The Influence of Agrotechnological Tools on cv. Rubin Apples Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Determination of Soluble Sugars by UPLC
2.3. Determination of Organic Acid by HPLC
2.4. Determination of Micro- and Macro- Elements by ICP—OES Spectrometer
2.5. Determination of Total Starch by Calorimetric Method
2.6. Determination of Total Phenolic Compounds by Calorimetric Method
2.7. Determination of DPPH Free Radical Scavenging Activity by the Calorimetric Method
2.8. Determination of the ABTS Radical Scavenging Activity by Calorimetric Method
2.9. Statistical Analysis
2.10. Meteorological Conditions
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 10 February 2021).
- Mika, A.; Buler, Z.; Treder, W. Mechanical pruning of apple trees as an alternative to manual pruning. Acta Sci. Pol. Hortorum Cultus 2016, 15, 113–121. [Google Scholar]
- Laužikė, K.; Sirgedaitė-Šėžienė, V.; Uselis, N.; Samuolienė, G. The Impact of Stress Caused by Light Penetration and Agrotechnological Tools on Photosynthetic Behavior of Apple Trees. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K. Comparison of three storage techniques for post-harvest quality preservation of six commercially available cultivars of apple. Int. J. Fruit Sci. 2018, 18, 268–286. [Google Scholar] [CrossRef]
- Karakasova, L.; Stefanoski, A.; Rafajlovska, V.; Klopceska, J. Technological characteristics of some apple cultivars. In I Balkan Symposium on Fruit Growing; ISHS: Plovdiv, Bulgaria, 2007; Volume 825, pp. 559–564. [Google Scholar]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Ma, C.; Sun, K.; Liu, Q.; Zhao, N.; Sun, Y.; Pan, L. Relationship between optical properties and soluble sugar contents of apple flesh during storage. Postharvest Biol. Technol. 2020, 159, 111021. [Google Scholar] [CrossRef]
- Harker, F.R.; Gunson, F.A.; Jaeger, S.R. The case for fruit quality: An interpretive review of consumer attitudes, and preferences for apples. Postharvest Biol. Technol. 2003, 28, 333–347. [Google Scholar] [CrossRef]
- Quilot-Turion, B.; Causse, M. 14 Natural Diversity and Genetic Control of Fruit Sensory Quality. In Fruit Ripening: Physiology, Signalling and Genomics; CABI Publishing: Oxfordshire, UK, 2014; Volume 228. [Google Scholar]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Tech. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Sharma, S.K. Nutritional characterization of apple as a function of genotype. J. Food Sci. Technol. 2018, 55, 2729–2738. [Google Scholar] [CrossRef]
- Král, M.; Tauferová, A.; Tremlová, B.; Šnirc, M.; Árvay, J.; Walczycka, M.; Florkiewicz, A. Macro-and Micro-elements in Locally Produced and Imported Fruits on Czech Market: A Quantitative Assessment. Erwerbs Obstbau 2020, 62, 361–367. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G.; Lopez, M.L. Fruit color development, anthocyanin content, standard quality, volatile compound emissions and consumer acceptability of several ‘Fuji’apple strains. Sci. Hortic. 2012, 137, 138–147. [Google Scholar] [CrossRef]
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef] [Green Version]
- Denardi, F.; Kvitschal, M.V.; Hawerroth, M.C. Yield performance of apple rootstocks of the Geneva series on replanting soil. Pesqui. Agropecuária Bras. 2018, 53, 924–933. [Google Scholar] [CrossRef]
- Reig, G.; Lordan, J.; Fazio, G.; Grusak, M.A.; Hoying, S.; Cheng, L.; Robinson, T. Horticultural performance and elemental nutrient concentrations on ‘Fuji’grafted on apple rootstocks under New York State climatic conditions. Sci. Hortic. 2018, 227, 22–37. [Google Scholar] [CrossRef]
- de Macedo, T.A.; da Silva, P.S.; Sander, G.F.; Welter, J.F.; Rufato, L.; de Rossi, A. Productivity and quality of ‘Fuji Suprema’ apple fruit in different rootstocks and growing conditions. Sci. Hortic. 2019, 256, 108651. [Google Scholar] [CrossRef]
- Dalhaus, T.; Schlenker, W.; Blanke, M.M.; Bravin, E.; Finger, R. The Effects of Extreme Weather on Apple Quality. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Qiang, Z.; Qin-ping, W.; Song-zhong, L.; Xiao-wei, W.; Zhi-hua, S.; Jin-jin, L. Formation of canopy structure, yield and fruit quality of ‘Fuji’apple with SH6 dwarf interstock from juvenility to fruiting early stage. Sci. Agric. Sin. 2013, 46, 1874–1880. [Google Scholar]
- Rudell, D.R.; Serra, S.; Sullivan, N.; Mattheis, J.P.; Musacchi, S. Survey of ‘d’Anjou’pear metabolic profile following harvest from different canopy positions and fruit tissues. Hortscience 2017, 52, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Yamaki, S. Metabolism and Accumulation of Sugars Translocated to Fruit and Their Regulation. J. Jpn. Soc. Hortic. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Fei, L.; Li, Y.; Zeng, J.; Dai, Z. Response of fruit yield, fruit quality, and water use efficiency to water deficits for apple trees under surge-root irrigation in the Loess Plateau of China. Agric. Water Manag. 2019, 222, 221–230. [Google Scholar] [CrossRef]
- Reid, M.; Kalcsits, L. Water Deficit Timing Affects Physiological Drought Response, Fruit Size, and Bitter Pit Development for ‘Honeycrisp’ Apple. Plants 2020, 9, 874. [Google Scholar] [CrossRef]
- Šircelj, H.; Tausz, M.; Grill, D.; Batič, F. Detecting different levels of drought stress in apple trees (Malus domestica Borkh.) with selected biochemical and physiological parameters. Sci. Hortic. 2007, 113, 362–369. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef]
- Suni, M.; Nyman, M.; Eriksson, N.A.; Björk, L.; Björck, I. Carbohydrate composition and content of organic acids in fresh and stored apples. J. Sci. Food Agric. 2000, 80, 1538–1544. [Google Scholar] [CrossRef]
- Palmer, J. The future role of crop physiologists, a personal view. In X International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems; ISHS: Stellenbosch, South Africa, 2012; Volume 1058, pp. 209–219. [Google Scholar]
- Jivan, C.; Sala, F. Relationship between tree nutritional status and apple quality. Hortic. Sci. 2014, 41, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC–ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Cheng, W.; Zhao, Z.; Cao, J. HPLC method for the simultaneous quantification of the major organic acids in Angeleno plum fruit. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; Volume 62, p. 012035. [Google Scholar]
- Arauújo, G.C.L.; Gonzalez, M.H.; Ferreira, A.G.; Nogueira, A.R.A.; Nóbrega, J.A. Effect of acid concentration on closed-vessel microwave-assisted digestion of plant materials. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 2121–2132. [Google Scholar] [CrossRef]
- Barbosa, J.T.P.; Santos, C.M.M.; Peralva, V.N.; Flores, E.M.M.; Korn, M.; Nóbrega, J.A.; Korn, M.G.A. Microwave-assisted diluted acid digestion for trace elements analysis of edible soybean products. Food Chem. 2015, 175, 212–217. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdel-Aal, E.S.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Biddlecombe, C.T.; Dalton, A. To investigate the effect of four timings of mechanical pruning on yield and fruit quality compared to a hand pruned control in an intensive ‘Gala’M9 orchard planted as a fruit wall. In Proceedings of the XI International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems, Bologna, Italy, 28 August–2 September 2016; Volume 1228, pp. 97–104. [Google Scholar]
- Franzen, J.B.; Hirst, P.M. Optimal pruning of apple and effects on tree architecture, productivity, and fruit quality. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Brisbane, Australia, 17 August 2014; Volume 1130, pp. 307–310. [Google Scholar]
- Kviklys, D.; Viskelis, J.; Lanauskas, J.; Uselis, N.; Liaudanskas, M.; Janulis, V. Effects of growth control on yield and fruit quality of the apple cultivar ‘Rubin’. Agric. Food Sci. 2020, 29, 257–264. [Google Scholar]
- Tahir, I.I.; Johansson, E.; Olsson, M.E. Improvement of quality and storability of apple cv. Aroma by adjustment of some pre-harvest conditions. Sci. Hortic. 2007, 112, 164–171. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Phytochemistry: Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–67. [Google Scholar]
- Viškelis, J.; Liaudanskas, M.; Uselis, N.; Kviklys, D. Internal fruit quality depends on the regulation of apple-tree vegetative growth. In Proceedings of the XXX International Horticultural Congress IHC2018: International Symposium on Cultivars, Rootstocks and Management Systems of 1281, Istanbul, Turkey, 12–16 August 2018; pp. 643–648. [Google Scholar]
- Drogoudi, P.D.; Pantelidis, G. Effects of position on canopy and harvest time on fruit physico-chemical and antioxidant properties in different apple cultivars. Sci. Hortic. 2011, 129, 752–760. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus × domestica) cultivars. Hortic. Res. 2014, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cebulj, A.; Cunja, V.; Mikulic-Petkovsek, M.; Veberic, R. Importance of metabolite distribution in apple fruit. Sci. Hortic. 2017, 214, 214–220. [Google Scholar] [CrossRef]
- Duran, A.; Tuzen, M.; Soylak, M. Trace element levels in some dried fruit samples from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 581–589. [Google Scholar] [CrossRef]
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Speisky, H. Determination of antioxidant capacity, total phenolic content and mineral composition of different fruit tissue of five apple cultivars grown in Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar] [CrossRef]


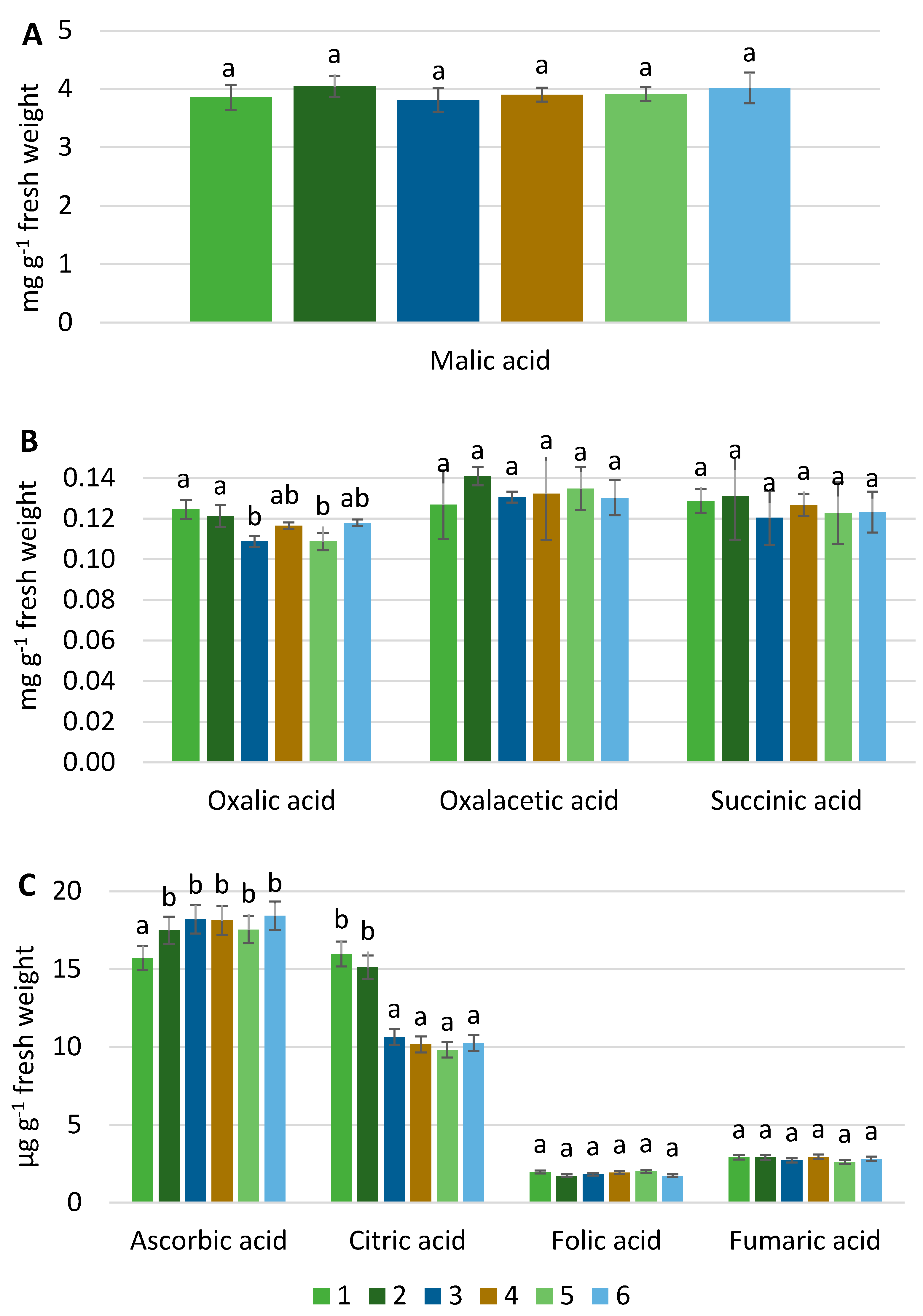



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laužikė, K.; Uselis, N.; Samuolienė, G. The Influence of Agrotechnological Tools on cv. Rubin Apples Quality. Agronomy 2021, 11, 463. https://doi.org/10.3390/agronomy11030463
Laužikė K, Uselis N, Samuolienė G. The Influence of Agrotechnological Tools on cv. Rubin Apples Quality. Agronomy. 2021; 11(3):463. https://doi.org/10.3390/agronomy11030463
Chicago/Turabian StyleLaužikė, Kristina, Nobertas Uselis, and Giedrė Samuolienė. 2021. "The Influence of Agrotechnological Tools on cv. Rubin Apples Quality" Agronomy 11, no. 3: 463. https://doi.org/10.3390/agronomy11030463
APA StyleLaužikė, K., Uselis, N., & Samuolienė, G. (2021). The Influence of Agrotechnological Tools on cv. Rubin Apples Quality. Agronomy, 11(3), 463. https://doi.org/10.3390/agronomy11030463






