Effects of Modified Atmosphere Packaging and Chitosan Treatment on Quality and Sensorial Parameters of Minimally Processed cv. ‘Italia’ Table Grapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation and Packaging
- -
- MPA: modified passive atmosphere (air), used as control.
- -
- MAP10 C: modified active atmosphere (10 kPa CO2 and 20 kPa O2) plus Chitosan.
- -
- MAP10: modified active atmosphere (10 kPa CO2 and 20 kPa O2).
2.3. Headspace Gas Composition
2.4. Quality Parameters: Weight Loss, Total Soluble Solids, Titratable Acidity, Decay, Browning, and Berry Abscission
2.5. Sensory Evaluation and Rachis Score
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results
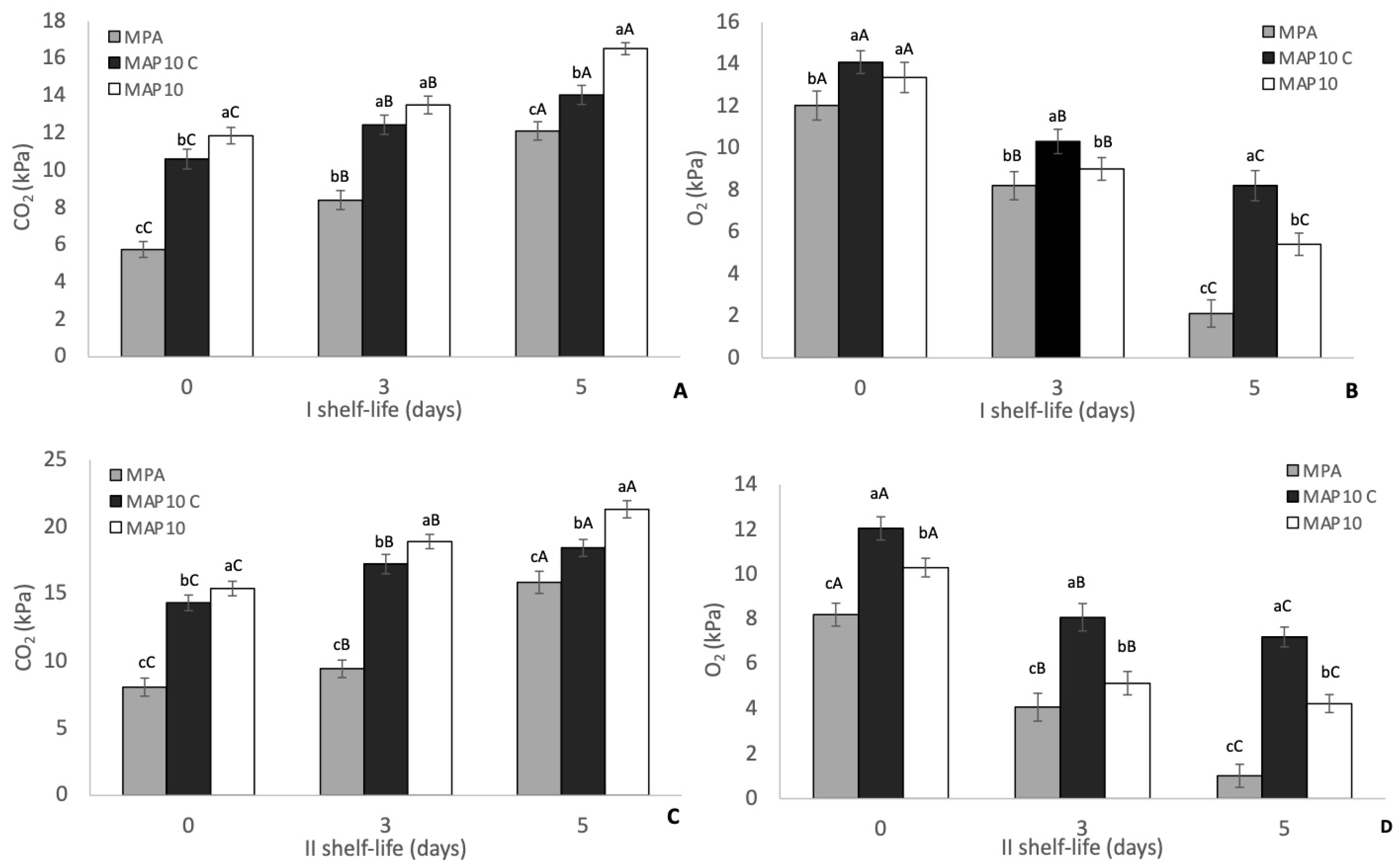
3.1. Headspace Gas Composition
3.2. Quality Parameters: Weight Loss, Total Soluble Solids, Titratable Acidity, Decay, Browning, and Berry Abscission
3.3. Sensory Evaluation and Rachis Score
3.4. Microbiological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rolle, R.S.; Chism, G.W. Physiological consequences of minimally processed fruits and vegetables. J. Food Qual. 1987, 10, 157–177. [Google Scholar] [CrossRef]
- Sabir, A.; Sabir, F.K.; Kara, Z. Effects of modified atmosphere packing and honey dip treatments on quality maintenance of minimally processed grape cv. Razaki (V. vinifera L.) during cold storage. J. Food Sci. Technol. 2011, 48, 312–318. [Google Scholar] [PubMed] [Green Version]
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Liguori, G.; D’Aquino, S.; Sortino, G.; De Pasquale, C.; Inglese, P. Effects of passive and active modified atmosphere packaging conditions on quality parameters of minimally processed table grapes during cold storage. J. Berry Res. 2015, 5, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Rosales, R.; Romero, I.; Fernandez-Caballero, C.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Low Temperature and Short-Term High-CO2 Treatment in Postharvest Storage of Table Grapes at Two Maturity Stages: Effects on Transcriptome Profiling. Front. Plant Sci. 2016, 7, 1020. [Google Scholar] [CrossRef] [Green Version]
- Romero, I.; Vazquez-Hernandez, M.; Maestro-Gaitan, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Table Grapes during Postharvest Storage: A Review of the Mechanisms Implicated in the Beneficial Effects of Treatments Applied for Quality Retention. Int. J. Mol. Sci. 2020, 21, 9320. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Conte, A.; Mastromatteo, M.; Speranza, B.; Antonacci, A.; Del Nobile, M.A. Effects of passive and active modified atmosphere packaging conditions on ready-to-eat table grape. J. Food Eng. 2011, 102, 115–121. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Mitchell, F.G. Postharvest handling systems: Table grapes. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California: Berkeley, CA, USA, 2002; pp. 357–363. [Google Scholar]
- Crisosto, C.H.; Garner, D.; Crisosto, G. High carbon dioxide atmospheres affect stored ‘Thompson seedless’ table grapes. HortScience 2002, 37, 1074–1078. [Google Scholar] [CrossRef] [Green Version]
- Karabulut, O.A.; Gabler, F.M.; Mansour, M.; Smilanick, J.L. Postharvest ethanol and hot water treatments of table grapes to control gray mold. Postharvest Biol. Technol. 2004, 34, 169–177. [Google Scholar] [CrossRef]
- Schena, L.; Nigro, F.; Pentimone, I.; Ligorio, A.; Ippolito, A. Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biol. Technol. 2002, 30, 209–220. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Salerno, M. Effect of short hypobaric treatments on postharvest rots of sweet cherries, strawberries and table grapes. Postharvest Biol. Technol. 2001, 22, 1–6. [Google Scholar] [CrossRef]
- Martinez-Romero, D.; Guillen, F.; Castillo, S.; Valero, D.; Serrano, M. Modified atmosphere packaging maintains quality of table grapes. J. Food Sci. 2003, 68, 1838–1843. [Google Scholar] [CrossRef]
- Kader, A.A.; Zagory, D.; Kerbel, E.L. Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1983, 28, 1–30. [Google Scholar] [CrossRef]
- Sanz, C.; Pérez, A.G.; Olías, R.; Olías, J.M. Quality of strawberries packed with perforated polypropylene. J. Food Sci. 1999, 64, 748–752. [Google Scholar] [CrossRef]
- Chamara, D.; Illeperuma, K.; Galappatty, P.T.; Saranda, K.H. Modified atmosphere packaging of ‘Kolikuttu’ bananas at low temperature. J. Hortic. Sci. Biotechnol. 2000, 75, 92–96. [Google Scholar] [CrossRef]
- Bai, J.H.; Saftner, R.A.; Watada, A.E.; Lee, Y.S. Modified atmosphere maintains quality of fresh-cut cantaloupe (Cucumis melo L.). J. Food Sci. 2001, 66, 1207–1211. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Roselló, C. Modification of cell wall composition of apricot (Prunus armeniaca) during drying and storage under modified atmospheres. J. Agric. Food Chem. 1998, 46, 5228–5253. [Google Scholar] [CrossRef]
- Beaudry, R.M. Effect of O2 and CO2 partial pressure on selected phenomena affecting fruit and vegetable quality. Postharvest Biol. Technol. 1999, 15, 293–303. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B. High CO2-modified atmosphere to preserve sensory and nutritional quality of organic table grape (cv. ‘Italia’) during storage and shelf-life. Eur. J. Hortic. Sci. 2016, 81, 197–203. [Google Scholar] [CrossRef]
- Lichter, A.; Zutkhy, Y.; Sonego, L.; Dvir, O.; Kaplunov, T.; Sarig, P.; Ben-Arie, R. Ethanol controls postharvest decay of table grapes. Postharvest Biol. Technol. 2001, 24, 301–308. [Google Scholar] [CrossRef]
- Xu, W.T.; Huang, K.L.; Guo, F.; Qu, W.; Yang, J.J.; Liang, Z.H.; Lou, Y.B. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol. Technol. 2007, 46, 86–94. [Google Scholar] [CrossRef]
- Shiri, A.; Bakhshi, D.; Ghasemnezhad, M.; Dadi, M.; Papachatzis, A.; Kalorizou, H. Chitosan coating improved the shelf life and postharvest quality of table grape (Vitis vinifera) cultivar ‘Shahrudi’. Turk. J. Agric. For. 2013, 37, 148–156. [Google Scholar]
- Al-Qurashi, A.D.; Awad, M.A. Postharvest chitosan treatment affects quality, antioxidant capacity, antioxidant compounds and enzymes activities of ‘El-Bayadi’ table grapes after storage. Sci. Hortic. 2015, 197, 392–398. [Google Scholar] [CrossRef]
- Reglinski, T.; Elmer, P.A.G.; Taylor, J.T.; Parry, F.J.; Marsden, R.; Wood, P.N. Suppression of Botrytis bunch rot in Chardonnay grapevines by induction of host resistance and fungal antagonism. Australas. Plant Path. 2005, 34, 481–488. [Google Scholar] [CrossRef]
- Ben-Shalom, N.; Ardi, R.; Pinto, R.; Aki, C.; Fallik, E. Controlling gray mould caused by Botrytis cinerea in cucumber plants by means of chitosan. Crop Prot. 2003, 22, 285–290. [Google Scholar] [CrossRef]
- Chien, P.J.; Sheu, F.; Lin, H.R. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007, 100, 1160–1164. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Di Venere, D.; Salerno, M. Effect of pre and postharvest chitosan treatments to control storage grey mould of table grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Effect of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn) fruit. Postharvest Biol. Technol. 1997, 12, 195–202. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Antifungal effects of chitosan coating on fresh strawberries and raspberries during storage. J. Hortic. Sci. Biotechnol. 1998, 73, 763–767. [Google Scholar] [CrossRef]
- Du, J.; Gemma, H.; Iwahori, S. Effects of chitosan coating on the storage of peach, Japanese pear and kiwifruit. J. Jpn. Soc. Hortic. Sci. 1997, 66, 15–22. [Google Scholar] [CrossRef]
- Bautista-Banos, S.; Hernandez-Lauzardo, A.N.; Velazquez-del Valle, M.G.; Hernandez-Lopez, M.; Ait Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Jinasena, D.; Pavithra Pathirathna, P.; Wickramarachchi, S.; Marasinghe, E. Use of chitosan to control anthracnose on Embul banana. In Proceedings of the International Conference on Asia Agriculture and Animal, Bangkok, Thailand, 5–7 June 2011; pp. 56–60. [Google Scholar]
- Valverde, J.M.; Valero, D.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. Novel edible coating based on aloe vera gel to maintain table grape quality and safety. J. Agric. Food Chem. 2005, 53, 7807–7813. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, F.; Bellincontro, A.; Di Renzo, G. GRAPE: Post-harvest Operations. In Agricultural and Food Engineering Technologies Service; Mejıa, D., Ed.; Food and Agriculture Organization of the United Nations: Roma, Italy, 2005. [Google Scholar]
- Romanazzi, G.; Lichter, A.; Mlikota Gabler, F.; Smilanick, J. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012, 54, 118–121. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Scrocco, C.; Brescia, I.; Speranza, B.; Sinigaglia, M.; Perniola, R.; Antonacci, D. A study on quality loss of minimally processed grapes as affected by film packaging. Postharvest Biol. Technol. 2009, 51, 21–26. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest biology and technology: An overview. In Postharvest Technology of Horticultural Crops 1; Kader, A.A., Ed.; Publication 331; University of California and Agricultural and Natural Resources: Berkeley, CA, USA, 2002; pp. 39–47. [Google Scholar]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F. Microbiological spoilage of fruits and vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages, Food Microbiology and Food Safety; Sperber, W.H., Doyle, M.P., Eds.; Springer Science Business Media: Berlin/Heidelberg, Germany, 2009; pp. 135–183. [Google Scholar]





| Treatments | Weight loss | TSS | TA | Decay | Browning | Berry Abscission |
|---|---|---|---|---|---|---|
| (%) | (°Brix) | (% TA) | (%) | (%) | (%) | |
| Harvest | ||||||
| / | 18.21 ± 0.34 | 0.40 ± 0.05 | / | / | ||
| 7 days at 5 °C | ||||||
| MPA | 4.40 ± 0.034 a | 17.52 ± 0.24 ns | 0.38 ± 0.001 ns | 2.0 ± 0.03 a | 2.6 ± 0.01 a | 2.6 ± 0.12 a |
| MAP10 | 1.82 ± 0.029 b | 18.11 ± 0.38 ns | 0.39 ± 0.004 ns | 1.0 ± 0.02 b | 1.1 ± 0.02 b | 0.9 ± 0.07 b |
| MAP10 C | 0.76 ± 0.052 c | 18.17 ± 0.27 ns | 0.39 ± 0.002 ns | 0.8 ± 0.05 b | 1.0 ± 0.01 b | 0.9 ± 0.09 b |
| 14 days at 5 °C | ||||||
| MPA | 6.32 ± 0.148 a | 16.17 ± 0.31 c | 0.37 ± 0.003 ns | 3.5 ± 0.07 a | 3.0 ± 0.03 a | 5.8 ± 0.13 a |
| MAP10 | 2.62 ± 0.136 b | 17.18 ± 0.22 b | 0.38 ± 0.002 ns | 1.2 ± 0.07 b | 1.3 ± 0.02 b | 1.5 ± 0.09 b |
| MAP10 C | 1.85 ± 0.114 c | 17.90 ± 0.29 a | 0.38 ± 0.001 ns | 0.9 ± 0.05 b | 1.1 ± 0.01 b | 1.1 ± 0.08 b |
| I SHELF-LIFE 7 days at 5 °C + 5 days at 20 °C | ||||||
| MPA | 8.32 ± 0.143 a | 16.46 ± 0.23 b | 0.35 ± 0.003 ns | 4.4 ± 0.03 a | 4.6 ± 0.07 a | 11.9 ± 0.41 a |
| MAP10 | 3.61 ± 0.019 b | 17.80 ± 0.19 a | 0.37 ± 0.002 ns | 1.2 ± 0.02 b | 2.6 ± 0.01 b | 2.1 ± 0.11 b |
| MAP10 C | 2.25 ± 0.103 c | 17.94 ± 0.11 a | 0.37 ± 0.002 ns | 1.0 ± 0.01 c | 1.8 ± 0.01 c | 1.8 ± 0.09 b |
| II SHELF-LIFE 14 days at 5 °C + 5 days at 20 °C | ||||||
| MPA | 11.38 ± 0.068 a | 14.66 ± 0.21 c | 0.33 ± 0.001 ns | 5.2 ± 0.06 a | 5.4 ± 0.08 a | 17.1 ± 0.23 a |
| MAP | 5.31 ± 0.108 b | 16.79 ± 0.12 b | 0.36 ± 0.003 ns | 1.8 ± 0.01 b | 2.6 ± 0.02 b | 2.5 ± 0.11 b |
| MAP10 C | 4.07 ± 0.068 c | 17.56 ± 0.14 a | 0.36 ± 0.002 ns | 1.2 ± 0.01 c | 1.9 ± 0.02 c | 2.1 ± 0.14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, G.; Sortino, G.; Gullo, G.; Inglese, P. Effects of Modified Atmosphere Packaging and Chitosan Treatment on Quality and Sensorial Parameters of Minimally Processed cv. ‘Italia’ Table Grapes. Agronomy 2021, 11, 328. https://doi.org/10.3390/agronomy11020328
Liguori G, Sortino G, Gullo G, Inglese P. Effects of Modified Atmosphere Packaging and Chitosan Treatment on Quality and Sensorial Parameters of Minimally Processed cv. ‘Italia’ Table Grapes. Agronomy. 2021; 11(2):328. https://doi.org/10.3390/agronomy11020328
Chicago/Turabian StyleLiguori, Giorgia, Giuseppe Sortino, Gregorio Gullo, and Paolo Inglese. 2021. "Effects of Modified Atmosphere Packaging and Chitosan Treatment on Quality and Sensorial Parameters of Minimally Processed cv. ‘Italia’ Table Grapes" Agronomy 11, no. 2: 328. https://doi.org/10.3390/agronomy11020328
APA StyleLiguori, G., Sortino, G., Gullo, G., & Inglese, P. (2021). Effects of Modified Atmosphere Packaging and Chitosan Treatment on Quality and Sensorial Parameters of Minimally Processed cv. ‘Italia’ Table Grapes. Agronomy, 11(2), 328. https://doi.org/10.3390/agronomy11020328








