Influence of Application of Organic Residues of Different Biochemical Quality on Phosphorus Fractions in a Tropical Sandy Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Characteristics
2.2. Experimental Design and Soil Sampling
2.3. Chemical Analysis
2.4. Sequential Extraction Procedure
2.5. Statistical Analysis
3. Results and Discussion
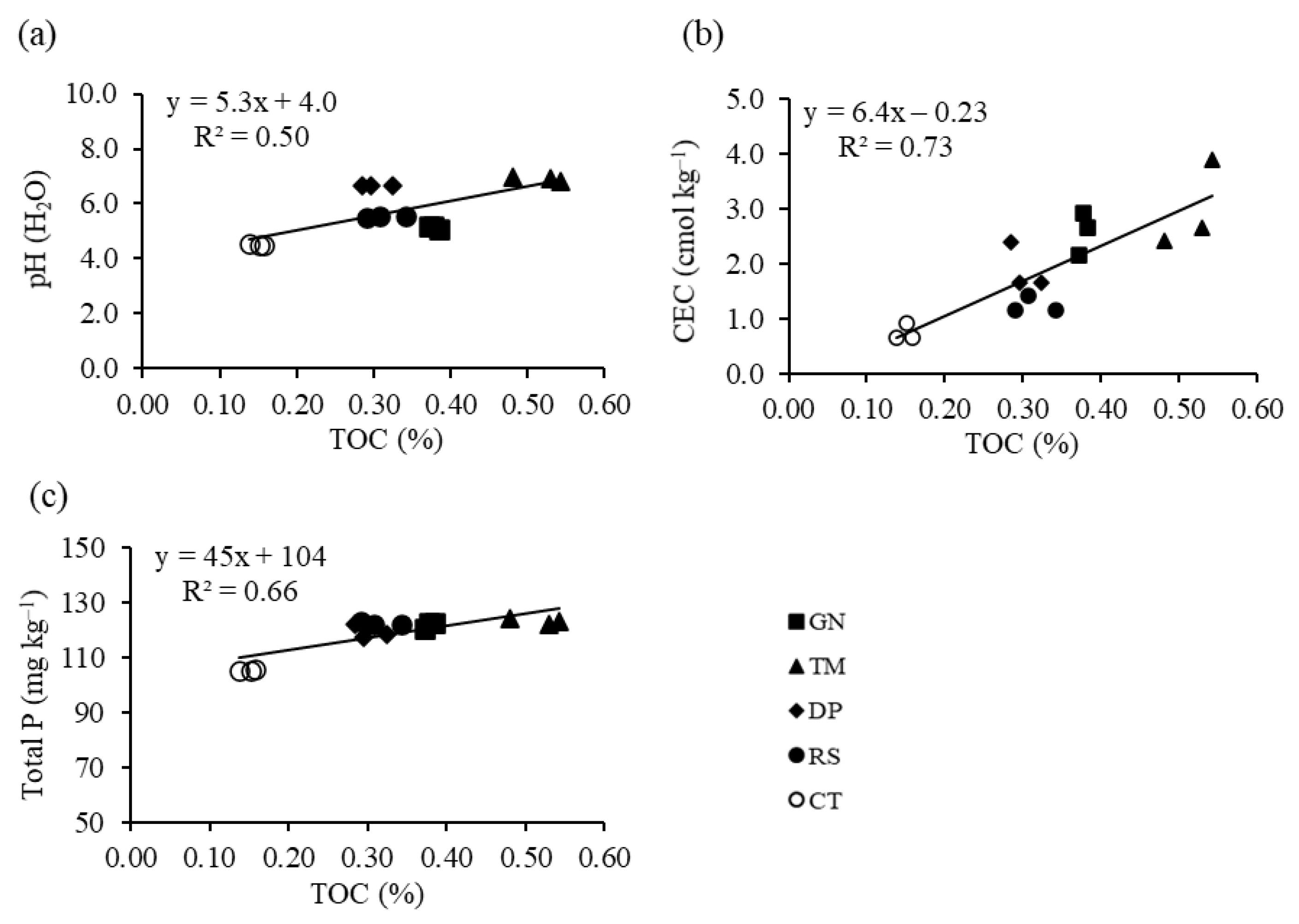
3.1. Application Effects of Organic Residues of Different Quality on Soil Chemical and Mineralogical Properties
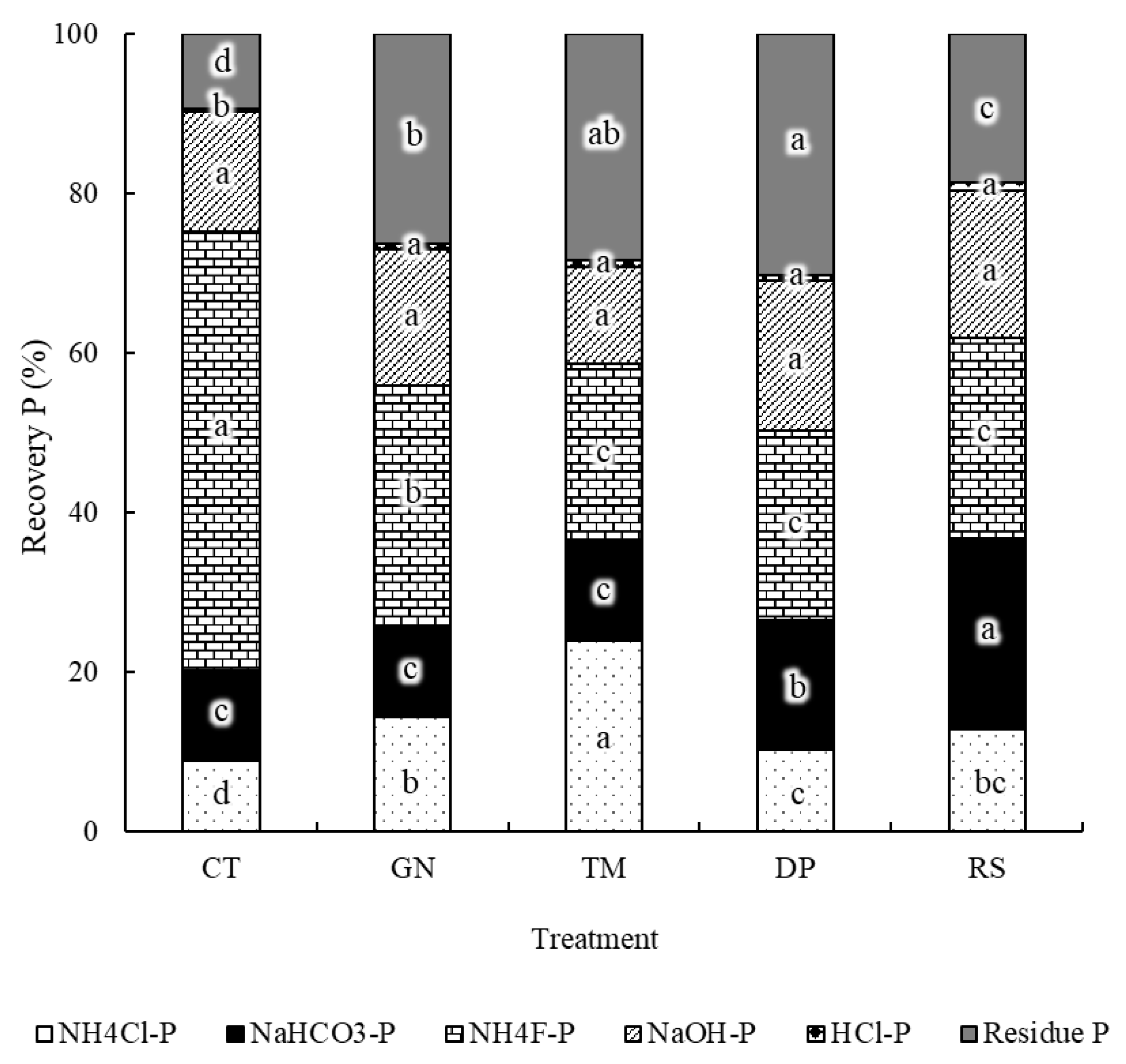
3.2. Phosphorus Fractions
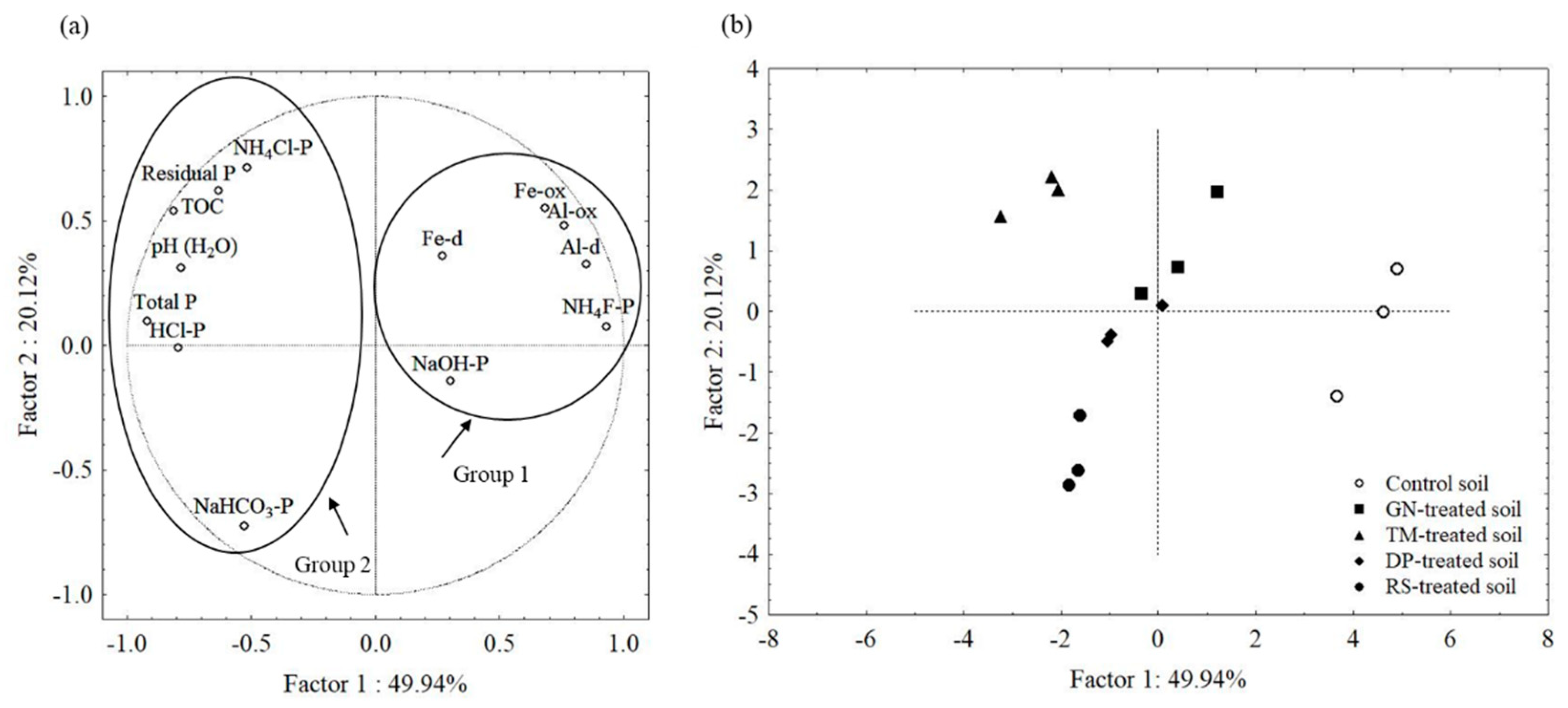
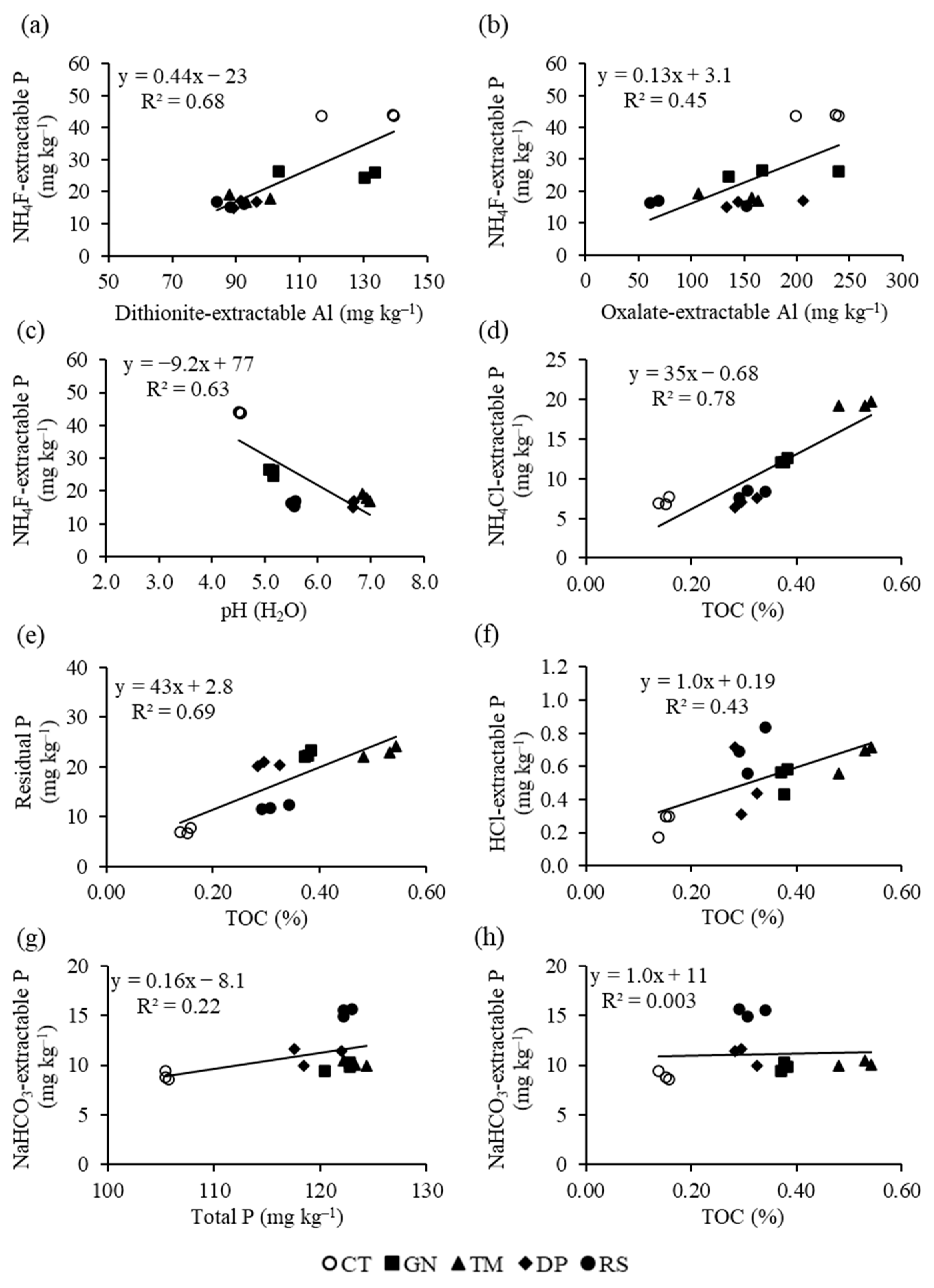
3.3. Relationships between Phosphorus Fractions and Soil Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vityakon, P.; Meepech, S.; Cadisch, G.; Toomsan, B. Soil organic matter and nitrogen transformation mediated by plant residues of different qualities in sandy acid upland and paddy soils. Neth. J. Agric. Sci. 2000, 48, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Fujii, K.; Hayakawa, C.; Panitkasate, T.; Maskhao, I.; Funakawa, S.; Kosaki, T.; Nawata, E. Acidification and buffering mechanisms of tropical sandy soil in northeast Thailand. Soil Tillage Res. 2017, 165, 80–87. [Google Scholar] [CrossRef]
- Singh, B.; Gilkes, R.J. Properties and distribution of iron oxides and their association with minor elements in the soils of south-western Australia. Eur. J. Soil Sci. 1992, 43, 77–98. [Google Scholar] [CrossRef]
- Holford, I.C.R. Soil phosphorus: Its measurement, and its uptake by plants. Aust. J. Soil Res. 1997, 35, 227–240. [Google Scholar] [CrossRef]
- Abdala, D.B.; da Silva, I.R.; Verguts, L.; Sparks, D.L. Long-term manure application effects on phosphorus speciation, kinetics and distribution in highly weathered agricultural soils. Chemosphere 2015, 119, 504–514. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil-phosphorus fractions induced by cultivation practices and by laboratory incubation. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Hesterberg, D.; Zhou, W.Q.; Hitchison, K.J.; Beauchemin, S.; Sayers, D.E. XAFS study of adsorbed and mineral forms of phosphate. J. Synchrotron Radiat. 1999, 6, 636–638. [Google Scholar] [CrossRef]
- Hesterberg, D. Macroscale chemical properties and X-ray absorption spectroscopy of soil phosphorus. In Synchrotron-Based Techniques in Soils and Sediments; Singh, B., Grafe, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 313–356. [Google Scholar]
- Eriksson, A.K.; Gustadsson, J.P.; Hesterberg, D. Phosphorus speciation of clay fractions from long-term fertility experiments in Sweden. Geoderma 2015, 241, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Frossard, E.; Brossard, M.; Hedley, M.J.; Metherell, A. Reactions controlling the cycling of P in soils. In Phosphorus in the Global Environment; Tiessen, H., Ed.; John Wiley & Sons: New York, NY, USA, 1995; pp. 107–137. [Google Scholar]
- Eriksson, A.K.; Hesterberg, D.; Klysubun, W.; Gustafsson, J.P. Phosphorus dynamics in Swedish agricultural soils as influenced by fertilization and mineralogical properties: Insights gained from batch experiments and XANES spectroscopy. Sci. Total Environ. 2016, 566, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Laakso, J.; Uusitalo, R.; Yli-Halla, M. Phosphorus speciation in agricultural catchment soils and in fresh and dried sediments of five constructed wetlands. Geoderma 2016, 271, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Yusiharni, E.; Gilkes, R. Minerals in the ash of Australian native plants. Geoderma 2012, 189, 369–380. [Google Scholar] [CrossRef]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekui, W. Forms and solubility of plant nutrient elements in tropical plant waste biochars. J. Plant Nutr. Soil Sci. 2015, 178, 732–740. [Google Scholar] [CrossRef]
- Sukitprapanon, T.; Jantamenchai, M.; Tulaphitak, D.; Vityakon, P. Nutrient composition of diverse organic residues and their long-term effects on available nutrients in a tropical sandy soil. Heliyon 2020, 6, e05601. [Google Scholar] [CrossRef]
- Quilty, J.R.; Cattle, S.R. Use and understanding of organic amendments in Australian agriculture: A review. Soil Res. 2011, 49, 1–26. [Google Scholar] [CrossRef]
- Noack, S.R.; McLaughlin, M.J.; Smernik, R.J.; McBeath, T.M.; Armstrong, R.D. Crop residue phosphorus: Speciation and potential bio-availability. Plant Soil. 2012, 359, 375–385. [Google Scholar] [CrossRef]
- Mackay, J.E.; Macdonald, L.M.; Smernik, R.J.; Cavagnaro, T.R. Organic amendments as phosphorus fertilizers: Chemical analysis, biological processes and plant P uptake. Soil Biol. Biochem. 2017, 107, 50–59. [Google Scholar] [CrossRef]
- Noack, S.R.; McBeath, T.M.; McLaughlin, M.J.; Smernik, R.J.; Armstrong, R.D. Management of crop residues affects the transfer of phosphorus to plant and soil roots: Results from a dual-labelling experiment. Soil Biol. Biochem. 2014, 71, 31–39. [Google Scholar] [CrossRef]
- Palm, C.A.; Gachengo, C.N.; Delve, R.J.; Cadisch, G.; Giller, K.E. Organic inputs for soil fertility management in tropical agroecosystems: Application of an organic resource database. Agric. Ecosyst. Environ. 2001, 83, 27–42. [Google Scholar] [CrossRef]
- Schmieder, F.; Bergström, L.; Riddle, M.; Gustaffson, J.; Klysubun, W.; Zehetner, F.; Condron, L.; Kirchmann, H. Phosphorus speciation in a long-term manure-amended soil profile-Evidence from wet chemical extraction, 31P-NMR and P K-edge XANES spectroscopy. Geoderma 2018, 322, 19–27. [Google Scholar] [CrossRef]
- Samahadthai, P.; Vitkakon, P.; Saenjan, P. Effects of different quality plant residues on soil carbon accumulation and aggregate formation in a tropical sandy soils in Northeast Thailand as revealed by a 10-year field experiment. Land Degrad. Dev. 2010, 21, 463–473. [Google Scholar] [CrossRef]
- Chivenge, P.; Vanlauwe, B.; Gentile, R.; Six, J. Organic resource quality influences short-term aggregate dynamics and soil organic carbon and nitrogen accumulation. Soil Biol. Biochem. 2011, 43, 657–666. [Google Scholar] [CrossRef]
- Puttaso, A.; Vityakon, P.; Rasche, F.; Saenjan, P.; Treloges, V.; Cadisch, G. Does organic residues quality influence carbon retention in a tropical sandy soils? Soil Sci. Soc. Am. J. 2013, 77, 1001–1011. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop residues and management practices: Effect on soil quality, soil nitrogen dynamics, crop yield, and nitrogen recovery. Adv. Agron. 1999, 68, 197–319. [Google Scholar]
- Sukitprapanon, T.; Suddhiprakarn, A.; Kheoruenromne, I.; Gilkes, R.J. Rare earth elements in acid sulfate soils under long-term paddy rice cultivation in Thailand. Geoderma Reg. 2019, 17, e00216. [Google Scholar] [CrossRef]
- Lin, J.; Chen, S. The relationship between adsorption of heavy metal and organic matter in river sediments. Environ. Int. 1998, 24, 345–352. [Google Scholar] [CrossRef]
- Vischetti, C.; Manaci, E.; Casucci, C.; Bernardi, A.D.; Carninali, A. Adsorption and degradation of tree pesticides in vineyard sol and in an organic biomix. Environment 2020, 7, 113. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Meteorological Department. Climate of Thailand; Meteorological Department, Ministry of Information and Communication Technology: Bangkok, Thailand, 2015.
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United State Department of Agriculture, Natural Resource Conservation Service: Washington, DC, USA, 2014.
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods-Australasia; CSIRO Publishing: Melbourne, Australia, 2011. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3. Chemical Method; Sparks, D.L., Ed.; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association-American Water Works Association: Baltimore, MD, USA, 1998. [Google Scholar]
- Chao, T.T. Use of partial dissolution techniques in geochemical exploration. J. Geochem. Explor. 1984, 20, 101–135. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification; Mineralogical Society Monograph No. 5; Mineralogical Society: London, UK, 1980. [Google Scholar]
- Zhang, H.; Kovar, J.L. Phosphorus fractionation. In Methods for Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; Pierzysnki, G.M., Ed.; North Carolina State University Press: Raleigh, NC, USA, 2000; pp. 50–59. [Google Scholar]
- Kovar, J.L.; Pierzynski, G.M. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, 2nd ed.; Southern Cooperative Series Bulletin No. 408; Virginia Tech University: Blacksburg, VA, USA, 2009. [Google Scholar]
- Cleemput, O.V.; Boeckx, P. Nitrogen and its transformation. In Encyclopedia of Soil Science, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Lawongsa, P.; Rungthong, R.; Kamolmanit, B.; Saenjan, P.; Vityakon, P. Responses of soil bacterial population to the appearance of allelopathic substances. Khon Kaen Agr. J. 2016, 44 (Suppl. 1), 983–990. [Google Scholar]
- Ellerbrock, R.H.; Gerke, H.H. Characterization of organic matter composition of soil and flow path surfaces based on physiochemical principles—A review. In Advances in Agronomy; Spark, D.L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 117–177. [Google Scholar]
- Weng, L.; Riemsdijk, W.H.V.; Hiemstra, T. Factors controlling phosphate interaction with iron oxides. J. Environ. Qua. 2012, 41, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Abdala, D.B.; Ghosh, A.K.; Silva, I.R.; Novais, R.F.; Venegas, V.H.A. Phosphorus saturation of a tropical soil and related P leaching caused by poultry litter addition. Agric. Ecosyst. Environ. 2012, 16, 15–23. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, H.; Hu, Y.; Wei, X.; Chen, X.; Ge, T.; Wu, J.; Su, Y. Cellulose and lignin regulate partitioning of soil phosphorus fractions and alkaline phosphomonoesterase encoding bacterial community in phosphorus-deficient soils. Biol. Fertil. Soils 2018, 55, 31–42. [Google Scholar] [CrossRef]
- Sukitprapanon, T.; Suddhiprakarn, A.; Kheoruenromne, I.; Anusontpornperm, S.; Gilkes, R.J. A comparison of potential, active and post-active acid sulfate soils in Thailand. Geoderma Reg. 2016, 7, 346–356. [Google Scholar] [CrossRef]
- Jakobsen, S.T. Interaction between plant nutrients: IV. Interaction between calcium and phosphate. Acta Agric. Scand. Sect. B 1993, 43, 6–10. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Miles, R.J. Soil phosphorus fractions after 111 years of animal manure and fertilizer applications. Biol. Fertil. Soils 2002, 36, 35–42. [Google Scholar]
- Luo, L.; Ma, Y.; Sanders, R.L.; Xu, C.; Li, J.; Myneni, S.C. Phosphorus speciation and transformation in long-term fertilized soil: Evidence from chemical fractionation and P K-edge XANES spectroscopy. Nutr. Cycl. Agroecosyst. 2017, 107, 215–226. [Google Scholar] [CrossRef]
- Bache, B.W.; Crooke, W.M. Interactions between aluminum, phosphorus, and pH in the response of barley to soil acidity. Plant Soil 1981, 61, 365–375. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press, Taylor and Francis: New York, NY, USA, 2011. [Google Scholar]
- Samadi, A.; Gilkes, R.J. Forms of phosphorus in virgin and fertilized calcareous soils of Western Australia. Soil Res. 1998, 36, 585–602. [Google Scholar] [CrossRef]
- Lindsay, W.L. Iron oxide solubilization by organic matter and its effect on iron availability. Plant Soil 1991, 130, 27–34. [Google Scholar] [CrossRef]
- Noble, A.D.; Zenneck, I.; Randall, P.J. Lead litter ash alkalinity and neutralization of soil acidity. Plant Soil. 1996, 179, 293–302. [Google Scholar] [CrossRef]
- Weyers, E.; Strawn, D.G.; Peak, D.; Moore, A.D.; Baker, L.L.; Cade-Menun, B. Phosphorus speciation in calcareous soils following annual dairy manure amendments. Soil Sci. Soc. Am. J. 2016, 80, 1531–1542. [Google Scholar] [CrossRef]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Breuning-Madsen, H.; Andersen, M.N. Phosphorus retention and availability in three contrasting soil amended with rice husk and corn cob biochar at varying pyrolysis temperature. Geoderma 2019, 341, 10–17. [Google Scholar] [CrossRef]
- Gilkes, R.J.; Prakongkep, N. How the unique properties of soil kaolin affect the fertility of tropical soils. Appl. Clay Sci. 2016, 131, 100–106. [Google Scholar] [CrossRef]
- Li, W.; Johnson, C.E. Relationships among pH, aluminum solubility and aluminum complexation with organic matter in acid forest soils of the Northeastern United States. Geoderma 2016, 271, 234–242. [Google Scholar] [CrossRef]
- Maranguit, D.; Guillaume, T.; Kuzyakov, Y. Land-use change affects phosphorus fractions in highly weathered tropical soils. Catena 2017, 149, 385–393. [Google Scholar] [CrossRef]
- Recena, R.; Cade-Menun, B.J.; Delgado, A. Organic phosphorus form in agricultural soils under Mediterranean climate. Soil Sci. Soc. Am. J. 2018, 82, 783–795. [Google Scholar] [CrossRef]
- Butnan, S.; Vityakon, P. The interactive effects of soil disturbance and residue quality on soil nitrogen mineralization in a tropical sandy soil. Soil Res. 2020, 58, 277–288. [Google Scholar] [CrossRef]
- Boesch, D.; Hecky, R.; O’Melia, C.; Schindler, D.; Seitzinger, S. Eutropication of Swedish Seas, Report 5509; Swedish Environmental Protection Agency: Stockholm, Sweden, 2006.
- Myers, R.J.K.; van Noordwijk, M.; Vityakon, P. Synchrony of nutrient release and plant demand: Plant litter quality, soil environment and farmer management options. In Driven by Nature: Plant Litter Quality and Decomposition; Cadisch, G., Giller, K.E., Eds.; CAB International: Cambridge, UK, 1997; pp. 215–229. [Google Scholar]
| Residue 1 | TOC 2 | N 2 | C/N 2 | P 2 | Ca 2 | L 3 | Pp 3 |
|---|---|---|---|---|---|---|---|
| % | % | mg kg−1 | mg kg−1 | % | % | ||
| GN | 39 | 1.7 | 22 | 2169 | 3490 | 9.9 | 1.6 |
| TM | 40 | 1.7 | 23 | 763 | 19,837 | 17 | 4.6 |
| DP | 42 | 1.0 | 40 | 480 | 4130 | 25 | 5.0 |
| RS | 36 | 0.40 | 86 | 655 | 1222 | 3.6 | 1.5 |
| Treatment | pH | TOC | CEC | Total P | P-ox | Al-ox | Fe-ox | Al-d | Fe-d | DPS |
|---|---|---|---|---|---|---|---|---|---|---|
| (H2O) | (%) | (cmol kg−1) | (-------------------------mg kg−1------------------------) | (%) | ||||||
| CT | 4.5 e | 0.15 d | 0.75 c | 106 b | 84 a | 225 a | 417 | 132 a | 1210 | 13 a |
| GN | 5.1 d | 0.38 b | 2.6 a | 122 a | 78 a | 181 ab | 358 | 122 a | 1207 | 14 a |
| TM | 6.9 a | 0.52 a | 3.0 a | 123 a | 57 ab | 143 b | 333 | 94 b | 1194 | 12 a |
| DP | 5.7 b | 0.30 c | 1.9 ab | 119 a | 50 b | 161 ab | 334 | 92 b | 1182 | 10 b |
| RS | 5.5 c | 0.31 c | 1.2 b | 122 a | 40 b | 94 b | 256 | 88 b | 1174 | 9.1 b |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | 0.011 | 0.031 | 0.085 | <0.001 | 0.983 | <0.001 |
| Treatment | Bulk Sample | Clay Fraction | |||||
|---|---|---|---|---|---|---|---|
| Quartz | Feldspars | Muscovite | Anatase | Quartz | Kaolinite | Anatase | |
| Control soil | xxxx | tr | tr | tr | xxx | x | tr |
| GN-treated soil | xxxx | tr | tr | nd | xxx | x | tr |
| TM-treated soil | xxxx | tr | nd | nd | xxx | x | tr |
| DP-treated soil | xxxx | tr | tr | nd | xxx | x | tr |
| RS-treated soil | xxxx | tr | nd | nd | xxx | x | tr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukitprapanon, T.-S.; Jantamenchai, M.; Tulaphitak, D.; Prakongkep, N.; Gilkes, R.J.; Vityakon, P. Influence of Application of Organic Residues of Different Biochemical Quality on Phosphorus Fractions in a Tropical Sandy Soil. Agronomy 2021, 11, 248. https://doi.org/10.3390/agronomy11020248
Sukitprapanon T-S, Jantamenchai M, Tulaphitak D, Prakongkep N, Gilkes RJ, Vityakon P. Influence of Application of Organic Residues of Different Biochemical Quality on Phosphorus Fractions in a Tropical Sandy Soil. Agronomy. 2021; 11(2):248. https://doi.org/10.3390/agronomy11020248
Chicago/Turabian StyleSukitprapanon, Tanabhat-Sakorn, Metawee Jantamenchai, Duangsamorn Tulaphitak, Nattaporn Prakongkep, Robert John Gilkes, and Patma Vityakon. 2021. "Influence of Application of Organic Residues of Different Biochemical Quality on Phosphorus Fractions in a Tropical Sandy Soil" Agronomy 11, no. 2: 248. https://doi.org/10.3390/agronomy11020248







