Germination of Salicornia bigelovii (Torr.) under Shrimp Culture Effluents and the Application of Vermicompost Leachate for Mitigating Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
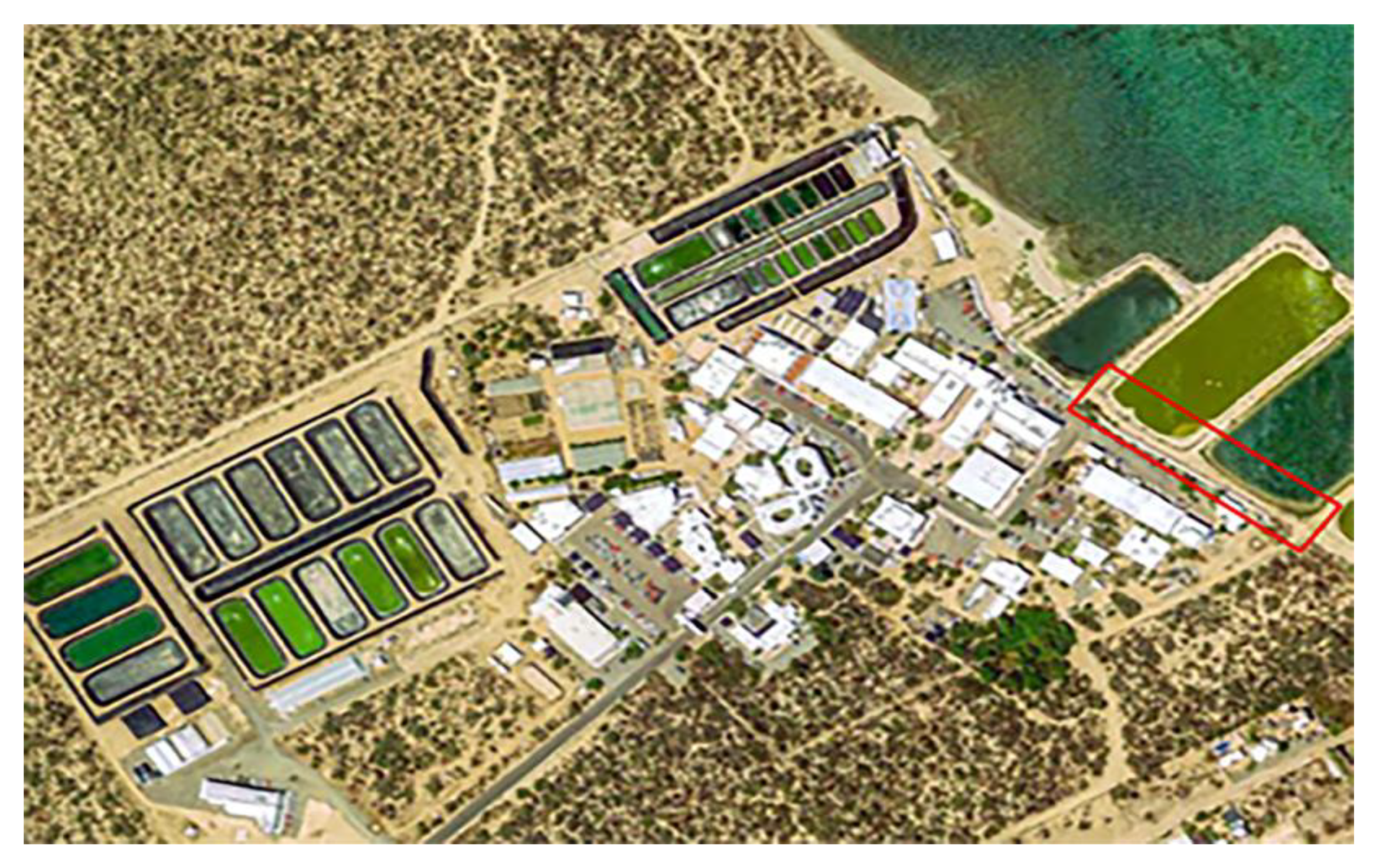
2.1. Study Site and Plant Material
2.2. Experimental Design
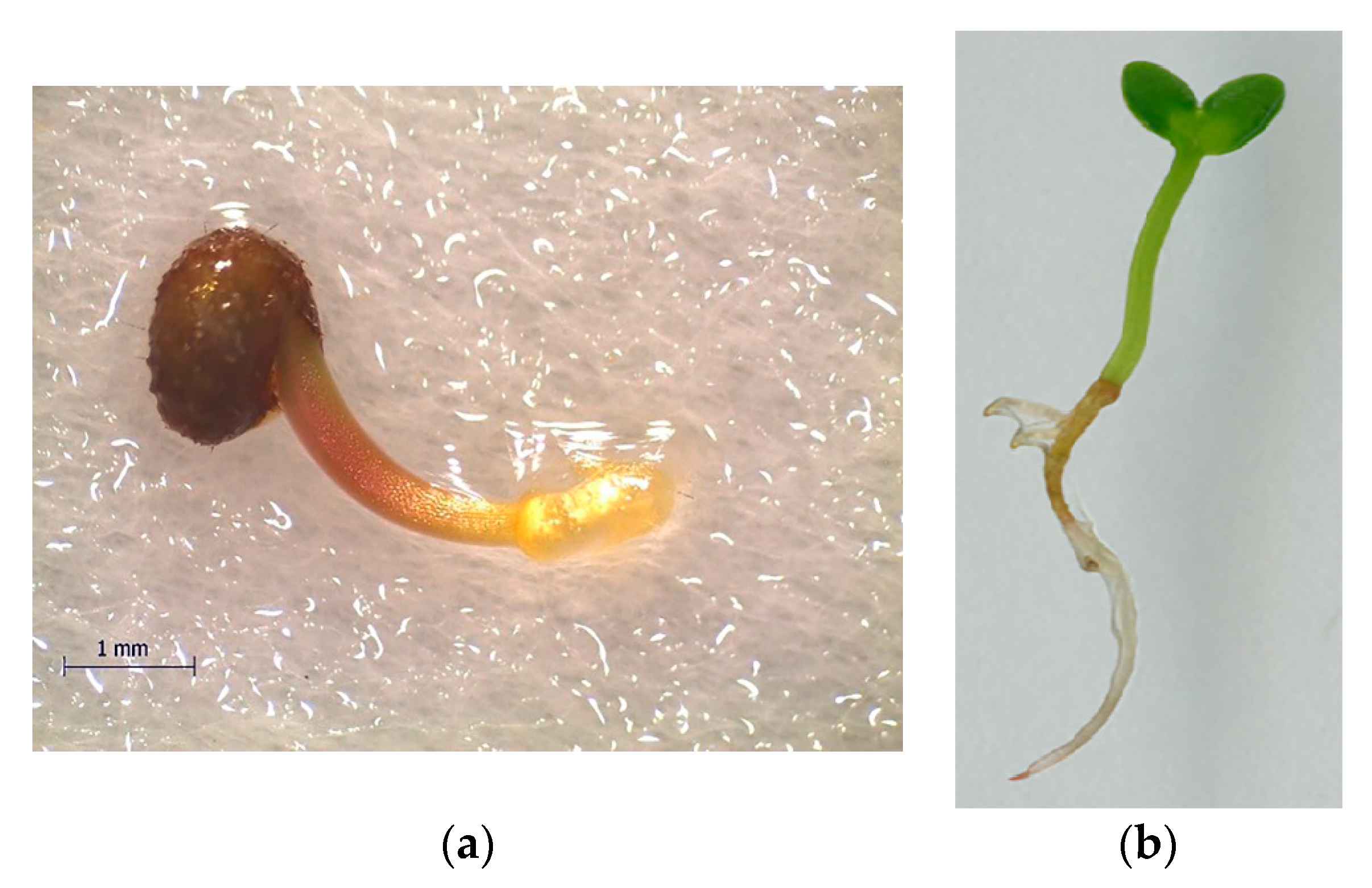
2.3. Germination Indices
2.4. Seedling Water Status Relations
2.5. Seedling Biomass
2.6. Statistical Analyses
3. Results
3.1. Effect of Vermicompost Leachate on the Physico-Chemical Properties of Treatments
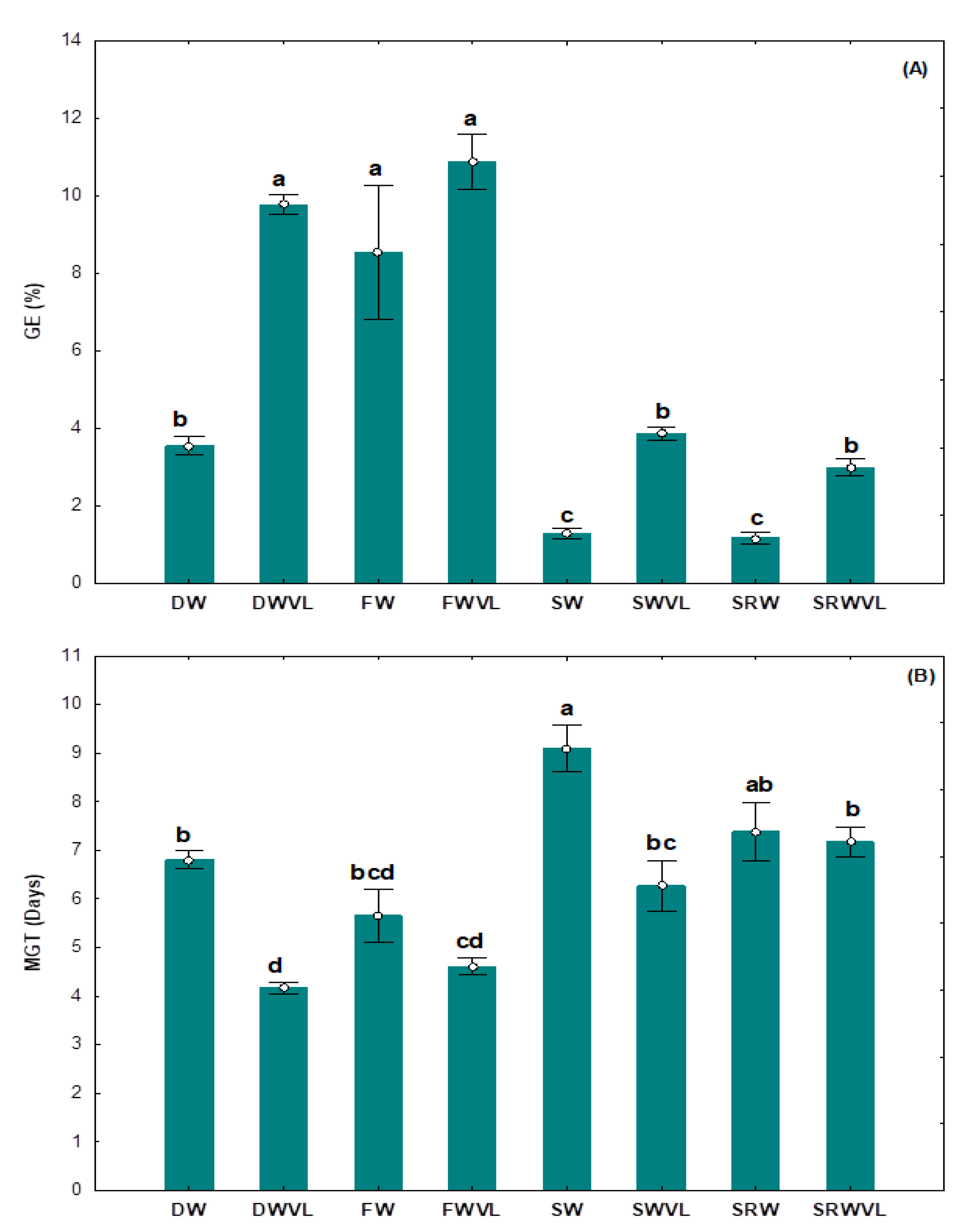
3.2. Germination Percentage, Germination Rate, Mean Germination Time, and Germination Energy
3.3. Seedling Biomass
4. Discussion
4.1. Germination Indices
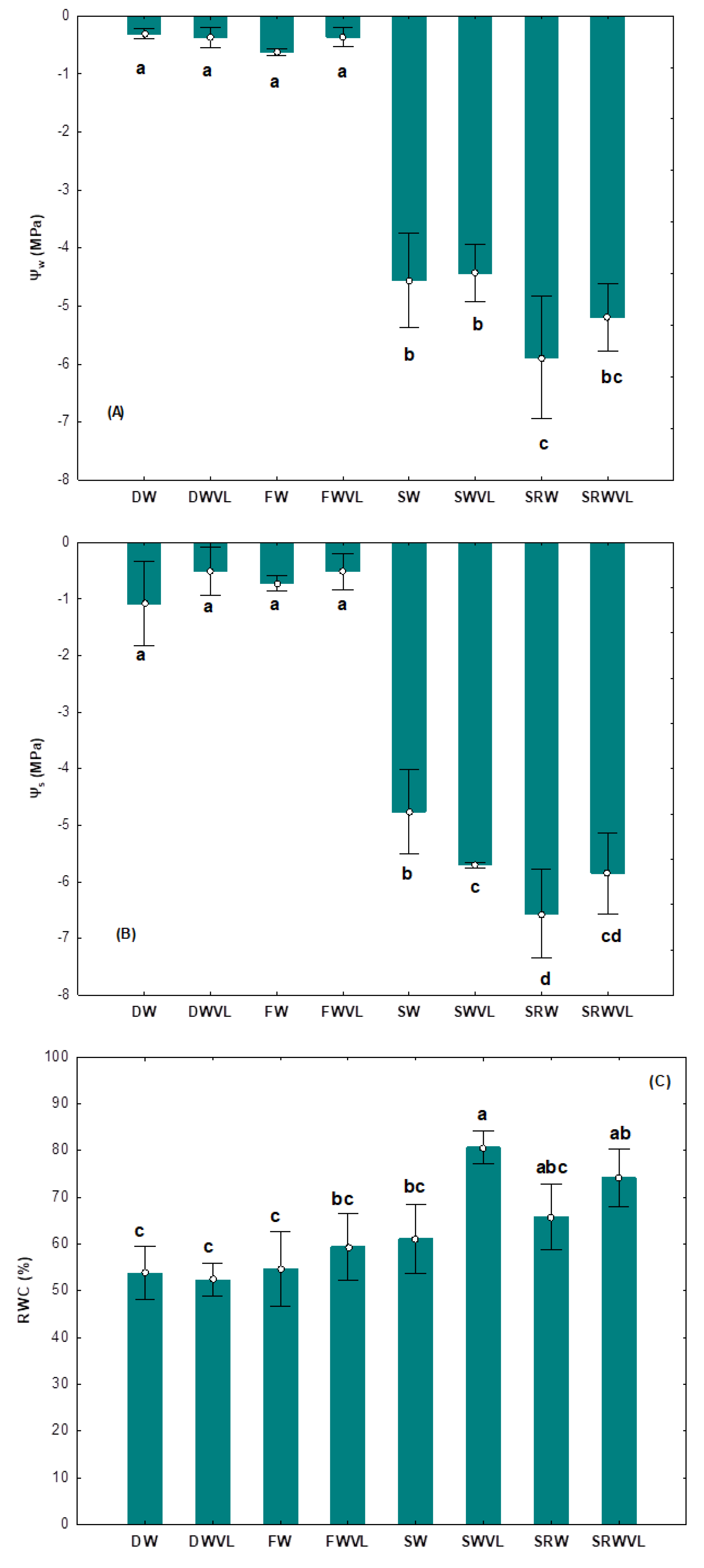
4.2. Seedling Water Status
4.3. Plant Biomass
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayat, K.; Bundschuh, J.; Jan, F.; Menhas, S.; Hayat, S.; Haq, F.; Shah, M.A.; Chaudhary, H.J.; Ullah, A.; Zhang, D.; et al. Combating soil salinity with combining saline agriculture and phytomanagement with salt-accumulating plants. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1085–1115. [Google Scholar] [CrossRef]
- Herrera-Romero, J.A.; Bojórquez-Serrano, J.I.; Can-Chulim, A.; Madueño-Molina, A.; García-Paredes, J.D. Salinity and soil properties of beach ridge in national marshlands in Mexico. Rev. Bio Cienc. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Sphigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hort. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Glenn, E.P.; Coates, W.E.; Riley, J.J.; Kuehl, R.O.; Swingle, R.S. Salicornia bigelovii Torr.: A seawater-irrigated forage for goats. Anim. Feed Sci. Technol. 1992, 40, 21–30. [Google Scholar] [CrossRef]
- Attia, F.M.; Alsobayel, A.A.; Kriadees, M.S.; Al-Saiady, M.Y.; Bayoumi, M.S. Nutrient composition and feeding value of Salicornia bigelovii Torr. meal in broiler diets. Anim. Feed Sci. Technol. 1997, 65, 257–263. [Google Scholar] [CrossRef]
- Eganathan, P.S.R.; Subramanian, H.M.; Latha, R.; Srinivasa Rao, C. Oil analysis in seeds of Salicornia brachiata. Ind. Crops Prod. 2006, 23, 177–179. [Google Scholar] [CrossRef]
- Kadereit, G.; Ball, P.; Beer, S.; Mucina, L.; Sokoloff, D.; Teege, P.; Yaprak, A.E.; Freitag, H. A taxonomic nightmare comes true: Phylogeny and biogeography of glassworts (Salicornia L., Chenopodiaceae). Taxon 2007, 56, 1143–1170. [Google Scholar] [CrossRef] [Green Version]
- Troyo-Diéguez, E.; Solis-Cámara, A.M.B. Germinación de Salicornia bigelovii Torr. (Chenopodiaceae) bajo diferentes concentraciones de agua marina. Southwest. Nat. 1992, 37, 22–27. [Google Scholar] [CrossRef]
- Glenn, E.P.; Miyamoto, S.; Moore, D.; Brown, J.J.; Lewis-Thompson, T.; Brown, P. Water requirements for cultivating Salicornia bigelovii Torr. with seawater on sand in a coastal desert environment. J. Arid Environ. 1997, 36, 711–730. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W.; Yang, Y.; Yang, C.; Yuan, Z.; Xiong, S.; Diana, J. Environmental impact of aquaculture and countermeasures to aquaculture pollution in China. Environ. Sci. Pollut. Res. 2007, 14, 452–462. [Google Scholar] [CrossRef]
- Buhmann, A.; Papenbrock, J. Biofiltering of aquaculture effluents by halophytic plants: Basic principles, current uses and future perspectives. Environ. Exp. Bot. 2013, 92, 122–133. [Google Scholar] [CrossRef]
- Webb, J.M.; Quinta, R.; Papadimitriou, S.; Norman, L.; Rigby, M.; Thomas, D.N.; Le Vay, L. Halophyte filter beds for treatment of saline wastewater from aquaculture. Water Res. 2012, 46, 5102–5114. [Google Scholar] [CrossRef]
- Rueda-Puente, E.O.; Murillo-Amador, B.; Ortega-García, J.; Rangel-Preciado, P.; Nieto-Garibay, A.; Holguín-Peña, R.J.; López-Ahumada, G.A.; Rodríguez-Félix, F.; Vargas-López, J.M.; Wong-Corral, F.J. Desarrollo natural de la halófita Salicornia bigelovii (Torr.) en zona costera del estado de Sonora. Trop. Subtrop. Agroecosyst. 2017, 20, 1–9. Available online: http://www.revista.ccba.uady.mx/ (accessed on 2 December 2020).
- Bidabadi, S.S.; Dehghanipoodeh, S.; Wright, G.C. Vermicompost leachate reduces some negative effects of salt stress in pomegranate. Int. J. Recycl. Org. Waste Agric. 2017, 6, 255–263. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Seed germination in the Great Basin halophyte Salsola iberica. Can. J. Bot. 2002, 80, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Gul, B. Halophyte seed germination. In Ecophysiology of High Salinity Tolerant Plants, 1st ed.; Khan, M.A., Weber, D.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 11–30. [Google Scholar]
- Khan, M.A. An ecological overview of halophytes from Pakistan. In Tasks for Vegetation Science, 1st ed.; Lieth, H., Moschenko, M., Eds.; Kluwer Academic Press: Dordrecht, The Netherlands, 2003; Volume 38, pp. 167–188. [Google Scholar]
- Mogahieb, R.E.A.; Saneoka, H.; Fujita, K. Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritima. Plant Sci. 2004, 166, 1345–1349. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Salt stress effects on growth, pigments, proteins and lipid peroxidation in Salicornia persica and S. erupaea. Biol. Plant. 2009, 53, 243–248. [Google Scholar] [CrossRef]
- Gutiérrez-Miceli, F.A.; Oliva Llaven, M.A.; Mendoza Nazar, P.; Ruíz Sesma, B.; Álvarez-Solís, J.D.; Dendooven, L. Optimization of vermicompost and worm-bed leachate for the organic cultivation of radish. J. Plant Nutr. 2011, 34, 1642–1653. [Google Scholar] [CrossRef]
- Aremu, A.O.; Stirk, W.A.; Kulkarni, M.G.; Tarkowská, D.; Turecková, V.; Gruz, J.; Subrtová, M.; Pencik, A.; Novák, A.; Dolezal, K.; et al. Evidence of phytohormones and phenolic acids variability in garden-waste-derived vermicompost leachate, a well-known plant growth stimulant. Plant Growth Regul. 2015, 75, 483–492. [Google Scholar] [CrossRef]
- Benazzouk, S.; Dobrev, P.I.; Djazouli, Z.E.; Motyka, V.; Lutts, S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L.) at the seedling stage: A phytohormonal approach. Plant Soil. 2020, 446, 145–162. [Google Scholar] [CrossRef]
- Chinsamy, M.; Kulkarni, M.G.; Van Staden, J. Garden-waste-vermicompost leachate alleviates salinity stress in tomato seedlings by mobilizing salt tolerance mechanisms. Plant Growth Regul. 2013, 71, 41–47. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; ISTA: Zurich, Switzerland, 1999. [Google Scholar]
- Bendscheider, K.; Robinson, R.J. A New Spectrophotometric Method for the Determination of Nitrite in Sea Water; Technical Report No. 8; Oceanographic Laboratories, University of Washington: Washington, DC, USA, 1952; Volume 183, pp. 1–18. Available online: https://digital.lib.washington.edu/researchworks/bitstream/handle/1773/15938/52-1.pdf?sequence=1&isAllowed=y (accessed on 26 November 2020).
- Morris, A.W.; Riley, J.P. The determination of nitrate in sea water. Anal. Chim. Acta 1963, 29, 272–279. [Google Scholar] [CrossRef]
- Chesnin, L.; Yen, C.H. Turbidimetric determination of available sulphates. Soil Sci. Soc. Am. J. 1950, 15, 149–151. [Google Scholar] [CrossRef]
- Culkin, F.; Cox, R.A. Sodium, potassium, magnesium, calcium and strontium in sea water. Deep Sea Res. Oceanogr. Abstr. 1966, 13, 789–804. [Google Scholar] [CrossRef]
- Valderrama, J.C. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 1981, 10, 109–122. [Google Scholar] [CrossRef]
- Acosta-Durán, C.M.; Solís-Pérez, O.; Villegas-Torres, O.G.; Cardoso-Vigueros, L. Precomposteo de residuos orgánicos y su efecto en la dinámica poblacional de Eisenia foetida. Agron. Costarric. 2013, 37, 127–139. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall, Inc: Englewood Cliffs, NJ, USA, 1958; pp. 66–81. [Google Scholar]
- NOM-021-SEMARNAT. Que Establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreo y Análisis. Ministry of the Environment and Natural Resources (Secretaria de Medio Ambiente y Recursos Naturales), 2nd ed.; Federation Official Gazette (Diario Oficial de la Federación): Mexico City, Mexico, 2000; pp. 12–13. Available online: http://www.ordenjuridico.gob.mx/Documentos/Federal/wo69255.pdf (accessed on 26 November 2020).
- Bremner, J.M. Total Nitrogen. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1091–1100. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada Bulletin: Ottawa, ON, Canada, 1972; p. 310.
- Jackson, M.L. Análisis Químico de Suelos; Ediciones Omega: Barcelona, Spain, 1976; pp. 283–301. [Google Scholar]
- Al-Mudaris, M.A. Notes on various parameters recording the speed of seed germination. J. Agric. Trop. Subtrop. 1998, 99, 147–154. Available online: https://www.jarts.info/index.php/tropenlandwirt/article/view/1495/671 (accessed on 14 December 2020).
- Orchard, T.J. Estimating the parameters of plant seedling emergence. Seed Sci. Technol. 1977, 5, 61–69. [Google Scholar]
- Wani, M.R.; Singh, S.S. Correlation dynamics of germination value, germination energy index and germination speed of Pongamia pinnata (L.) Pierre seeds of Pendra Provenance, Chhattisgarh, India. Int. J. Res. Agric. For. 2016, 3, 28–32. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Ford, R. Demonstrating osmotic potential: One factor in the Plant Water Potential equation. In Tested Studies for Laboratory Teaching, Proceedings of the 36th Conference of the Association for Biology Laboratory Education (ABLE); Article 29; McMahon, K., Ed.; 2015; Volume 36, p. 29. Available online: http://www.ableweb.org/volumes/vol-36/?art=29 (accessed on 17 December 2020).
- Yamasaki, S.; Dillenburg, L. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. Available online: https://www.researchgate.net/profile/Lucia_Dillenburg/publication/264876205_Measurements_of_leaf_relative_water_content_in_Araucaria_Angustifolia/links/551936f50cf21b5da3b7dc75.pdf (accessed on 4 December 2020).
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L. (Chenopodiaceae). Plant Divers. 2016, 38, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Guillén, S.; Terrazas, T.; De la Barrera, E.; Casas, A. Germination differentiation patterns of wild and domesticated columnar cacti in a gradient of artificial selection intensity. Genet. Resour. Crop Evol. 2011, 58, 409–423. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.N.; Wong, W.S.; Ng, C.Y.L.; Teo, C.H.; Ge, L.; Chen, X.; Yong, J.W.H. Mass spectrometric evidence for the occurrence of plant growth promoting cytokinins in vermicompost tea. Biol. Fert. Soils. 2014, 50, 401–403. [Google Scholar] [CrossRef]
- Canellas, L.P.; Lopes-Olivares, F.; Okorokova-Facanha, A.L.; Rocha-Facanha, A. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeller, H.; Perez, S.C.J.G.A. Effects of water and salt stress and the gibberellin action in Senna spectabilis seeds. Cienc. Florest. 2001, 11, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Arancon, N.; Pant, A.; Radovich, T.; Hue, N.V.; Potter, J.K.; Converse, C.E. Seed germination and seedling growth of tomato and lettuce as affected by vermicompost water extracts (Teas). Hort. Sci. 2012, 47, 1722–1728. [Google Scholar] [CrossRef] [Green Version]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 305–345. [Google Scholar] [CrossRef]
- Singh, S.; Kulkarni, M.G.; Van Staden, J. Biochemical changes associated with gibberellic acid-like activity of smoke-water, karrikinolide and vermicompost leachate during seedling development of Phaseolus vulgaris L. Seed Sci. Res. 2014, 24, 63–70. [Google Scholar] [CrossRef]
- Ayala, F.; O’Leary, J. Growth and physiology of Salicornia bigelovii Torr. at suboptimal salinity. Int. J. Plant. Sci. 1995, 156, 197–205. Available online: www.jstor.org/stable/2474958 (accessed on 4 December 2020). [CrossRef]
- Kudo, N.; Fujiyama, H. Responses of halophyte Salicornia bigelovii to different forms of nitrogen source. Pedosphere 2010, 20, 311–317. [Google Scholar] [CrossRef]
- Haque, M.I.; Rathore, M.S.; Gupta, H.; Jha, B. Inorganic solutes contribute more than organic solutes to the osmotic adjustment in Salicornia brachiata (Roxb.) under natural saline conditions. Aquat. Bot. 2017, 142, 78–86. [Google Scholar] [CrossRef]
- Ermakov, E.I.; Ktitorova, I.N.; Skobeleva, O.V. Effect of humic acid in the mechanical properties of cell walls. Rus. J. Plant Physiol. 2000, 47, 518–525. [Google Scholar]
- Benazzouk, S.; Djazouli, Z.E.; Lutts, S. Vermicompost leachate as a promising agent for priming and rejuvenation of salt-treated germinating seeds in Brassica napus. Commun. Soil Sci. Plan. 2019, 11, 1344–1357. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis and translocation. Ann. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungar, I.A. Halophyte seed germination. Bot. Rev. 1978, 44, 233–264. [Google Scholar] [CrossRef]
- Rueda-Puente, E.O.; García-Hernández, J.L.; Preciado-Rangel, P.; Murillo-Amador, B.; Tarazón-Herrera, M.A.; Flores-Hernández, A.; Holguin-Peña, J.; Aybar, A.N.; Barrón Hoyos, J.M.; Weimers, D.; et al. Germination of Salicornia bigelovii ecotypes under stressing conditions of temperature and salinity and ameliorative effects of plant growth-promoting bacteria. J. Agron. Crop Sci. 2007, 193, 167–176. [Google Scholar] [CrossRef]



| Treatments | E.C. | TDS | Salinity | pH | NO2− | NO3− | PO43− | SO42− | Ca | K+ | Na+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| dS/m | ppt | psu | µM | mg/L | |||||||
| DW | 0.0027 | 0.0018 | 0.061 | 5.74 | ND | ND | ND | ND | ND | ND | ND |
| DWVL | 0.87 | 0.348 | 0.164 | 6.57 | 0.952 | 849.05 | 12.4 | 42.75 | 52 | 6.2 | 36 |
| FW | 1.36 | 0.669 | 0.728 | 8 | <0.1 | 416 | 0.481 | 58.9 | 114 | 5.8 | 240 |
| FWVL | 1.84 | 0.894 | 0.967 | 8.33 | 1.150 | 1182.6 | 9.25 | 99.3 | 173 | 20 | 280 |
| SW | 55.83 | 27.36 | 36.77 | 7.39 | <0.1 | 1.32 | 1.16 | 2181.8 | 480 | 6.2 | 3348 |
| SWVL | 53.92 | 26.42 | 35.37 | 7.7 | 0.995 | 809.01 | 13.15 | 3250.9 | 400 | 20 | 3218 |
| SRW | 59.85 | 29.33 | 38.7 | 6.88 | 63 | 1305.75 | 57.6 | 3013.6 | 348 | 17.1 | 3894 |
| SRWVL | 58.98 | 28.9 | 38.06 | 6.91 | 98.5 | 2255.25 | 66.5 | 3600 | 192 | 20 | 3801 |
| VL | 11.93 | 5.85 | 6.65 | 8.75 | * | 1500 | * | * | 110 | * | 640 |
| Biomaterial | EC | TDS | pH | OM | Ntotal | NO2− | NO3− | P | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | g L−1 | % | mg kg−1 | |||||||
| VC | 4.83 | 2.53 | 8.49 | 12.5 | 0.792 | 12.7 | 495.7 | 65.4 | 130.3 | 19,370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Galindo, E.; Nieto-Garibay, A.; Troyo-Diéguez, E.; Lucero-Vega, G.; Murillo-Amador, B.; Ruiz-Espinoza, F.H.; Fraga-Palomino, H.C. Germination of Salicornia bigelovii (Torr.) under Shrimp Culture Effluents and the Application of Vermicompost Leachate for Mitigating Salt Stress. Agronomy 2021, 11, 424. https://doi.org/10.3390/agronomy11030424
García-Galindo E, Nieto-Garibay A, Troyo-Diéguez E, Lucero-Vega G, Murillo-Amador B, Ruiz-Espinoza FH, Fraga-Palomino HC. Germination of Salicornia bigelovii (Torr.) under Shrimp Culture Effluents and the Application of Vermicompost Leachate for Mitigating Salt Stress. Agronomy. 2021; 11(3):424. https://doi.org/10.3390/agronomy11030424
Chicago/Turabian StyleGarcía-Galindo, Emilio, Alejandra Nieto-Garibay, Enrique Troyo-Diéguez, Gregorio Lucero-Vega, Bernardo Murillo-Amador, Francisco Higinio Ruiz-Espinoza, and Héctor Cirilo Fraga-Palomino. 2021. "Germination of Salicornia bigelovii (Torr.) under Shrimp Culture Effluents and the Application of Vermicompost Leachate for Mitigating Salt Stress" Agronomy 11, no. 3: 424. https://doi.org/10.3390/agronomy11030424







