis of Root Traits and its Effect on Yield for Drought Tolerance breeding in Rice (Oryza sativa). In Proceedings of the International Symposium: Exposing the Hidden Half: Root Research at the Forefront of Science, Yearim Hotel, Israel, 8–12 July 2018. [Google Scholar]
- Wang, Z.K.; Ni, Z.F.; Wu, H.L.; Nie, X.L.; Sun, Q.X. Heterosis in root development and differential gene expression between hybrids and their parental inbreds in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Shaoxia, Z.; Yongcai, F.; Zhen, S.; Xiangkun, W.; Chuanqing, S. Identification of a drought tolerant introgression line derived from Dongxiang common wild rice (O. rufipogon Griff.). Plant Molec. Biol. 2006, 62, 247–259. [Google Scholar]
- Srivastava, H.K. Nuclear control and mitochondrial transcript processing with Relevance to cytoplasmic male sterility in higher plants. Crop Sci. 2000, 79, 176–186. [Google Scholar]
- Syed, M.A.; Alam, M.A.; Hossain, A.; Islam, M.R.; Vemuri, H.; Jahan, N. Rice Breeding and Genomics Approaches for Improving Water and Nitrogen Use Efficiency. In Rice Research for Quality Improvement: Genomics and Genetic Engineering; Roychoudhury, A., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Seetharam, K.; Krishna, G.; Krishnamurthy, L.; Gajanan, S.; Babu, R.; Zerka, M.; Vinayan, M.T.; Vivek, B.S. Genomic Regions associated with root traits under drought stress in tropical maize (Zea mays L.). PLoS ONE 2016, 11, e0164340. [Google Scholar] [CrossRef]
- Cormier, F.; Le Gouis, J.; Dubreuil, P.; Lafarge, S.; Praud, S. A genome -wide identification of chromosomal regions determining nitrogen use efficiency components in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2014, 127, 2679–2693. [Google Scholar] [CrossRef]
- Liu, C.; Pinto, F.; Cossani, C.M.; Sukumaran, S.; Reynolds, M.P. Spectral refectance indices as proxies for yield potential and heat stress tolerance in spring wheat: Heritability estimates and marker-trait associations. Front. Agric. Sci. Eng. 2019, 6, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Jamil, M.; Ali, A.; Gul, A.; Ghafoor, A.; Napar, A.A.; Ibrahim, A.M. Genome-wide association studies of seven agronomic traits under two sowing conditions in bread wheat. BMC Plant Biol. 2019, 19, 149. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genetic dissection of drought and heat-responsive agronomic traits in wheat. Plant Cell Environ. 2019, 42, 2540–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesta, P.; Mora, F.; Del Pozo, A. Association mapping of drought tolerance indices in wheat: QTL-rich regions on chromosome 4A. Sci. Agric. 2020, 77, e20180153. [Google Scholar] [CrossRef] [Green Version]
- Gahlaut, V.; Jaiswal, V.; Singh, S.; Balyan, H.S.; Gupta, P.K. Multi-locus genome wide association mapping for yield and its con-tributing traits in hexaploid wheat under diferent water regimes. Sci. Rep. 2019, 9, 19468. [Google Scholar] [CrossRef] [PubMed]
- Genc, Y.; Taylor, J.; Lyons, G.; Li, Y.; Cheong, J.; Appelbee, A.; Oldach, K.; Sutton, T. Bread wheat with high salinity and sodicity tolerance. Front. Plant Sci. 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkesford, M.J.; Griffiths, S. Exploiting genetic variation in nitrogen use efficiency for cereal crop improvement. Curr. Opin. Plant Biol. 2019, 49, 35–42. [Google Scholar] [CrossRef]
- Swamy, B.P.M.; Shamsudin, N.A.A.; Rahman, S.N.A.; Mauleon, R.; Ratnam, W.; Cruz, M.T.S.; Kumar, A. Association Mapping of Yield and Yield-related Traits under Reproductive Stage Drought Stress in Rice (Oryza sativa L.). Rice 2017, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Hassan, F.S.C.; Solouki, M.; Fakheri, B.A.; Nezhad, N.M.; Masoudi, B. Mapping QTLs for physiological and biochemical traits related to grain yield under control and terminal heat stress conditions in bread wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2018, 24, 1231–1243. [Google Scholar] [CrossRef]
- Tura, H.; Edwards, J.; Gahlaut, V.; Garcia, M.; Sznajder, B.; Baumann, U.; Shahinnia, F.; Reynolds, M.; Langridge, P.; Balyan, H.S.; et al. QTL analysis and fne mapping of a QTL for yield-related traits in wheat grown in dry and hot environments. Theor. Appl. Genet. 2020, 13, 239–257. [Google Scholar] [CrossRef]
- Shamaya, N.J.; Shavrukov, Y.; Langridge, P.; Roy, S.J.; Tester, M. Genetics of Na+ exclusion and salinity tolerance in Afghani durum wheat landraces. BMC Plant. Biol. 2017, 17, 209. [Google Scholar] [CrossRef] [Green Version]
- Asif, M.A.; Schilling, R.K.; Tilbrook, J.; Brien, C.; Dowling, K.; Rabie, H. Mapping of novel salt tolerance QTL in an Excali-bur × Kukri doubled haploid wheat population. Theor Appl Genet. 2018, 131, 2179–2196. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Holl, J.B.; McMullen, M.D.; Buckler, E.S. Genetic design and statistical power of nested association mapping in maize. Genetics 2008, 178, 539–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, I.J.; Bansept-Basler, P.; Barber, T.; Bentley, A.R.; Cockram, J.; Gosman, N.; Greenland, A.J.; Horsnell, R.; Howells, R.; O’Sullivan, D.M.; et al. An eight-parent multiparent advanced generation inter-cross population for winter-sown wheat: Creation, properties, and validation. G3 (Bethesda) 2014, 4, 1603–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandillo, N.; Raghavan, C.; Muyco, P.A.; Sevilla, M.A.; Lobina, I.T.; Dilla-Ermita, C.J.; Tung, C.W.; McCouch, S.; Thomson, M.; Mauleon, R.; et al. Multi-parent advanced generation inter-cross (MAGIC) populations in rice: Progress and potential for genetics research and breeding. Rice 2013, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.E.; Verbyla, K.L.; Verbyla, A.P.; Raghavan, C.; Singh, V.K.; Gaur, P.; Leung, H.; Varshney, R.K.; Cavanagh, C.R. MAGIC populations in crops: Current status and future prospects. Theor. Appl. Genet. 2015, 128, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-assisted backcrossing: A useful method for rice improvement. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, B.; Sammler, R.; Backhaus, A.; Beschow, H.; Schumann, E.; Mock, H.P.; von Wiren, N.; Seiffert, U.; Pillen, K. Genetic regulation of growth and nutrient content under phosphorus deficiency in the wild barley introgression library S42IL. Plant Breed. 2017, 136, 892–907. [Google Scholar] [CrossRef]
- Surapaneni, M.; Balakrishnan, D.; Mesapogu, S.; Addanki, K.R.; Yadavalli, V.R.; Tripura, V.G.N.; Neelamraju, S. Identification of major effect QTLs for agronomic traits and CSSLs in rice from Swarna/Oryza nivara derived backcross inbred lines. Front. Plant. Sci. 2017, 8, 1027. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, W.; Lin, X.; Xu, S.; Xu, J.; Li, Z. Simultaneous Improvement and Genetic Dissection of Drought Tolerance Using Selected Breeding Populations of Rice. Front. Plant. Sci. 2018, 9, 320. [Google Scholar] [CrossRef]
- Venuprasad, R.; Bool, M.E.; Quiatchon, L.; Sta Cruz, M.T.; Amante, M.; Atlin, G.N. A large-effect QTL for rice grain yield under upland drought stress on chromosome 1. Mol. Breed. 2011, 30, 535–547. [Google Scholar] [CrossRef]
- De Vita, P.; Taranto, F. Durum wheat (Triticum turgidum ssp. durum) breeding to meet the challenge of climate change. In Advances in Plant Breeding Strategies: Cereals; Springer: Cham, Switzerland, 2019; pp. 471–524. [Google Scholar]
- Choudhary, M.; Yadav, M.; Saran, R. Advanced screening and breeding approaches for heat tolerance in wheat. J. Pharm. Phytochem. 2020, 9, 1047–1052. [Google Scholar]
- Perez-Clemente, R.; Gómez-Cadenas, A. In vitro Tissue Culture, a Tool for the Study and Breeding of Plants Subjected to Abiotic Stress Conditions. Recent Adv. Plant 2011, 91–108. [Google Scholar] [CrossRef] [Green Version]
- Dita, M.A.L.; Rispail, N.; Prats, E.; Rubiales, D.; Singh, K. Biotechnology approaches to overcome biotic and abiotic stress constraints in legumes. Euphytica 2006, 147, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Shen, Z.; Zhao, D.; Xu, L.; Zhang, L.; Zou, Q. Genome-Wide Analysis of LysM-Containing Gene Family in Wheat: Structural and Phylogenetic Analysis during Development and Defense. Genes 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic aspects of somaclonal variation in plants. Plant Mol. Biol. 2000, 43, 179–188. [Google Scholar] [CrossRef]
- Predieri, S. Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Organ. Cult. 2001, 64, 185–210. [Google Scholar] [CrossRef]
- Biswas, M.; Dutt, M.; Roy, U.; Islam, R.; Hossain, M. Development and evaluation of in vitro somaclonal variation in strawberry for improved horticultural traits. Sci. Hortic. 2009, 122, 409–416. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. Biotechnology 2016, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Abdelkareem, A. Somaclonal variation in bread wheat (Triticum aestivum L.). II. Field performance of somaclones. Cereal Res. Commun. 2005, 33, 485–492. [Google Scholar] [CrossRef]
- Mahmood, I.; Razzaq, A.; Ashraf, M.; Ahmad Hafiz, I.; Kaleem, S.; Qayyum, A.; Ahmad, M. In vitro selection of tissue culture induced somaclonal variants of wheat for drought tolerance. J. Agric. Res. 2012, 50, 03681157. [Google Scholar]
- Bell, R.; Haque, E.; Jahiruddin, M.; Rahman, M.; Begum, M.; Miah, M.A.M.; Islam, M.; Hossen, A.; Salahin, N.; Zahan, T.; et al. Conservation Agriculture for Rice-Based Intensive Cropping by Smallholders in the Eastern Gangetic Plain. Agriculture 2018, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Tsago, Y.; Andargie, M.; Takele, A. In vitro screening for drought tolerance in different sorghum (Sorghum bicolor L.) varieties. J. Stress Physiol. Biochem. 2013, 9, 72–83. [Google Scholar]
- Ahmed, H.U.; Mundt, C.C.; Hoffer, M.E.; Coakley, S.M. Selective influence of wheat cultivars on pathogenicity of Mycosphaerella graminicola (Anamorph Septoria tritici). Phytopathology 1996, 86, 454–458. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Svabova, L.; Lebeda, A. In vitro selection for improved plant resistance to toxin-producing pathogens. J. Phytopathol. 2005, 153, 52–64. [Google Scholar] [CrossRef]
- Tal, M. In vitro selection for salt tolerance in crop plants: Theoretical and practical considerations. Vitr. Cell. Dev. Biol. Plant 1994, 30, 175–180. [Google Scholar] [CrossRef]
- Kacem, N.S.; Delporte, F.; Muhovski, Y.; Djekoun, A.; Watillon, B. In vitro screening of durum wheat against water-stress mediated through polyethylene glycol. J. Genet. Eng. Biotech. 2017, 15, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sakhanokho, H.; Kelley, R. Influence of salicylic acid on in vitro propagation and salt tolerance in Hibiscus acetosella and Hibiscus moscheutos (cv ‘Luna Red’). Afr. J. Biotech. 2009, 8, 1474–1481. [Google Scholar]
- Dunwell, J.M. Crop genomics: Progress and prospects. J. Chem. Technol. Biotechnol. 2000, 75, 913–918. [Google Scholar] [CrossRef]
- Wu, Q.D.; VanEtten, H.D. Introduction of plant and fungal genes into pea (Pisum sativum L.) hairy roots reduces their ability to produce pisatin and affects their response to a fungal pathogen. Mol. Plant Microbe Interact. 2004, 17, 798–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, H.P.; Bainsla, N.K.; Yadav, D.K. Breeding for Abiotic Stress Tolerance in Crop Plants. In Recent Advances in Plant Stress Physiology; Daya Publishing House: New Delhi, India, 2017; pp. 329–378. [Google Scholar]
- Xia, L.; Ma, Y.; He, Y.; Jones, H.D. GM Wheat Development in China: Current Status and Challenges to Commercialization. J. Exp. Bot. 2012, 63, 1785–1790. [Google Scholar] [CrossRef] [Green Version]
- Brini, F.; Hanin, M.; Mezghani, I.; Berkowitz, G.A.; Masmoudi, K. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+ pyro phosphatase TVP1pyrophosphatase TVP1 improve salt and drought stress tolerance in Arabidopsis thaliana plants. J. Exp. Bot. 2007, 58, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [Green Version]
- Bhowmik, P.; Ellison, E.; Polley, B.; Bollina, V.; Kulkarni, M.; Ghanbarnia, K.; Song, H.; Gao, C.; Voytas, D.F.; Kagale, S. Targeted mutagenesis in wheat microspores using CRISPR/Cas9. Sci. Rep. 2018, 8, 6502. [Google Scholar] [CrossRef] [Green Version]
- Witcombe, J.R.; Hollington, P.A.; Howarth, C.J.; Reader, S.; Steele, K.A. Breeding for abiotic stresses for sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 703–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef]
- Pellegrineschi, A.; Reynolds, M.; Pacheco, M.; Brito, R.M.; Almeraya, R.; Yamaguchi-Shinozaki, K.; Hoisington, D. Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 2004, 47, 493–500. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Oliveira, L.K.; Melo, L.C.; Brondani, C.; Peloso, M.J.D.; Brondani, R.P.V. Backcross assisted by microsatellite markers in common bean. Genet. Mol. Res. 2008, 7, 1000–1010. [Google Scholar] [CrossRef]
- Gonzaga, Z.J.C.; Carandang, J.; Sanchez, D.L.; Mackill, D.J.; Septiningsih, E.M. Mapping additional QTLs from FR13A to increase submergence tolerance in rice beyond SUB1. Euphytica 2016, 209, 627–636. [Google Scholar] [CrossRef]
- Wani, S.; Choudhary, M.; Kumar, P.; Aisha, N.; Surekha, C.; Ahmad, P.; Gosal, S.S. Marker-Assisted Breeding for Abiotic Stress Tolerance in Crop Plants. In Biotechnologies of Crop Improvement; Springer: Cham, Switzerland, 2018; Volume 3, pp. 1–23. [Google Scholar] [CrossRef]
- Merchuk-Ovnat, L.; Barak, V.; Fahima, T.; Ordon, F.; Lidzbarsky, G.A.; Krugman, T.; Saranga, Y. Ancestral QTL alleles from wild emmer wheat improve drought resistance and productivity in modern wheat cultivars. Front. Plant Sci. 2016, 7, 452. [Google Scholar] [CrossRef] [Green Version]
- Barakat, M.N.; Saleh, M.S.; Al-Doss, A.A.; Moustafa, K.A.; Elshafei, A.A.; Zakri, A.M.; Al-Qurainy, F.H. Mapping of QTLs associated with abscisic acid and water stress in wheat. Biol. Plant 2015, 59, 291–297. [Google Scholar] [CrossRef]
- Turki, N.; Shehzad, T.; Harrabi, M.; Okuma, K. Detection of QTLs associated with salinity tolerance in durum wheat based on asso-ciation analysis. Euphytica 2015, 201, 29–41. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.; Li, L.; Zhang, X.; Xu, H.; An, D. Mapping QTLs for salt tolerance with addi-tive, epistatic and QTL 3 treatment interaction effects at seedling stage in wheat. Plant Breed. 2013, 132, 276–283. [Google Scholar] [CrossRef]
- Yu, M.; Chen, G. Conditional QTL mapping for waterlogging tolerance in two RILs popula-tions of wheat. Springerplus 2013, 2, 245. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Bai, G.; Zhang, D.; Hong, D. Validation of quantitative trait loci for aluminum tolerance in Chinese wheat landrace FSW. Euphytica 2013, 192, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, K.; Singh, A.K.; Basavaraj, S.H.; Cherukuri, D.P.; Charpe, A.M.; Krishnan, S.G.; Gupta, S.K.; Joseph, M.; Koul, S.; Mohapatra, T.; et al. Marker assisted selection for biotic stress resistance in wheat and rice. Indian J. Genet. Plant Breed. 2009, 69, 305–314. [Google Scholar]
- Dormatey, R.; Sun, C.; Ali, K.; Coulter, J.A.; Bi, Z.; Bai, J. Gene Pyramiding for Sustainable Crop Improvement against Biotic and Abiotic Stresses. Agronomy. 2020, 10, 1255. [Google Scholar] [CrossRef]
- Walker, D.R.; Narvel, J.M.; Boerma, H.R.; All, J.N.; Parrott, W.A. A QTL that enhances and broadens Bt insect resistance in soybean. Theor. Appl. Genet. 2004, 109, 1051–1057. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, M.Y.; Polle, E.A.; Konzak, C.F. Highly efficient doubled-haploid production in wheat (Triticum aestivum L.) via induced microspore embryogenesis. Crop. Sci. 2002, 42, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Martinez, S.A.; Thompson, A.L.; Wen, N.; Murphy, L.; Sanguinet, K.A.; Steber, C.M.; Garland Campbell, K.A. Registration of the Louise/Alpowa wheat recombinant inbred line mapping population. J. Plant Regist. 2018, 12, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, H.; Khurana, P. Use of doubled haploid technology for development for development of stable drought tolerant bread wheat (Triticum aestivum L.) transgenics. Plant Biotech. J. 2011, 9, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patial, M.; Pal, D.; Thakur, A.; Bana, R.S.; Patial, S. Doubled Haploidy Techniques in Wheat (Triticum aestivum L.): An Overview. Proc. Natl. Acad. Sci. USA 2019, 89, 27–41. [Google Scholar] [CrossRef]
- Brar, D.S.; Singh, K.; Khush, G.S. Frontiers in Rice Breeding. In The Future Rice Strategy for India; Academic Press: Cambridge, MA, USA, 2017; pp. 137–160. [Google Scholar]
- Li, Z.S.; Li, B.; Zheng, Q.; Li, H. Review and New Progress in Wheat Wide Hybridization for Improving the Resistance to Biotic and Abiotic Stresses. In Advances in Wheat Genetics: From Genome to Field; Ogihara, Y., Takumi, S., Handa, H., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef] [Green Version]
- Robsa, S.A. Review paper on the Role of Somatic Hybridization in Crop Improvement. Int. J. Res. Stud. Agric. Sci. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Göntér, I.; Szarka, B.; Lendvai, A.; Molnár-Láng, M.; Mórocz, S.; Dudits, D. Problems and possibilities of wheat-maize somatic hybridization. Acta Biol. Szeged. 2002, 46, 11–12. [Google Scholar]
- Arumugam, N.; Mukhopadhyay, V.A.; Gupta, Y.S.; Sodhi, J.K.; Verma, D.; Pradhan, A.K. Synthesis of somatic hybrids (RCBB) by fusing heat-tolerant Raphanus sativus (RR) and Brassica oleracea (CC) with Brassica nigra (BB). Plant Breed. 2002, 121, 168–170. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Kumar, S.; Jasrotia, P.; Singh, D.P.; Singh, G.P. Nanotechnology in Wheat Production and Protection. In Environmental Nanotechnology; Springer: Cham, Switzerland, 2020; Volume 4, pp. 165–194. [Google Scholar]
- Kashyap, P.L.; Kumar, S.; Srivastava, A.K. Nanodiagnostics for plant pathogens. Environ. Chem. Lett. 2017, 15, 7–13. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 3148–3160. [Google Scholar] [CrossRef]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodzadeh, H.; Aghilli, R. Effect on Germination and Early Growth Characteristics in Wheat Plants (Triticum aestivum L.) Seeds Exposed to TiO2 Nanoparticles. J. Chem. Health Risks 2013, 4, 29–36. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef] [Green Version]
- Eshi, Y.; Ezhang, Y.; Ehan, W.; Efeng, R.; Ehu, Y.; Eguo, J.; Gong, H.-J. Silicon Enhances Water Stress Tolerance by Improving Root Hydraulic Conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef]
- Sharma, S.; TSRHC Scoliosis Clinical Group; Londono, D.; Eckalbar, W.L.; Gao, X.; Zhang, D.; Mauldin, K.; Kou, I.; Takahashi, A.; Matsumoto, M.; et al. A PAX1 enhancer locus is associated with susceptibility to idiopathic scoliosis in females. Nat. Commun. 2015, 6, 6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miransari, M.; Smith, D.L. Overcoming the stressful effects of salinity and acidity on soybean nodulation and yields using signal molecule genistein under field conditions. J. Plant Nutr. 2007, 30, 1967–1992. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Tubiello, F.N.; Soussana, J.F.; Howden, S.M. Crop and pasture response to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19686–19690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velde, B.; Barré, P. Soils, Plants and Clay Minerals: Mineral and Biologic Interactions; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Pal, R.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Impact of sowing date on yield, dry matter and nitrogen accumulation, and nitrogen translocation in dry-seeded rice in North-West India. Field Crop. Res. 2017, 206, 138–148. [Google Scholar] [CrossRef]
- Sarkar, S.; Gaydon, D.S.; Brahmachari, K.; Nanda, M.K.; Ghosh, A.; Mainuddin, M. Modelling Yield and Seasonal Soil Salinity Dynamics in Rice-Grasspea Cropping System for the Coastal Saline Zone of West Bengal, India. Proceedings 2019, 36, 146. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Huang, J.; Zhu, L.; Shah, F.; Nie, L.; Cui, K.; Peng, S. Varietal difference in the response of rice chalkiness to temperature during ripening phase across different sowing dates. Field Crop. Res. 2013, 151, 85–91. [Google Scholar] [CrossRef]
- Shah, F.; Coulter, J.A.; Ye, C.; Wu, W. Yield penalty due to delayed sowing of winter wheat and the mitigatory role of increased seeding rate. Eur. J. Agron. 2020, 119, 126120. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Chapman, S.C.; Rattey, A.R.; Neal, J.; Song, Y.; Christopher, J.T.; Reynolds, M. Developmental and growth controls of tillering and water-soluble carbohydrate accumulation in contrasting wheat (Triticum aestivum L.) genotypes: Can we dissect them? J. Exp. Bot. 2012, 64, 143–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panhwar, Q.A.; Ali, A.; Naher, U.A.; Memon, M.Y. Fertilizer Management Strategies for Enhancing Nutrient Use Efficiency and Sustainable Wheat Production. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Organic Farming; Sarath Chandran, M.R., Unni, S.T., Eds.; Woodhead Publishing: Amsterdam, Netherlands, 2019. [Google Scholar] [CrossRef]
- Liu, S.; Lu, Y.; Yang, C.; Liu, C.; Ma, L.; Dang, Z. Effects of modified biochar on rhizosphere microecology of rice (Oryza sativa L.) grown in As-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 23815–23824. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Pathak, H.; Chakrabarti, B.; Singh, S.; Gupta, D.K.; Harit, R. Impact of terminal heat stress on wheat yield in India and options for adaptation. Agric. Syst. 2020, 181, 102826. [Google Scholar] [CrossRef]
- Jat, R.K.; Singh, P.; Jat, M.L.; Dia, M.; Sidhu, H.S.; Jat, S.L.; Bijarniya, D.; Jat, H.S.; Parihar, C.M.; Kumar, U.; et al. Heat stress and yield stability of wheat genotypes under different sowing dates across agro-ecosystems in India. Field Crop. Res. 2018, 218, 33–50. [Google Scholar] [CrossRef]
- Singh, B.-S.; Humphreys, E.; Yadav, S.; Gaydon, D.S. Options for increasing the productivity of the rice–wheat system of north-west India while reducing groundwater depletion. Part 1. Rice variety duration, sowing date and inclusion of mungbean. Field Crop. Res. 2015, 173, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Gou, F.; van Ittersum, M.K.; van der Werf, W. Simulating potential growth in a relay-strip intercropping system: Model description, calibration and testing. Field Crop. Res. 2017, 200, 122–142. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, A.; Brahmachari, K.; Ray, K.; Nanda, M.K.; Sarkar, D. Weather relation of rice-grass pea crop sequence in Indian Sundarbans. J. Agrometeorol. 2020, 22, 148–157. [Google Scholar]
- Sarkar, S.; Ghosh, A.; Brahmachari, K. Application of APSIM Model for Assessing the Complexities of Rice-based Cropping Systems of South-Asia. In Advanced Agriculture, 1st ed.; Maitra, S., Pramanick, B., Eds.; New Delhi Publishers: New Delhi, India, 2020; pp. 212–233. [Google Scholar]
- Andarzian, B.; Hoogenboom, G.; Bannayan, M.; Shirali, M.; Andarzian, B. Determining optimum sowing date of wheat using CSM-CERES-Wheat model. J. Saudi Soc. Agric. Sci. 2015, 14, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Tao, F. Sustainable intensification options to improve yield potential and eco-efficiency for rice-wheat rotation system in China. Field Crop. Res. 2017, 211, 89–105. [Google Scholar] [CrossRef]
- Seleiman, M.F. Use of Plant Nutrients in Improving Abiotic Stress Tolerance in Wheat. In Wheat Production in Changing Environments; Springer: Singapore, 2019; pp. 481–495. [Google Scholar]
- Seleiman, M.F. Wheat Production in Changing Environments; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Abedin, M.J.; Rouf, M.A.; Rashid, M.H.; Eaqub, M. Residual effects of TSP and Farmyard manure under renewed application of urea on the yield of crop and some chemical properties of soil. Bangladesh J. Agric. Sci. 1999, 10, 100–109. [Google Scholar]
- Son, T.T.; Singh, U.; Padilla, J.L.; Buresh, R.J. Management of urea on degraded soils of the Red River Delta [Vietnam]: Effect of growing season and cultural practice. In Proceedings of the Conference on Viet Nam and IRRI Partnership in Rice Research, Hanoi, Vietnam, 4–7 May 1994. [Google Scholar]
- Araya, A.; Prasad, P.; Gowda, P.; Afewerk, A.; Abadi, B.; Foster, A. Modeling irrigation and nitrogen management of wheat in northern Ethiopia. Agric. Water Manag. 2019, 216, 264–272. [Google Scholar] [CrossRef]
- Rahma, A.E.; Warrington, D.N.; Lei, T. Efficiency of wheat straw mulching in reducing soil and water losses from three typical soils of the Loess Plateau, China. Int. Soil Water Conserv. Res. 2019, 7, 335–345. [Google Scholar] [CrossRef]
- Badaruddin, M.; Reynolds, M.P.; Ageeb, O.A. Wheat Management in Warm Environments: Effect of Organic and Inorganic Fertilizers, Irrigation Frequency, and Mulching. Agron. J. 1999, 91, 975–983. [Google Scholar] [CrossRef]
- Wang, Y.; Janz, B.; Engedal, T.; Neergaard, A. Effect of irrigation regimes and nitrogen rates on water use efficiency and nitrogen uptake in maize. Agric. Water Manag. 2017, 179, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, H.; Ray, K.; Sarkar, S.; Puste, A.; Mozumder, M.; Rana, L. Impact of nitrogen nutrition on productivity and nutrient use efficiency of potato (Solanum tuberosum L.) In an inceptisol of west Bengal, India. SAARC J. Agric. 2016, 13, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Agami, R.A.; Alamri, S.A.; El-Mageed, T.A.; Abousekken, M.; Hashem, M. Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water Manag. 2018, 210, 261–270. [Google Scholar] [CrossRef]
- Brueck, H.; Erdle, K.; Gao, Y.; Giese, M.; Zhao, Y.; Peth, S.; Lin, S. Effects of N and water supply on water use-efficiency of a semiarid grassland in Inner Mongolia. Plant Soil 2010, 328, 495–505. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gong, S.; Xu, D.; Sui, J. Nitrogen fertigation effect on photosynthesis, grain yield and water use efficiency of winter wheat. Agric. Water Manag. 2017, 179, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Plant Nutrients. In Principles of Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001; pp. 1–13. [Google Scholar]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants § Summary-Zusammenfassung. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Sun, X.; Song, D.; Chen, W.; Zhang, A.; et al. Phosphorous Application Improves Drought Tolerance of Phoebe zhennan. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.F.; Pang, K.; Schnitzler, C.E.; Nguyen, A.-D.; Moreland, R.T.; Simmons, D.K.; Koch, B.J.; Francis, W.R.; Havlak, P.; Smith, S.A.; et al. The Genome of the Ctenophore Mnemiopsis leidyi and Its Implications for Cell Type Evolution. Science 2013, 342, 1242592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Rana, L.; Banerjee, H.; Ray, K.; Sarkar, S. System of wheat intensification (SWI)—A new approach for increasing wheat yield in small holder farming system. J. Appl. Nat. Sci. 2017, 9, 1453–1464. [Google Scholar] [CrossRef]
- Panda, R.K.; Behera, S.K.; Kashyap, P.S. Effective management of irrigation water for wheat under stressed conditions. Agric. Water Manag. 2003, 63, 37–56. [Google Scholar] [CrossRef]
- Ahmed, I.; Ullah, A.; ur Rahman, M.H.; Ahmad, B.; Wajid, S.A.; Ahmad, A.; Ahmed, S. Climate change impacts and adaptation strategies for agronomic crops. In Climate Change and Agriculture; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Xu, Y.; Ma, X.; Ahmad, I.; Jia, Q.; Akmal, M.; Hussain, Z.; Arif, M.; Cai, T.; Zhang, J.; et al. Deficit irrigation strategies to improve winter wheat productivity and regulating root growth under different planting patterns. Agric. Water Manag. 2019, 219, 1–11. [Google Scholar] [CrossRef]
- Doussan, C.; Pierret, A.; Garrigues, E.; Pagès, L. Water uptake by plant roots: II—Modelling of water transfer in the soil root-system with explicit account of flow within the root system—Comparison with experiments. In Plant and Soil; Springer: New York, NY, USA, 2016; pp. 99–117. [Google Scholar]
- Liu, Y.; Zhang, X.; Xi, L.; Liao, Y.; Han, J. Ridge-furrow planting promotes wheat grain yield and water productivity in the irrigated sub-humid region of China. Agric. Water Manag. 2020, 231, 105935. [Google Scholar] [CrossRef]
- Si, Z.; Zain, M.; Mehmood, F.; Wang, G.; Gao, Y.; Duan, A. Effects of nitrogen application rate and irrigation regime on growth, yield, and water-nitrogen use efficiency of drip-irrigated winter wheat in the North China Plain. Agric. Water Manag. 2020, 231, 106002. [Google Scholar] [CrossRef]
- Jha, S.K.; Ramatshaba, T.S.; Wang, G.; Liang, Y.; Liu, H.; Duan, A.; Duan, A. Response of growth, yield and water use efficiency of winter wheat to different irrigation methods and scheduling in North China Plain. Agric. Water Manag. 2019, 217, 292–302. [Google Scholar] [CrossRef]
- Mondal, S.; Dutta, S.; Crespo-Herrera, L.; Huerta-Espino, J.; Braun, H.J.; Singh, R.P. Fifty years of semi-dwarf spring wheat breeding at CIMMYT: Grain yield progress in optimum, drought and heat stress environments. Field Crop. Res. 2020, 250, 107757. [Google Scholar] [CrossRef]
- Javadipour, Z.; Balouchi, H.; Dehnavi, M.M.; Yadavi, A. Roles of methyl jasmonate in improving growth and yield of two varieties of bread wheat (Triticum aestivum) under different irrigation regimes. Agric. Water Manag. 2019, 222, 336–345. [Google Scholar] [CrossRef]
- Selim, D.A.-F.H.; Nassar, R.M.A.; Boghdady, M.S.; Bonfill, M. Physiological and anatomical studies of two wheat cultivars irrigated with magnetic water under drought stress conditions. Plant Physiol. Biochem. 2019, 135, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Guerra, L.; Merlin, O.; Er-Raki, S. Irrigation retrieval from Landsat optical/thermal data integrated into a crop water balance model: A case study over winter wheat fields in a semi-arid region. Remote Sens. Environ. 2020, 239, 111627. [Google Scholar] [CrossRef] [Green Version]
- Gaydon, D.S.; Singh, B.-; Wang, E.; Poulton, P.; Ahmad, B.; Ahmed, F.; Akhter, S.; Ali, I.; Amarasingha, R.; Chaki, A.; et al. Evaluation of the APSIM model in cropping systems of Asia. Field Crop. Res. 2017, 204, 52–75. [Google Scholar] [CrossRef]
- Sun, S.; Yang, X.; Lin, X.; Sassenrath, G.F.; Li, K. Climate-smart management can further improve winter wheat yield in China. Agric. Syst. 2018, 162, 10–18. [Google Scholar] [CrossRef]
- Bell, R.W.; Haque, M.E.; Islam, M.A.; Alam, M.K.; Jahiruddin, M. Long-Term Impact of Conservation Agriculture in Rice-Based Cropping Systems. In Proceedings of the World Congress on Conservation Agriculture, Rosario, Agrentina, 1–4 August 2017. [Google Scholar]
- Rehman, H.; Nawaz, A.; Wakeel, A.; Saharawat, Y.; Farooq, M. Conservation Agriculture in South Asia. In Conservation Agriculture; Farooq, M., Siddique, K., Eds.; Springer: Cham, Switzerland, 2015; pp. 249–283. [Google Scholar] [CrossRef]
- Kakraliya, S.; Jat, H.; Singh, I.; Sapkota, T.B.; Singh, L.K.; Sutaliya, J.M.; Sharma, P.C.; Jat, R.; Choudhary, M.; Lopez-Ridaura, S.; et al. Performance of portfolios of climate smart agriculture practices in a rice-wheat system of western Indo-Gangetic plains. Agric. Water Manag. 2018, 202, 122–133. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M.; Misheva, S. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop. Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Brestič, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef]
- Chovancek, E.; Zivcak, M.; Botyanszka, L.; Hauptvogel, P.; Yang, X.; Misheva, S.; Hussain, S.; Brestic, M. Transient Heat Waves May Affect the Photosynthetic Capacity of Susceptible Wheat Genotypes Due to Insufficient Photosystem I Photoprotection. Plants 2019, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Jones, S.; Ganapathysubramanian, B.; Sarkar, S.; Mueller, D.; Sandhu, K.; Nagasubramanian, K. Challenges and Opportunities in Machine-Augmented Plant Stress Phenotyping. Trends Plant Sci. 2020, 26, 53–69. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. https://doi.org/10.3390/agronomy11020241
Hossain A, Skalicky M, Brestic M, Maitra S, Ashraful Alam M, Syed MA, Hossain J, Sarkar S, Saha S, Bhadra P, et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy. 2021; 11(2):241. https://doi.org/10.3390/agronomy11020241
Chicago/Turabian StyleHossain, Akbar, Milan Skalicky, Marian Brestic, Sagar Maitra, M. Ashraful Alam, M. Abu Syed, Jamil Hossain, Sukamal Sarkar, Saikat Saha, Preetha Bhadra, and et al. 2021. "Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate" Agronomy 11, no. 2: 241. https://doi.org/10.3390/agronomy11020241
APA StyleHossain, A., Skalicky, M., Brestic, M., Maitra, S., Ashraful Alam, M., Syed, M. A., Hossain, J., Sarkar, S., Saha, S., Bhadra, P., Shankar, T., Bhatt, R., Kumar Chaki, A., EL Sabagh, A., & Islam, T. (2021). Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy, 11(2), 241. https://doi.org/10.3390/agronomy11020241














