A Tool Derived from the Vicia faba Micronucleus Assay, to Assess Genotoxicity, Cytotoxicity or Biostimulation of Novel Compounds Used in Agriculture
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Culture
2.2. Hydroponic Conditions
2.3. Humic Substances and Fulvic Acids
2.4. Other Commercial Products
2.5. Effects of BHS® on Vicia faba Roots Exposed to Contaminants
2.6. Growth Measurement
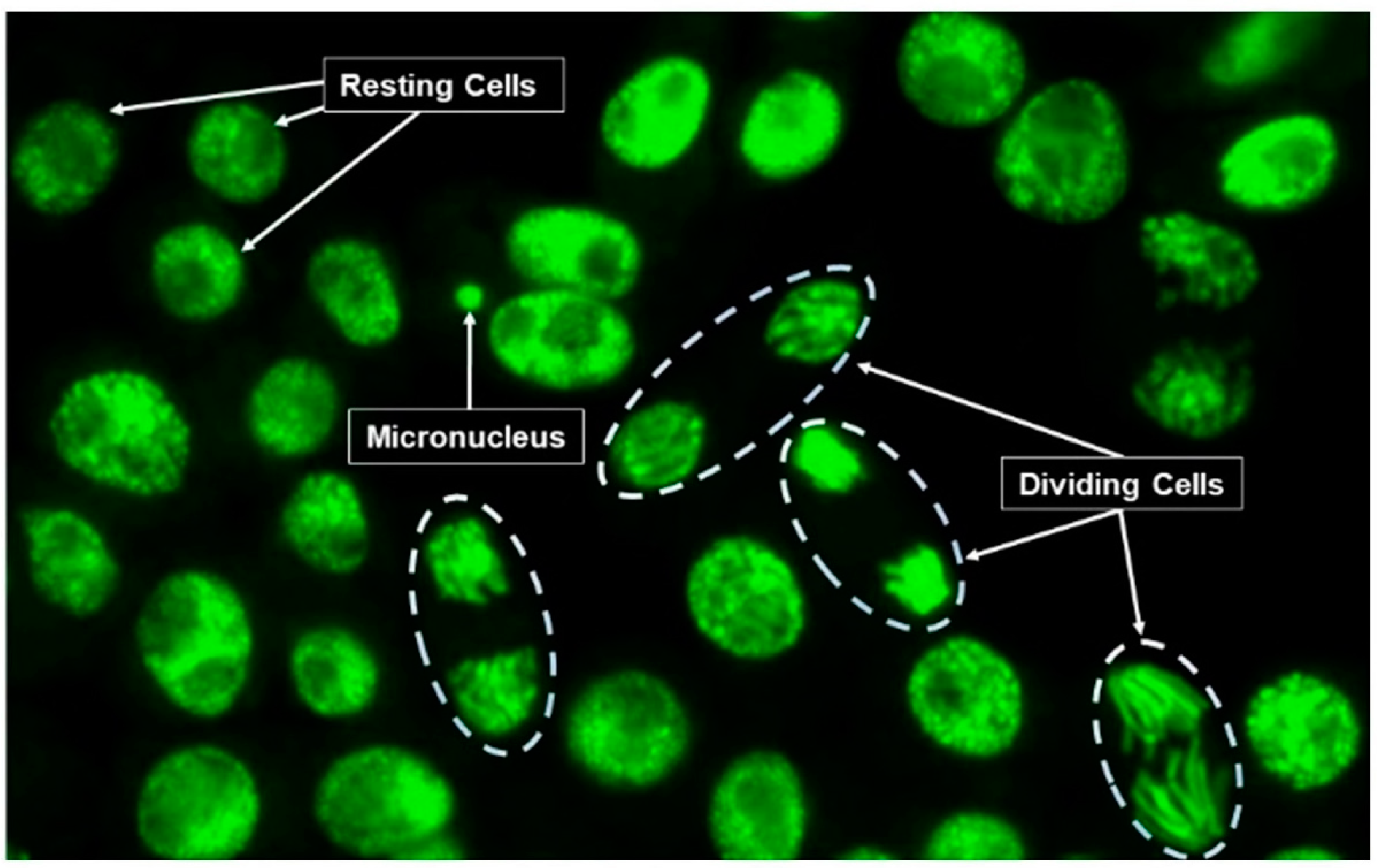
2.7. Micronucleus Assay
2.8. Statistical Analysis
3. Results and Discussion
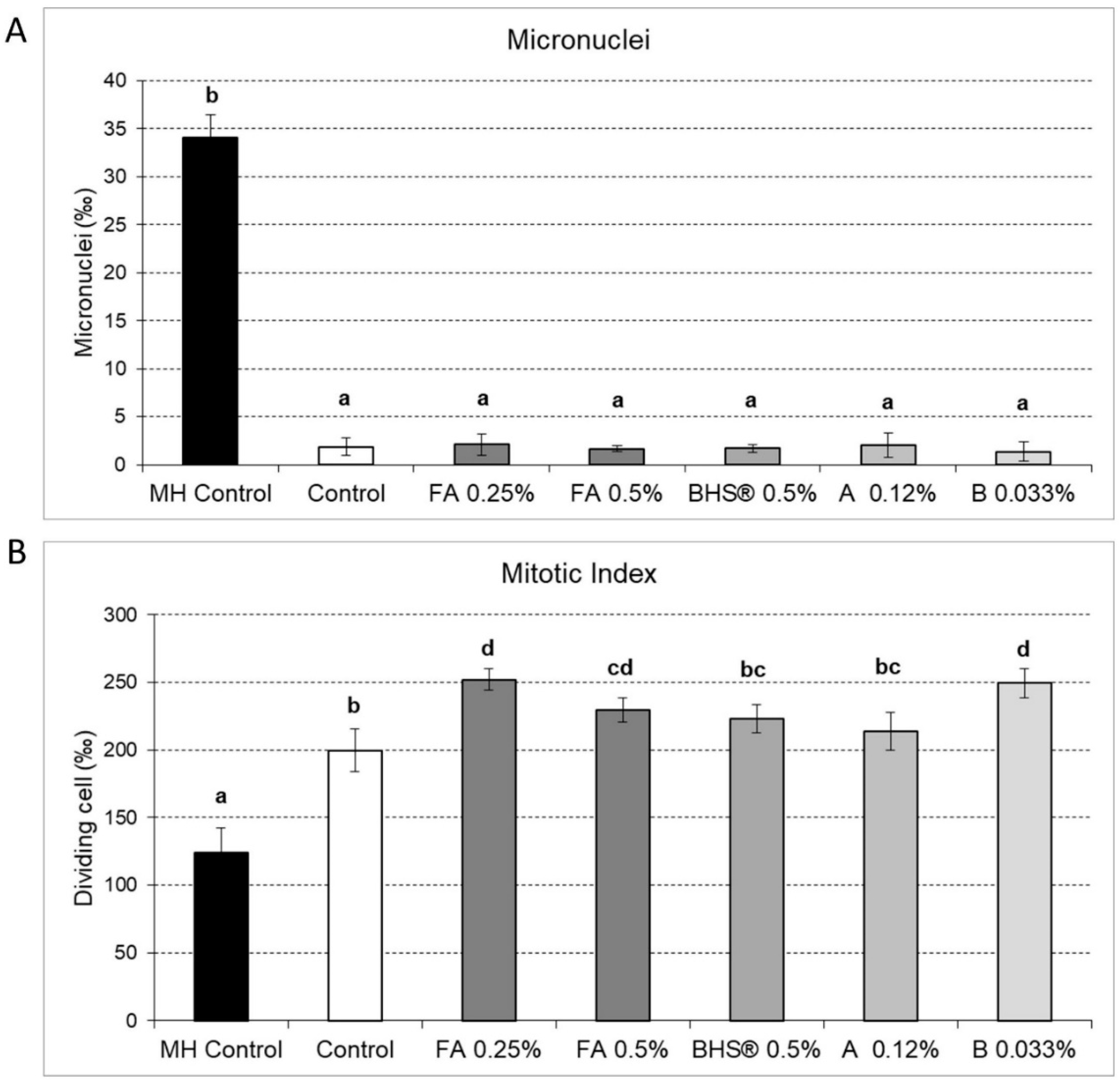
3.1. Impacts of Biostimulants on Micronuclei Formation and Cell Division Frequency in Vicia Faba Roots Cells
- Genotoxic effects, if the micronuclei number is greater than the control (MN > MN Control);
- Cytotoxic effects, if the mitotic index is less than the control (MI < MI control);
- Biostimulant effects, if the mitotic index is greater than control (MI > MI Control).
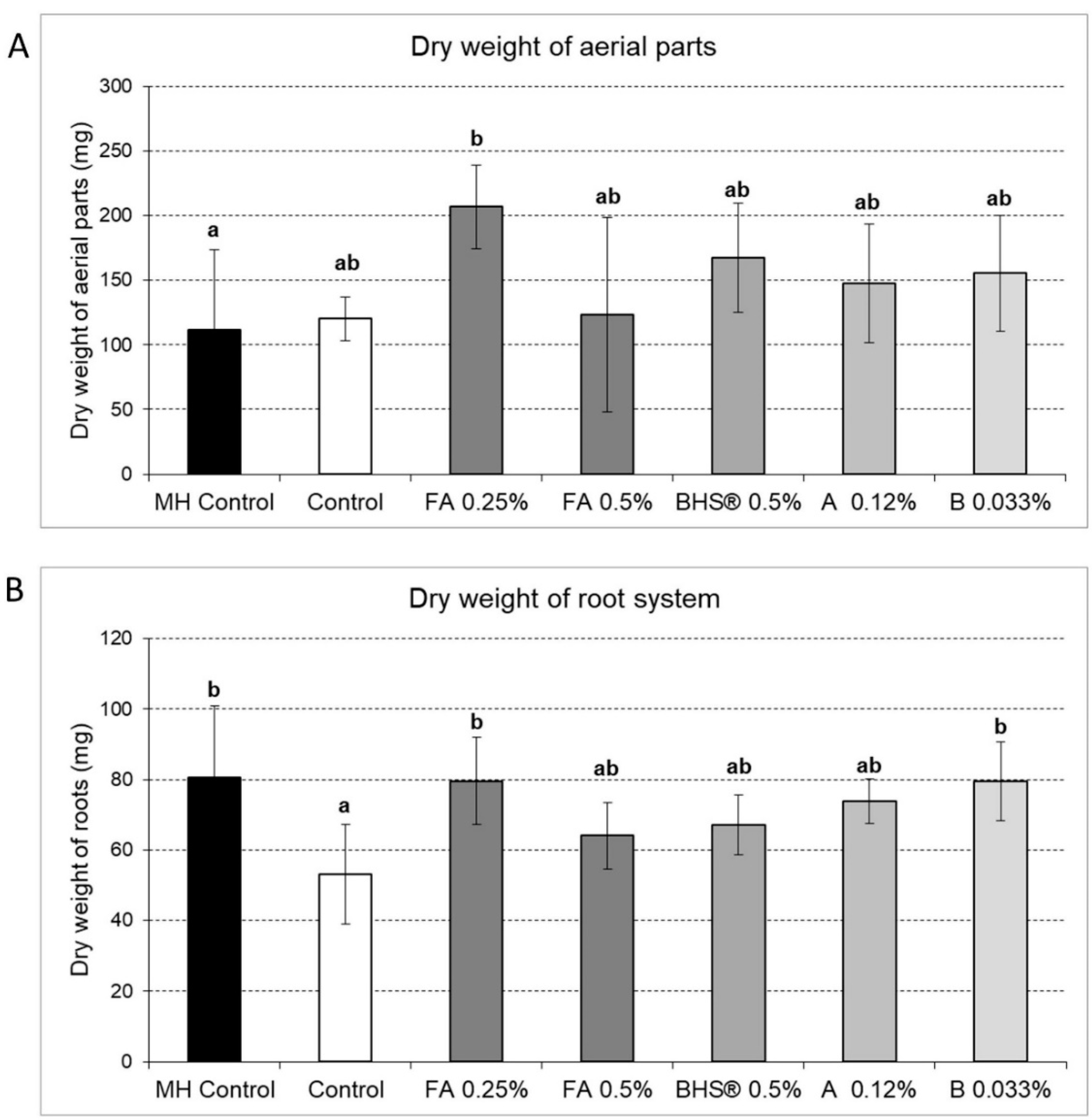
3.2. Impacts of Biostimulants on Root and Plant Aerial Biomass
- -
- Test growth effects within 10 days contrarily to those implying longer growth experimentation durations needed for biomass production;
- -
- Compare agronomical efficiencies of different commercial products;
- -
- Define the optimal dose required for the same product within short spans of time.
3.3. Impacts of Biostimulants on Length and Surface Area of the Root System
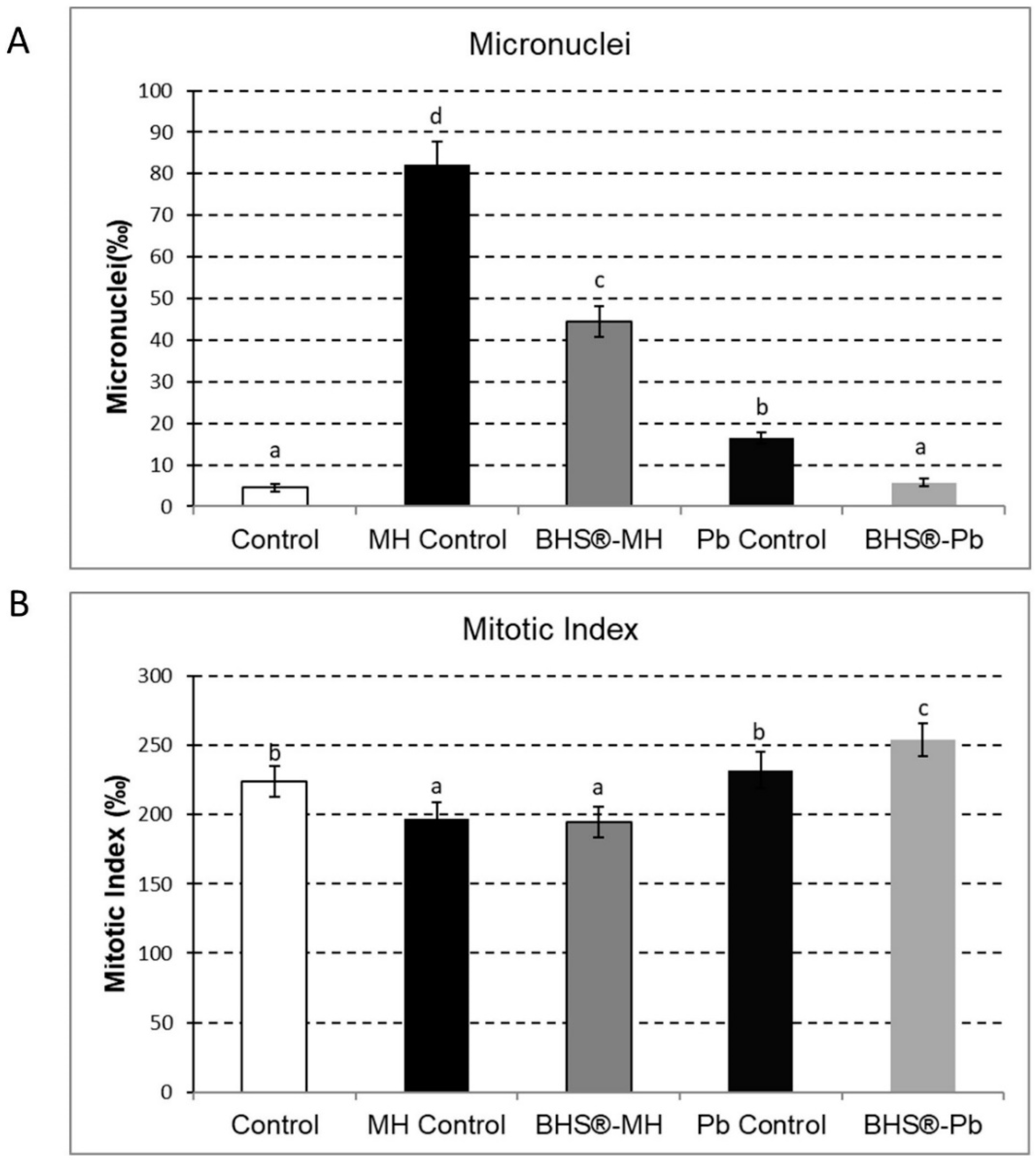
3.4. Protective Effects of Biostimulants towards Exposure to Contaminants
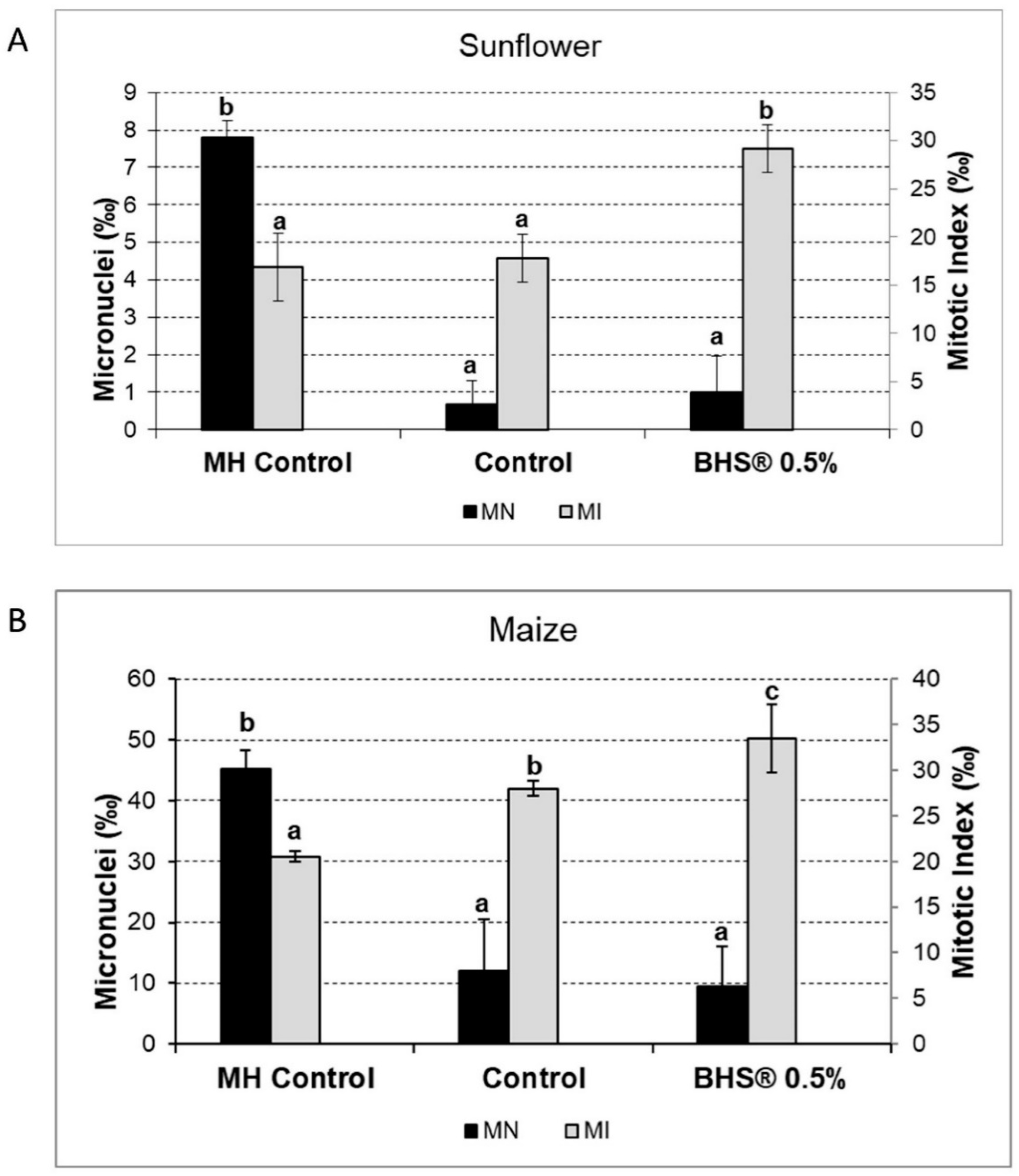
3.5. Extension to Other Agronomic Crops
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Laurent, E.-A.; Ahmed, N.; Durieu, C.; Grieu, P.; Lamaze, T. Marine and fungal biostimulants improve grain yield, nitrogen absorption and allocation in durum wheat plants. J. Agric. Sci. 2020, 158, 279–287. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Findura, P.; Treder, K. Modification of Yield and Fiber Fractions Biosynthesis in Phaseolus vulgaris L. by Treatment with Biostimulants Containing Amino Acids and Seaweed Extract. Agronomy 2020, 10, 1338. [Google Scholar] [CrossRef]
- Ioppolo, A.; Laudicina, V.A.; Badalucco, L.; Saiano, F.; Palazzolo, E. Wastewaters from citrus processing industry as natural biostimulants for soil microbial community. J. Environ. Manag. 2020, 273, 111137. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inocu-lants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef] [Green Version]
- Daniel, E.C.; Fabio, G. An assessment of seaweed extracts: Innovation for sustainable agriculture. Agronomy 2020, 10, 1433. [Google Scholar] [CrossRef]
- Masondo, N.A.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol. Environ. Saf. 2018, 147, 43–48. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation 2019/1009 of the European Parliament. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 4 November 2020).
- Du Jardin, P. Plant Biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Szczałba, M.; Kopta, T.; Gąstoł, M.; Sekara, A. Comprehensive insight into arbuscular mycorrhizal fungi, Trichoderma spp. and plant multilevel interactions with emphasis on biostimulation of horticultural crops. J. Appl. Microbiol. 2019, 127, 630–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, Ecological, and Biochemical Implications in Tomato Plants of Two Plant Biostimulants: Arbuscular Mycorrhizal Fungi and Seaweed Extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Hörz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Chen, Y.; Aviad, T. Humic Substances in Soil and Crop Sciences: Selected Readings. Humic Subst. Soil Crop Sci. Sel. Read. 1990, 161–186. [Google Scholar] [CrossRef]
- Vaughan, D.; Macdonald, I. Some effects of humic acid on cation uptake by parenchyma tissue. Soil Biol. Biochem. 1976, 8, 415–421. [Google Scholar] [CrossRef]
- Eyheraguibel, B. Caractérisation des Substances Humiques Biomimétiques: Effets sur les Végétaux; INPT: Toulouse, France, 2004; Volume 3. [Google Scholar]
- Baldotto, M.A.; Muniz, R.C.; Baldotto, L.E.B.; Dobbss, L.B. Root growth of Arabidopsis thaliana (L.) Heynh. treated with humic acids isolated from typical soils of Rio de Janeiro state, Brazil. Rev. Ceres 2011, 58, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Canellas, N.O.A.; Olivares, F.L.; Canellas, L.P. Metabolite fingerprints of maize and sugarcane seedlings: Searching for markers after inoculation with plant growth-promoting bacteria in humic acids. Chem. Biol. Technol. Agric. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S.; Francioso, O.; Pizzeghello, D.; Tinti, A.; Schiavon, M. Metabolite-Targeted Analysis and Physiological Traits of Zea Mays L. in Response to Application of a Leonardite-Humate and Lignosulfonate-Based Products for Their Evaluation as Potential Biostimulants. Agronomy 2019, 9, 445. [Google Scholar] [CrossRef] [Green Version]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Canterino, S.; Cerutti, A.K.; Bounous, G. Improving the nutritional value of kiwifruit with the application of agroindustry waste extracts. J. Appl. Bot. Food Qual. 2013, 86, 11–15. [Google Scholar] [CrossRef]
- Antón-Herrero, R.; García-Delgado, C.; Alonso-Izquierdo, M.; Cuevas, J.; Carreras, N.; Rivilla, B.; Camacho-Arévalo, R.; Eymar, E. New Uses of Treated Urban Waste Digestates on Stimulation of Hydroponically Grown Tomato (Solanum lycopersicon L.). Waste Biomass Valorization 2020, 1–13. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; La Fuente, M.C.-D.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [Green Version]
- Spaccini, R.; Cozzolino, V.; Di Meo, V.; Savy, D.; Drosos, M.; Piccolo, A. Bioactivity of humic substances and water extracts from compost made by ligno-cellulose wastes from biorefinery. Sci. Total. Environ. 2019, 646, 792–800. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, E.R.; De Camargo, R.; Lana, R.M.Q.; Madeiros, M.H.; Menezes, F.G.; Giorgenon, E.P. Yield and biometry of fertilized sugar cane with organomineral fertilizer of sewage sludge and biostimulant. Biosci. J. 2020, 36, 1564–1576. [Google Scholar] [CrossRef]
- El Fels, L.; Zamama, M.; El Asli, A.; Hafidi, M. Assessment of biotransformation of organic matter during co-composting of sewage sludge-lignocelullosic waste by chemical, FTIR analyses, and phytotoxicity tests. Int. Biodeterior. Biodegrad. 2014, 87, 128–137. [Google Scholar] [CrossRef]
- El Fels, L.; Hafidi, M.; Silvestre, J.; Kallerhoff, J.; Merlina, G.; Pinelli, E. Efficiency of co-composting process to remove genotoxicity from sewage sludge contaminated with hexavalent chromium. Ecol. Eng. 2015, 82, 355–360. [Google Scholar] [CrossRef]
- Palumbo, G.; Schiavon, M.; Nardi, S.; Ertani, A.; Celano, G.; Colombo, C.M. Biostimulant Potential of Humic Acids Extracted From an Amendment Obtained via Combination of Olive Mill Wastewaters (OMW) and a Pre-treated Organic Material Derived From Municipal Solid Waste (MSW). Front. Plant Sci. 2018, 9, 1028. [Google Scholar] [CrossRef]
- Silva, T.D.S.; Silva, A.P.S.E.; Santos, A.D.A.; Ribeiro, K.G.; De Souza, D.C.; Bueno, P.A.A.; Marques, M.M.M.; De Almeida, P.M.; Peron, A.P. Cytotoxicity, Genotoxicity, and Toxicity of Plant Biostimulants Produced in Brazil: Subsidies for Determining Environmental Risk to Non-Target Species. Water Air Soil Pollut. 2020, 231, 1–8. [Google Scholar] [CrossRef]
- Vijayaraj, V.; Liné, C.; Cadarsi, S.; Salvagnac, C.; Baqué, D.; Elger, A.; Barret, M.; Mouchet, F.; LaRue, C. Transfer and Ecotoxicity of Titanium Dioxide Nanoparticles in Terrestrial and Aquatic Ecosystems: A Microcosm Study. Environ. Sci. Technol. 2018, 52, 12757–12764. [Google Scholar] [CrossRef] [PubMed]
- Evariste, L.; Lagier, L.; Gonzalez, P.; Mottier, A.; Mouchet, F.; Cadarsi, S.; Lonchambon, P.; Daffe, G.; Chimowa, G.; Sarrieu, C.; et al. Thermal Reduction of Graphene Oxide Mitigates Its In Vivo Genotoxicity Toward Xenopus laevis Tadpoles. Nanomaterials 2019, 9, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shobha, G.; Shashidhara, K.S.; Naik, C. Cuprous Oxide Nanoparticles Induced Antioxidant Response and Genotoxicity in Lycopersicum esculentum. BioNanoScience 2020, 10, 1128–1137. [Google Scholar] [CrossRef]
- Wise, K.; Gill, H.; Selby-Pham, J. Willow bark extract and the biostimulant complex Root Nectar® increase propagation efficiency in chrysanthemum and lavender cuttings. Sci. Hortic. 2020, 263, 109108. [Google Scholar] [CrossRef]
- Eyheraguibel, B.; Silvestre, J.; Morard, P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour. Technol. 2008, 99, 4206–4212. [Google Scholar] [CrossRef] [Green Version]
- AFNOR EN ISO 29200. Soil Quality-Assessment of Genotoxic Effects on Higher Plants-Vicia Faba-Micronucleus Test; Association Française de Normalisation: La Plaine Saint-Denis, France, 2020. [Google Scholar]
- Iqbal, M. Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere 2016, 144, 785–802. [Google Scholar] [CrossRef]
- Cotelle, S.; Dhyèvre, A.; Muller, S.; Chenon, P.; Manier, N.; Pandard, P.; Echairi, A.; Silvestre, J.; Guiresse, M.; Pinelli, E.; et al. Soil genotoxicity assessment—Results of an interlaboratory study on the Vicia micronucleus assay in the context of ISO standardization. Environ. Sci. Pollut. Res. 2014, 22, 988–995. [Google Scholar] [CrossRef]
- De Simone, C.; Angelucci, R.; Errichetti, M.F.; Marconi, S.; Rossi, M.; Selvi, S. A statistical approach to evaluate compost genotoxicity. Biol. Fertil. Soils 2004, 41, 9–14. [Google Scholar] [CrossRef]
- Marcato-Romain, C.; Pinelli, E.; Pourrut, B.; Silvestre, J.; Guiresse, M. Assessment of the genotoxicity of Cu and Zn in raw and anaerobically digested slurry with the Vicia faba micronucleus test. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 672, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hajjouji, H.; Pinelli, E.; Guiresse, A.M.; Merlina, G.; Revel, J.-C.; Hafidi, M. Assessment of the genotoxicity of olive mill waste water (OMWW) with the Vicia faba micronucleus test. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 634, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Khadra, A.; Pinelli, E.; Lacroix, M.; Bousquet-Mélou, A.; Hamdi, H.; Merlina, G.; Guiresse, M.; Hafidi, M. Assessment of the genotoxicity of quinolone and fluoroquinolones contaminated soil with the Vicia faba micronucleus test. Ecotoxicol. Environ. Saf. 2012, 76, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Njagi, G.D.E.; Gopalan, H.N.B. Mutagenicity testing of herbicides, fungicides and insecticides. I. Chromosome aberrations in Vicia faba. Cytology 1981, 46, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Souguir, D.; Ferjani, E.; Ledoigt, G.; Goupil, P. Exposure of Vicia faba and Pisum sativum to copper-induced genotoxicity. Protoplasma 2008, 233, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Morard, P.; Eyheraguibel, B.; Morard, M.; Silvestre, J. Direct effects of humic-like substance on growth, water, and mineral nutrition of various species. J. Plant Nutr. 2010, 34, 46–59. [Google Scholar] [CrossRef]
- Akinremi, O.O.; Janzen, H.H.; Lemke, R.L.; Larney, F.J. Response of canola, wheat and green beans to leonardite additions. Can. J. Soil Sci. 2000, 80, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.-H.; Xu, Z.; Xu, C.; McConnell, H.; Rabago, E.V.; Arreola, G.A.; Zhang, H. The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat. Res. Mutagen. Relat. Subj. 1995, 334, 185–195. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Update on Plant Lipoxygenases Plant Lipoxygenases. Physiological and Molecular Features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A Vegetal Biopolymer-Based Biostimulant Promoted Root Growth in Melon While Triggering Brassinosteroids and Stress-Related Compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Foltête, A.-S.; Masfaraud, J.-F.; Férard, J.-F.; Cotelle, S. Is there a relationship between early genotoxicity and life-history traits in Vicia faba exposed to cadmium-spiked soils? Mutat. Res. Toxicol. Environ. Mutagen. 2012, 747, 159–163. [Google Scholar] [CrossRef]
- Raskin, I.; Ensley, B.D.; Burt, D. Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; J. Wiley: Hoboken, NJ, USA, 2000; ISBN 9780471192541. [Google Scholar]
- Pourrut, B.; Jean, S.; Silvestre, J.; Pinelli, E. Lead-induced DNA damage in Vicia faba root cells: Potential involvement of oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 726, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Portuondo-Farías, L.; Martinez-Balmori, D.; Guridi-Izquierdo, F.; Calderin-Garcia, A.; Machado-Torres, J.P. Structural and Functional Evaluation of Humic Acids in Interaction with Toxic Metals in a Cultivar of Agricultural Interest. Rev. Ciencias Técnicas Agropecu. 2017, 26, 39–46. [Google Scholar]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z.; Romic, D. Humic acids decrease uptake and distribution of trace metals, but not the growth of radish exposed to cadmium toxicity. Ecotoxicol. Environ. Saf. 2018, 151, 55–61. [Google Scholar] [CrossRef] [PubMed]
- El-Ghamery, A.; Mousa, M. Salicylic acid triggers adaptation cadmium cytogenetic toxicity in roots of Nigella sativa L. Egypt J. Bot. 2018, 58, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Marcano, L.; Carruyo, I.; Del Campo, A.; Montiel, X. Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa L. Environ. Res. 2004, 94, 221–226. [Google Scholar] [CrossRef]
- Gichner, T.; Menke, M.; Stavreva, D.; Schubert, I. Maleic hydrazide induces genotoxic effects but no DNA damage detectable by the Comet assay in tobacco and field beans. Mutagenesis 2000, 15, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, S.; Masfaraud, J.-F.; Férard, J.-F. Assessment of the genotoxicity of contaminated soil with the Allium/Vicia-micronucleus and the Tradescantia-micronucleus assays. Mutat. Res. Mol. Mech. Mutagen. 1999, 426, 167–171. [Google Scholar] [CrossRef]
- Wu, L.; Yi, H.; Yi, M. Assessment of arsenic toxicity using Allium/Vicia root tip micronucleus assays. J. Hazard. Mater. 2010, 176, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Crooke, W.M.; Knight, A.H. Crop composition in relation to soil pH and root cation-exchange capacity. J. Sci. Food Agric. 1971, 22, 235–241. [Google Scholar] [CrossRef]


| Product Name | Biomimetic Humic Substances (BHS®) | Fulvic Acid (FA) | Product “A” | Product “B” |
|---|---|---|---|---|
| Origin | physico-chemical extraction of poplar wood waste | physico-chemical extraction of poplar wood waste | Leonardite extract | By-products of the of wood pulp production |
| Composition | 45% humic acids and 55% fulvic acid | 100% fulvic acid, extracted from BHS®. | Humic acid 12% w/w; fulvic acid 3% w/w | sulfonated lignin |
| Initial Carbon concentration (%) | 1.5% | 0.825% | 7.28% | 23% |
| Recommended commercial carbon dose | 0.5% | 0.25% and 0.5% | 0.12% | 0.033% |
| Onset of Experiment | Exposure | Recovery | |
|---|---|---|---|
| Phase Duration (56 h) | 24 h | 8 h | 24 h |
| control | nutrient solution | nutrient solution | nutrient solution |
| Pb | nutrient solution | Pb 1 mg.L−1 | nutrient solution |
| MH | nutrient solution | MH 1.12 mg.L−1 | nutrient solution |
| BHS®’s protection against Pb | BHS® 0.5% | Pb 1 mg.L−1 | BHS® 0.5% |
| BHS®’s protection against MH | BHS® 0.5% | MH 1.12 mg.L−1 | BHS® 0.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, P.; Chauvey, L.; Kallerhoff, J.; Pinelli, E.; Morard, M.; Silvestre, J. A Tool Derived from the Vicia faba Micronucleus Assay, to Assess Genotoxicity, Cytotoxicity or Biostimulation of Novel Compounds Used in Agriculture. Agronomy 2021, 11, 321. https://doi.org/10.3390/agronomy11020321
Klein P, Chauvey L, Kallerhoff J, Pinelli E, Morard M, Silvestre J. A Tool Derived from the Vicia faba Micronucleus Assay, to Assess Genotoxicity, Cytotoxicity or Biostimulation of Novel Compounds Used in Agriculture. Agronomy. 2021; 11(2):321. https://doi.org/10.3390/agronomy11020321
Chicago/Turabian StyleKlein, Perrine, Lorelei Chauvey, Jean Kallerhoff, Eric Pinelli, Marie Morard, and Jérome Silvestre. 2021. "A Tool Derived from the Vicia faba Micronucleus Assay, to Assess Genotoxicity, Cytotoxicity or Biostimulation of Novel Compounds Used in Agriculture" Agronomy 11, no. 2: 321. https://doi.org/10.3390/agronomy11020321






