Differing Phosphorus Crop Availability of Aluminium and Calcium Precipitated Dairy Processing Sludge Potential Recycled Alternatives to Mineral Phosphorus Fertiliser
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterisation of Dairy Processing Sludge (DPS)
2.2. Experimental Grassland Plots
2.3. Crop and Soil Analysis
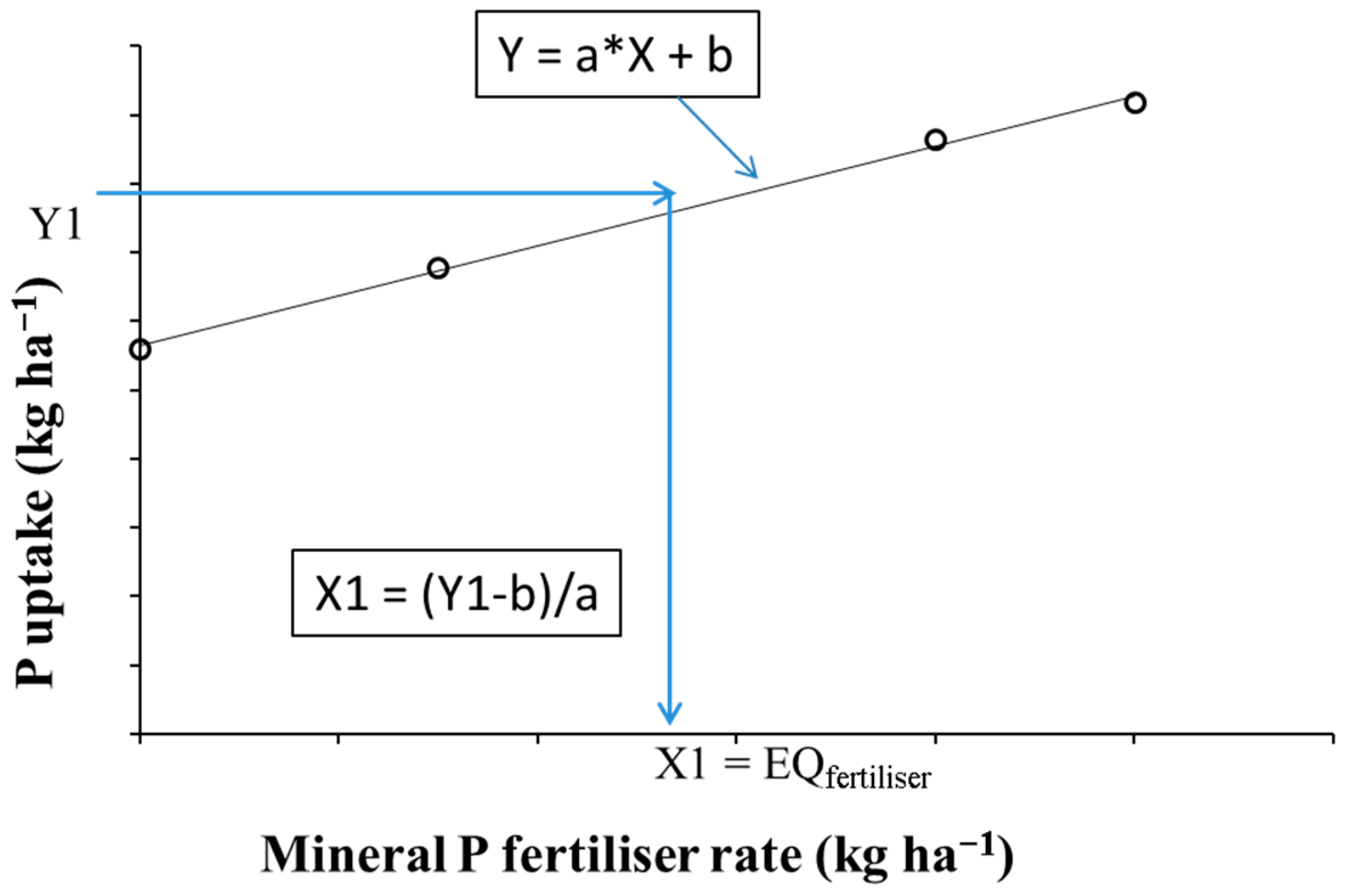
2.4. Calculation of P Bioavailability
2.5. Statistics
3. Results and Discussion
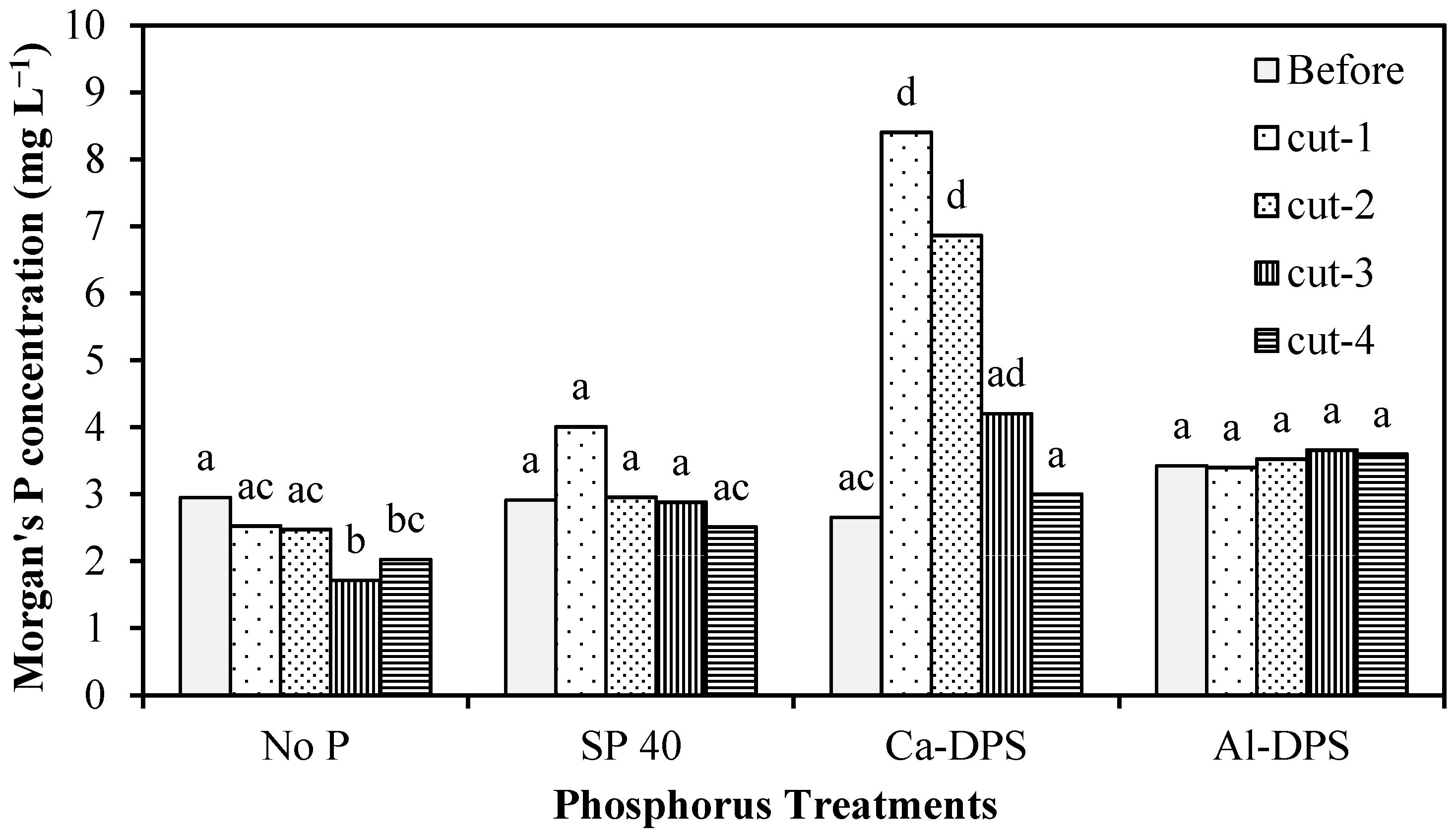
3.1. Soil P and pH Change
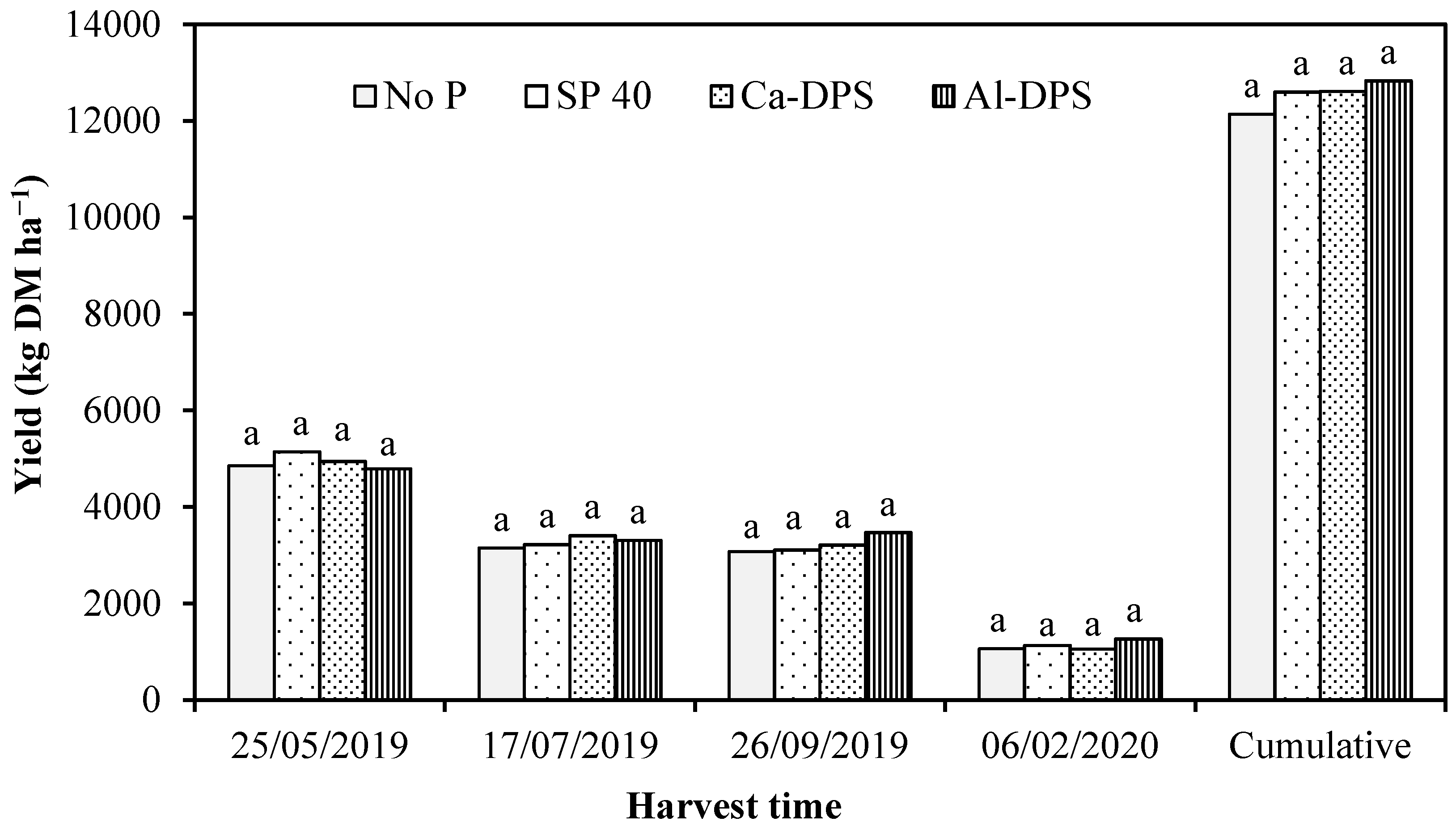
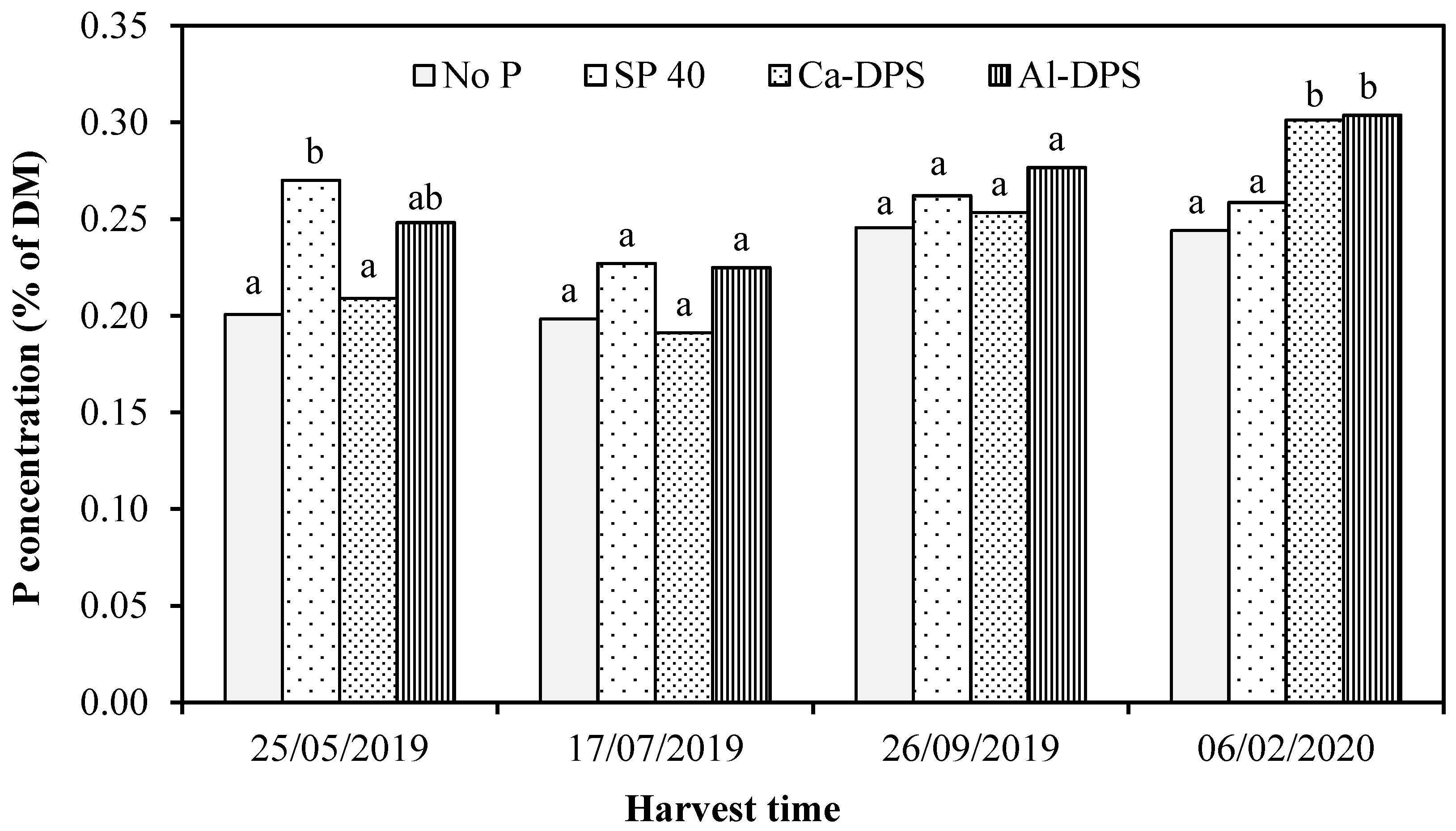
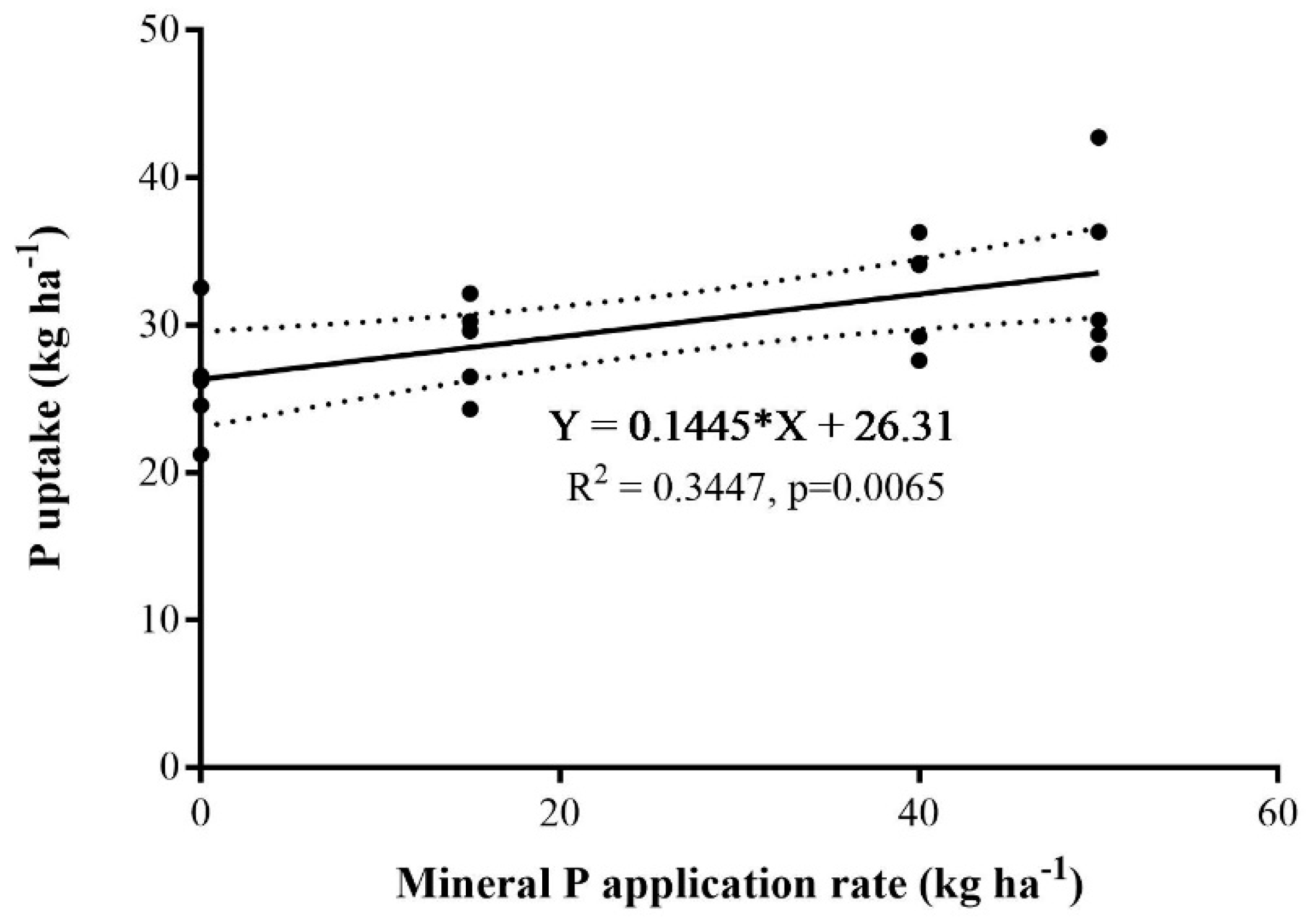
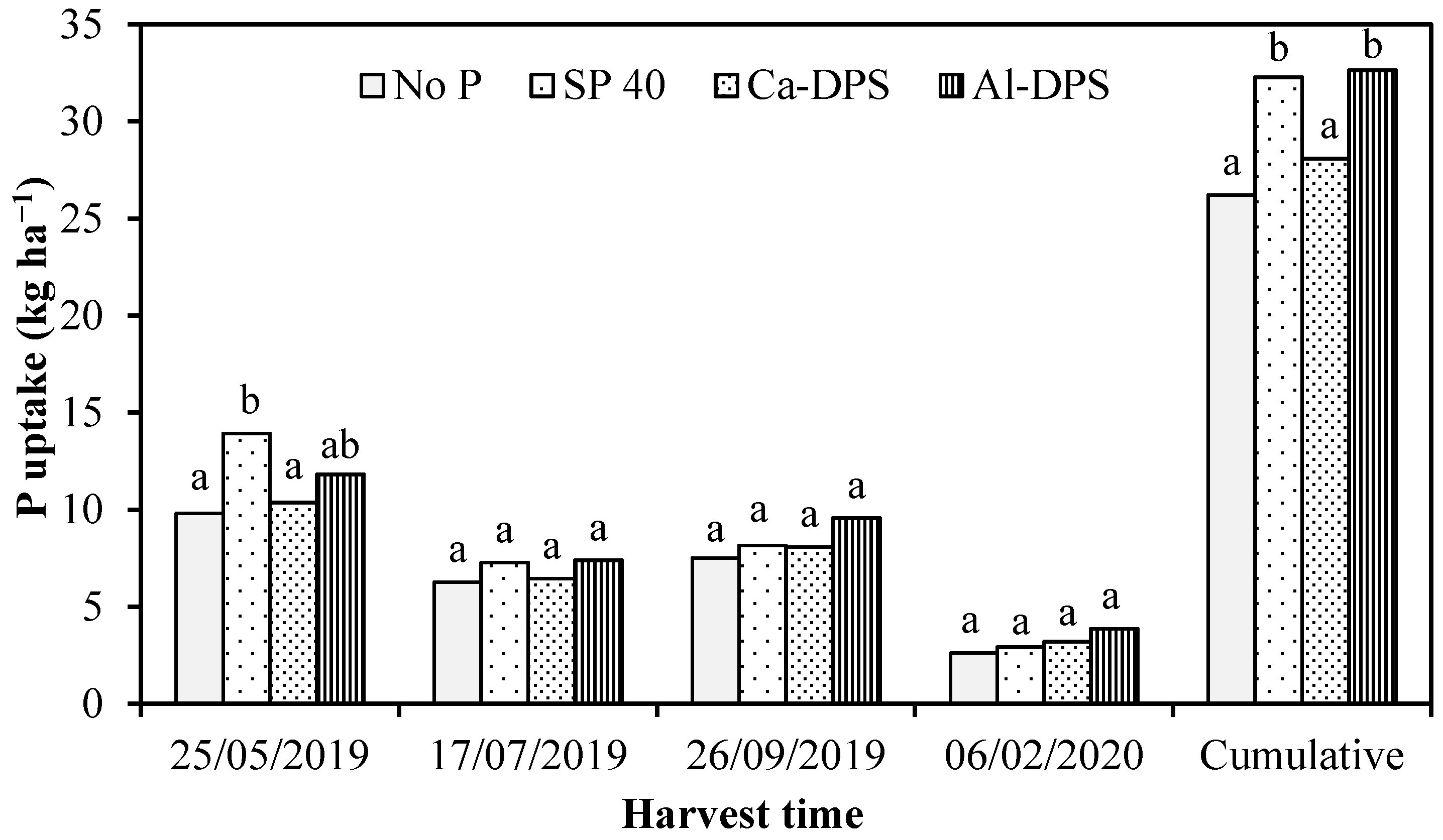
3.2. Grass Yield and P Uptake
3.3. P Bioavailability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nättorp, A.; Kabbe, C.; Matsubae, K.; Ohtake, H. Development of phosphorus recycling in Europe and Japan. In Phosphorus Recovery and Recycling; Springer: Singapore, 2019; pp. 3–27. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Kratz, S.; Vogel, C.; Adam, C. Agronomic performance of P recycling fertilizers and methods to predict it: A review. Nutr. Cycl. Agroecosyst. 2019, 115, 1–39. [Google Scholar] [CrossRef]
- EC (European Commission). Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System. 2020. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 1 November 2020).
- Slavov, A.K. General characteristics and treatment possibilities of dairy wastewater—A review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Daufin, G.; Escudier, J.P.; Carrère, H.; Bérot, S.; Fillaudeau, L.; Decloux, M. Recent and emerging applications of membrane processes in the food and dairy industry. Food Bioprod. Process. 2001, 79, 89–102. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Forrestal, P.; Richards, K.; Fenton, O. Dairy industry derived wastewater treatment sludge: Generation, type and characterization of nutrients and metals for agricultural reuse. J. Clean. Prod. 2019, 230, 1266–1275. [Google Scholar] [CrossRef]
- Erkan, H.S.; Gunalp, G.; Engin, G.O. Application of submerged membrane bioreactor technology for the treatment of high strength dairy wastewater. Braz. J. Chem. Eng. 2018, 35, 91–100. [Google Scholar] [CrossRef] [Green Version]
- EC (European Commission). Milk and Dairy Products. 2020. Available online: https://ec.europa.eu/info/food-farming-fisheries/animals-and-animal-products/animal-products/milk-and-dairy-products_en#:~:text=Total%20EU%20milk%20production%20is,yield%20per%20cow%20has%20improved (accessed on 27 November 2020).
- Lopez-Mosquera, M.E.; Cascallana, V.; Seoane, S. Comparison of the effects of dairy sludge and a mineral NPK fertilizer on an acid soil. Invest. Agr. Prod. Prot. Veg. 2002, 17, 87–99. [Google Scholar]
- López-Mosquera, M.E.; Moirón, C.; Carral, E. Use of dairy-industry sludge as fertiliser for grasslands in northwest Spain: Heavy metal levels in the soil and plants. Resour. Conserv. Recy. 2000, 30, 95–109. [Google Scholar] [CrossRef]
- Fehily Timoney and Company, Codes of Good Practice for the Use of Biosolids in Agriculture-Guidelines for Farmer. 1999. Available online: https://www.housing.gov.ie/sites/default/files/migrated-files/en/Publications/Environment/Water/FileDownLoad%2C17228%2Cen.pdf (accessed on 20 January 2021).
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.-C.; Show, P.L. Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef] [Green Version]
- Nolan, S.; Thorn, C.E.; Ashekuzzaman, S.M.; Kavanagh, I.; Nag, R.; Bolton, D.; Cummins, E.; O’Flaherty, V.; Abram, F.; Richards, K.; et al. Landspreading with co-digested cattle slurry, with or without pasteurisation, as a mitigation strategy against pathogen, nutrient and metal contamination associated with untreated slurry. Sci. Total Environ. 2020, 744, 140841. [Google Scholar] [CrossRef]
- Lucid, J.D.; Fenton, O.; Healy, M.G. Estimation of maximum biosolids and meat and bone meal application to a low P index soil and a method to test for nutrient and metal losses. Water Air Soil Pollut. 2013, 224, 1464–1475. [Google Scholar] [CrossRef] [Green Version]
- Teagasc. Major and micro nutrient advice for productive agricultural crops. In Johnstown Castle Wall DP; Plunkett, M., Ed.; Teagasc Environment Research Centre: Wexford, Ireland, 2016; p. 81. [Google Scholar]
- International Plant Nutrition Institute. Nutrient Source Specifics—Triple Superphosphate. 2020. Available online: http://www.ipni.net/publication/nss.nsf/0/35039C5F78D8740C852579AF0076567A/$FILE/NSS-14%20Triple%20Superphosphate.pdf (accessed on 12 October 2020).
- Schneider, K.D.; Martens, J.R.T.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef] [Green Version]
- Delin, S. Fertilizer value of phosphorus in different residues. Soil Use Manag. 2016, 32, 17–26. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Kwapinska, M.; Leahy, J.J.; Richards, K.; Fenton, O. Novel use of dairy processing sludge derived pyrogenic char (DPS-PC) to remove phosphorus in discharge effluents. Waste Biomass Valor. 2019, 11, 1453–1465. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Forrestal, P.; Richards, K.; Fenton, O. Potential loss of nutrients, carbon and metals in simulated runoff associated with dairy processing sludge application. Int. J. Environ. Sci. Technol. 2020, 17, 3955–3968. [Google Scholar] [CrossRef]
- Daly, K.; Styles, D.; Lalor, S.; Wall, D. Phosphorus sorption, supply potential and availability in soils with contrasting parent material and soil chemical properties. Eur. J. Soil Sci. 2015, 66, 792–801. [Google Scholar] [CrossRef]
- Wendling, L.A.; Blomberg, P.; Sarlin, T.; Priha, O.; Arnold, M. Phosphorus sorption and recovery using mineral-based materials: Sorption mechanisms and potential phytoavailability. Appl. Geochem. 2013, 37, 157–169. [Google Scholar] [CrossRef]
- Vanden Nest, T.; Amery, F.; Fryda, L.; Boogaerts, C.; Bilbao, J.; Vandecasteele, B. Renewable P sources: P use efficiency of digestate, processed animal manure, compost, biochar and struvite. Sci. Total Environ. 2021, 750. [Google Scholar] [CrossRef] [PubMed]
- Khiari, L.; Joseph, C.-A.; Gallichand, J.; Beecher, N.; Bouslama, S. Classification and Assessment Models of First Year Biosolids Phosphorus Bioavailability. Waste Biomass Valor. 2020, 11, 1443–1452. [Google Scholar] [CrossRef]
- Morgan, M.F. Chemical Soil Diagnosis by the Universal Soil Testing System. Connecticut Agricultural Experiment Station, Bulletin 450. 1941. Available online: https://portal.ct.gov/-/media/CAES/DOCUMENTS/Publications/Bulletins/B450pdf.pdf?la=en (accessed on 17 May 2020).
- Sheil, T.S.; Wall, D.P.; Culleton, N.; Murphy, J.; Grant, J.; Lalor, S.T.J. Long-term effects of phosphorus fertilizer on soil test phosphorus, phosphorus uptake and yield of perennial ryegrass. J Agric. Sci. 2016, 154, 1068–1081. [Google Scholar] [CrossRef]
- USEPA (undated) Memorandum. Subject: Fipronil ID#000264-00554: Updated Toxicology Hazard Assessment. Evaluation of an Acute Neurotoxicity Study with Fipronil and Reevaluation of the Developmental Neurotoxicity Study; USEPA: Washington, DC, USA, 1996.
- Daly, K.; Casey, A. Eutrophication from agricultural sources: Environmental soil phosphorus test. In EPA Research Report; Environmental Protection Agency: Wexford, Ireland, 2003; ISBN 1840951117. [Google Scholar]
- Delin, S. Fertilizer value of nitrogen in hen and broiler manure after application to spring barley using different application timing. Soil Use Manag. 2011, 27, 415–426. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Forrestal, P.; Richards, K.; Daly, K.; Fenton, O. Grassland Phosphorus and Nitrogen Fertiliser Replacement value of Dairy Processing Dewatered Sludge. Sustain. Prod. Consum. 2021, 25, 363–373. [Google Scholar] [CrossRef]
- Graca, J. An Evaluation of Phosphorus Supply Capacities in Irish Grassland Soils. Ph.D. Dissertation, University of Limerick, Limerick, Ireland, 2018. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954; p. 939.
- USDA (United States Department of Agriculture). Selection of an Appropriate Phosphorus Test for Soils. Soil Survey Laboratory, National Soil Survey Center: Lincoln. 2020. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051918.pdf (accessed on 17 February 2021).
- Arendas, T.; Csatho, P. Comparison of the effect of equivalent nutrients given in the form of farmyard manure or fertilizers in Hungarian long-term field trials. Commun. Soil Sci. Plan. 2002, 33, 2861–2878. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Tack, F.; Meers, E. Phosphorus Use Efficiency of Bio-based Fertilizers: Bioavailability and Fractionation. Pedosphere 2016, 26, 310–325. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Paikaray, N.K.; Ranjan, A.R. Availability and uptake of phosphorus from organic manures in groundnut (Arachis hypogea L.)-corn (Zea mays L.) sequence using radio tracer technique. Geoderma 2006, 133, 225–230. [Google Scholar] [CrossRef]
- Smith, G.S.; Cornforth, I.S.; Henderson, H.V. Critical leaf concentrations for deficiencies of nitrogen, potassium, phosphorus, sulfur, andmagnesium in perennial ryegrass. New Phytol. 1985, 101, 393–409. [Google Scholar] [CrossRef]
- Quintero, C.E.; Boschetti, N.G.; Benavidez, R.A. Effect of soil buffer capacity on soil test phosphorus interpretation and fertilizer requirement. Commun. Soil Sci. Plant Anal. 2003, 34, 1435–1450. [Google Scholar] [CrossRef]
- Schulte, R.; Herlihy, M. Quantifying responses to phosphorus in Irish grasslands: Interactions of soil and fertiliser with yield and P concentration. Eur. J. Agron. 2007, 26, 144–153. [Google Scholar] [CrossRef]
- Forrestal, P.J.; Harty, M.A.; Carolan, R.; Watson, C.J.; Lanigan, G.J.; Wall, D.P.; Hennessy, D.; Richards, K.G. Can the agronomic performance of urea equal calcium ammonium nitrate across nitrogen rates in temperate grassland? Soil Use Manag. 2017, 33, 243–251. [Google Scholar] [CrossRef]
- Murphy, P.; Murphy, P.N.C.; Wall, D.P. Evaluating the Role of Scale in the Sustainability of Nutrient Management of Grazed Pasture Dairy Systems. Ph.D. Thesis, University College Dublin, Dublin, Ireland, April 2020. [Google Scholar]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertiliser Phosphorus Use: Reconciling Changing Concepts of Soil Phosphorus with Agronomic Information. In FAO Fertilizer and Plant Nutrition Bulletin; FAO: Rome, Italy, 2008; p. 18. [Google Scholar]
| Sample | DM | OM | TC | pH | N | P | K | S | Ca | Al | Fe | Rate Applied * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % FW | % FW | % FW | g Kg−1 FW | FW Tonnes ha−1 | ||||||||
| Al-DPS | 12 | 8.1 | 4.3 | 6.9 | 7.3 | 5.9 | 2.1 | 0.6 | 3.7 | 3.7 | 0.2 | 6.8 |
| Ca-DPS | 25 | 7.2 | 8.1 | 7.7 | 6.4 | 25.3 | 1.4 | 0.7 | 60.1 | 0.3 | 1.3 | 1.6 |
| SP | nd | nd | nd | nd | 0 | 163 | 2.0 | 12.9 | 209 | 1.4 | nd | 0.25 |
| Soil P Index | Soil P Range (Morgan’s mg-P L−1) | Index Description | Response to P Fertiliser | P Fertiliser Application Recommendation |
|---|---|---|---|---|
| 1 | 0–3 | Very Low | Definite | Build up 1,2 + maintenance 3 |
| 2 | 3.1–5 | Low | Likely | Build up 1,2 + maintenance 3 |
| 3 | 5.1–8 | Medium/Adequate | Unlikely | Maintenance |
| 4 | >8 | Sufficient/High | None | P application not recommended |
| Treatment | P Rate | N Input * | Total N Uptake | NUE ** |
|---|---|---|---|---|
| kg ha−1 | kg ha−1 | kg ha−1 | % | |
| no P | 0 | 325 | 255 (34.8) | 79 (10.7) |
| SP | 15 | 325 | 256 (18.5) | 79 (5.7) |
| SP | 30 | 325 | 271 (32.0) | 83 (9.9) |
| SP | 40 | 325 | 279 (26.8) | 86 (8.3) |
| SP | 50 | 325 | 260 (24.5) | 80 (7.5) |
| SP | 60 | 325 | 257 (27.7) | 79 (8.5) |
| Ca-DPS | 40 | 327 # | 248 (25.5) | 76 (7.8) |
| Al-DPS | 40 | 329 # | 288 (35.1) | 88 (10.7) |
| Treatment | Rate | APR (%) | RPE (%) | FPE (%) | |||
|---|---|---|---|---|---|---|---|
| kg ha−1 | 1st Harvest | Total | 1st Harvest | Total | 1st Harvest | Total | |
| No P | 0 | − | − | − | − | − | − |
| SP | 15 | 8.2 | 16.0 | − | − | − | − |
| SP | 30 | 10.9 | 20.3 | − | − | − | − |
| SP | 40 | 10.3 | 15.2 | 100 | 100 | 99.3 | 103 |
| SP | 50 | 5.7 | 14.4 | − | − | − | − |
| SP | 60 | 8.2 | 11.8 | − | − | − | − |
| Ca-DPS | 40 | 1.4 | 4.7 | 13.8 | 30.9 | 16.0 | 30.8 |
| Al-DPS | 40 | 5.0 | 16.0 | 48.9 | 106 | 49.9 | 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashekuzzaman, S.M.; Fenton, O.; Meers, E.; Forrestal, P.J. Differing Phosphorus Crop Availability of Aluminium and Calcium Precipitated Dairy Processing Sludge Potential Recycled Alternatives to Mineral Phosphorus Fertiliser. Agronomy 2021, 11, 427. https://doi.org/10.3390/agronomy11030427
Ashekuzzaman SM, Fenton O, Meers E, Forrestal PJ. Differing Phosphorus Crop Availability of Aluminium and Calcium Precipitated Dairy Processing Sludge Potential Recycled Alternatives to Mineral Phosphorus Fertiliser. Agronomy. 2021; 11(3):427. https://doi.org/10.3390/agronomy11030427
Chicago/Turabian StyleAshekuzzaman, S.M., Owen Fenton, Erik Meers, and Patrick J. Forrestal. 2021. "Differing Phosphorus Crop Availability of Aluminium and Calcium Precipitated Dairy Processing Sludge Potential Recycled Alternatives to Mineral Phosphorus Fertiliser" Agronomy 11, no. 3: 427. https://doi.org/10.3390/agronomy11030427






