Laser Light Treatment of Seeds for Improving the Biomass Photosynthesis, Chemical Composition and Biological Activities of Lemongrass Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Photosynthesis Analysis
2.3. Metabolite Analyses
2.3.1. Primary Metabolism
Sugar Analysis
Amino Acid Analysis
Organic Acid Analysis
Essential Oil Analysis
2.3.2. Secondary Metabolites
Determination of Phenolic Profile
2.4. Biological Activity
2.5. Antioxidant Capacity
2.6. Hypocholesterolemic Activity
2.6.1. Inhibition of Micellar Solubility of Cholesterol
2.6.2. Pancreatic α-Amylase Inhibition Assay
2.6.3. Pancreatic Lipase Inhibition Assay
2.7. Anti-Diabetic Activity
2.7.1. Determination of In Vitro Glycemic Index
2.7.2. α-Glucosidase Inhibition Assay
2.7.3. α-Amylase Inhibition Assay
2.8. Statistical Analyses
3. Results and Discussion
3.1. Increased Photosynthesis and Respiration by Laser Light Improved Cymbopogon proximus Growth
3.2. Improved Photosynthetic Reactions in Laser Light-Treated Cymbopogon proximus Sprouts Induced the Assumption of Bioactive Primary Metabolites
3.3. Laser Treatment Increased the Levels of Essential Oils in Cymbopogon proximus Sprouts
3.4. Improved Levels of Antioxidant Metabolites in Laser-Treated Cymbopogon proximus Sprouts
3.5. Phenolic Compound Accumulation by Laser Treatment Improved the Overall Antioxidant Capacity of Cymbopogon proximus Sprouts
3.6. Enhanced Cholesterol-Lowering Activity of Laser Light-Treated Cymbopogon proximus Sprouts
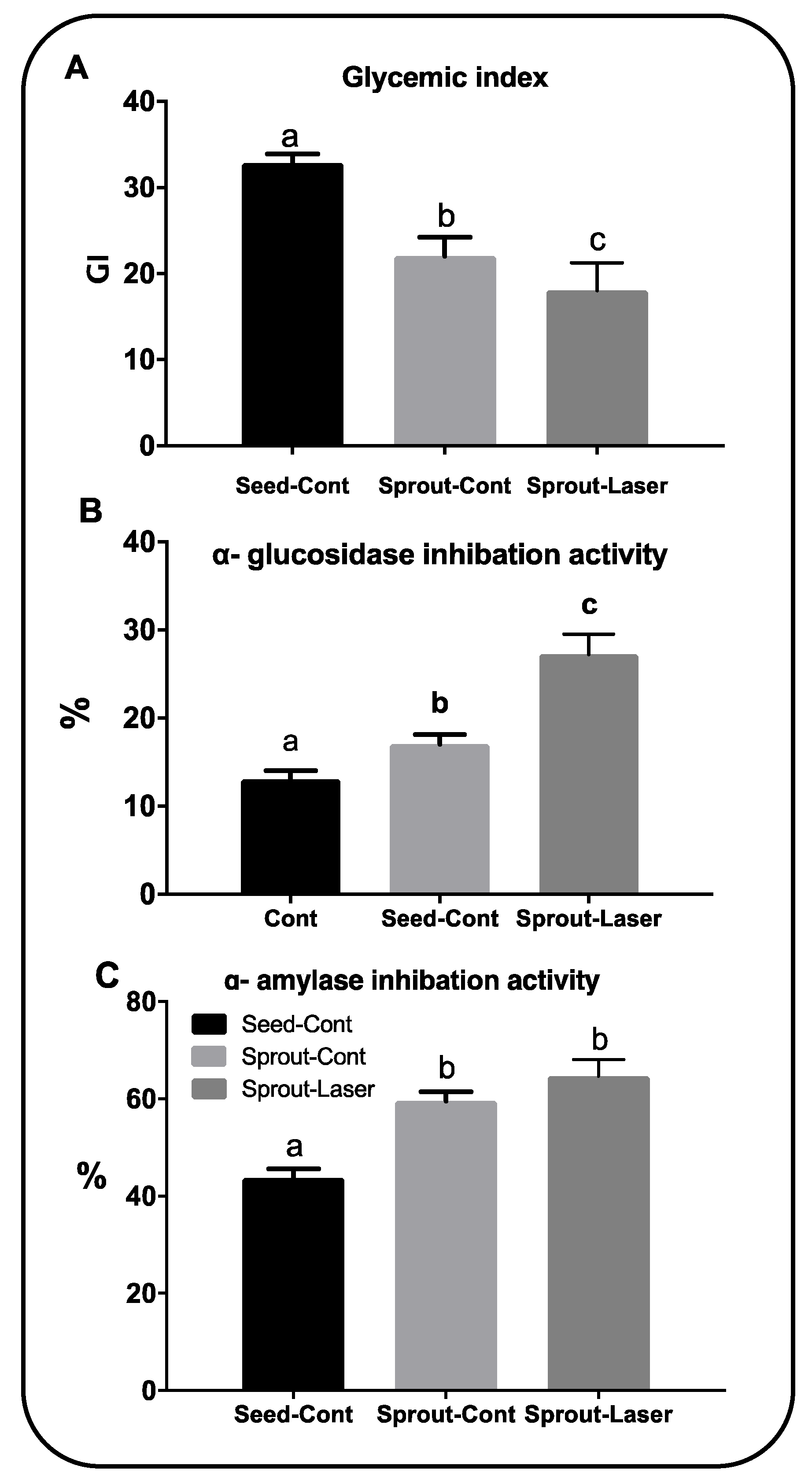
3.7. Improved Anti-Diabetic Activity of Laser-Treated Cymbopogon proximus Sprouts
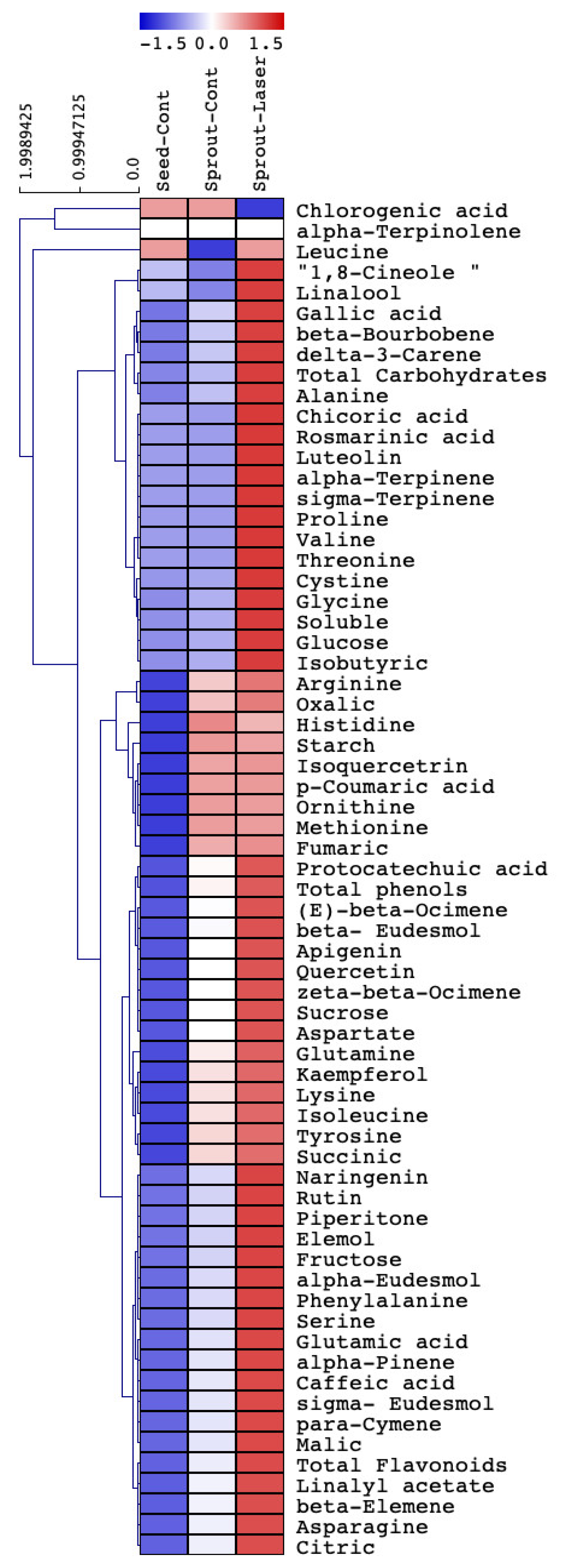
3.8. Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swieca, M.; Gawlik-Dziki, U.; Jakubczyk, A.; Bochnak, J.; Sikora, M.; Suliburska, J. Nutritional quality of fresh and stored legumes sprouts—Effect of Lactobacillus plantarum 299v enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Márton, M.; Mándoki, Z.S.; Csapo-Kiss, Z.S.; Csapó, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae Aliment. 2010, 3, 81–117. [Google Scholar]
- AbdulAzeez, M.A.; Abdullahi, A.S.; James, B.D. Lemongrass (Cymbopogon spp.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 509–516. [Google Scholar]
- Avoseh, O.; Oyedeji, O.; Rungqu, P.; Nkeh-Chungag, B.; Oyedeji, A. Cymbopogon species; ethnopharmacology, phyto-chemistry and the pharmacological importance. Molecules 2015, 20, 7438–7453. [Google Scholar] [CrossRef]
- Dutta, S.; Munda, S.; Lal, M.; Bhattacharyya, P.R. A short review on chemical composition therapeutic use and enzyme inhi-bition activities of Cymbopogon species. Indian J. Sci. Technol. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Ganjewala, D. Cymbopogon essential oils: Chemical compositions and bioactivities. Int. J. Essent. Oil Ther. 2009, 3, 56–65. [Google Scholar]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekpenyong, C.E.; Davies, K.; Antai, E.E. Cymbopogon citratus Stapf (DC) Extract Ameliorates Atherogenic Cardiovascular Risk in Diabetes-Induced Dyslipidemia in Rats. Br. J. Med. Med. Res. 2014, 4, 4695–4709. [Google Scholar] [CrossRef]
- Mansour, H.A.; Newairy, A.-S.; Yousef, M.; Sheweita, S. Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology 2002, 170, 221–228. [Google Scholar] [CrossRef]
- Adeneye, A.A.; Agbaje, E.O. Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. J. Ethnopharmacol. 2007, 112, 440–444. [Google Scholar] [CrossRef]
- Campos, J.; Schmeda-Hirschmann, G.; Leiva, E.; Guzmán, L.; Orrego, R.; Fernández, P.; González, M.; Radojkovic, C.; Zuñi-ga, F.A.; Lamperti, L.; et al. Lemon grass (Cymbopogon citratus (D.C) Stapf) polyphenols protect human umbilical vein endo-thelial cell (HUVECs) from oxidative damage induced by high glucose, hydrogen peroxide and oxidised low-density lipo-protein. Food Chem. 2014, 151, 175–181. [Google Scholar] [CrossRef]
- Khan, S.J.; Afroz, S.; Khan, R.A. Anti-hyper lipidemic and anti-hyperglycemic effects of Cymbopogon jwarancusa in high-fat high-sugar Diet model. Pak. J. Pharm. Sci. 2018, 31, 1341–1345. [Google Scholar]
- El-Askary, H.I.; Meselhy, M.R.; Galal, A.M. Sesquiterpenes from Cymbopogon proximus. Molecules 2003, 8, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, F.Y.; El-Khateeb, A. Effect of herbal beverages of Foeniculum vulgare and Cymbopogon proximus on inhibition of calcium oxalate renal crystals formation in rats. Ann. Agric. Sci. 2013, 58, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.A. Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Grasas Aceites 2011, 62, 55–61. [Google Scholar] [CrossRef]
- Warrag, N.M.; Tag Eldin, I.M.; Ahmed, E.M. Effect of Cymbopogon proximus (Mahareb) on ethylene glycol-induced nephro-lithiasis in rats. Afr. J. Pharm. Pharm. 2014, 8, 443–450. [Google Scholar]
- El-Nezhawy, A.O.H.; Maghrabi, I.A.; Mohamed, K.M.; Omar, H.A. Cymbopogon proximus extract decreases L-NAME-induced hypertension in rats. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 66–69. [Google Scholar]
- El Tahir, K.; Abdel-Kader, M. Chemical and pharmacological study of Cymbopogon proximus volatile oil. Res. J. Med. Plant 2008, 2, 53–60. [Google Scholar]
- Althurwi, H.N.; Abdel-Kader, M.S.; Alharthy, K.M.; Salkini, M.A.; Albaqami, F.F. Cymbopogon Proximus Essential Oil Protects Rats against Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis. Molecules 2020, 25, 1786. [Google Scholar] [CrossRef] [Green Version]
- Almuhayawi, M.; AbdElgawad, H.; Al Jaouni, S.; Selim, S.; Hassan, A.H.A.; Khamis, G. Elevated CO2 improves glucos-inolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.; Hassan, A.H.A.; Abdel-Mawgoud, M.; Khamis, G.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. Laser light as a promising approach to improve the nutritional value, antioxidant capacity and anti-inflammatory activity of flavo-noid-rich buckwheat sprouts. Food Chem. 2021, 345, 128788. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S.; Hassan, A.H.; Al Jaouni, S.K.; Alkhalifah, D.H.M.; Hozzein, W.N.; Selim, S.; Khamis, G. Influence of elevated CO2 on nutritive value and health-promoting prospective of three genotypes of Alfalfa sprouts (Medicago Sativa). Food Chem. 2021, 340, 128147. [Google Scholar] [CrossRef]
- Perveen, R.; Jamil, Y.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He-Ne Laser-Induced Improvement in Biochemical, Physiological, Growth and Yield Characteristics in Sunflower (Helianthus annuus L.). Photochem. Photobiol. 2011, 87, 1453–1463. [Google Scholar] [CrossRef]
- Chen, Y.P.; Yue, M. and Wang, X.L. Influence of He-Ne Laser Irradiation on Seeds Thermodynamic Parameters and Seed-lings Growth of Isatis indogotica. Plant Sci. 2005, 168, 601–606. [Google Scholar] [CrossRef]
- Ooi, A.; Wong, A.; Ng, T.K.; Marondedze, C.; Gehring, C.; Ooi, B.S. Growth and development of Arabidopsis thaliana under single-wavelength red and blue laser light. Sci. Rep. 2016, 6, 33885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, H. The Latest Development of Laser Application Research in Plant Factory. Agric. Agric. Sci. Procedia 2015, 3, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Haq, Z.U.; Abbas, M. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J. Photochem. Photobiol. B Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef]
- AbdelGawad, H.; Peshev, D.; Zinta, G.; Ende, W.V.D.; Janssens, I.A.; Asard, H. Climate Extreme Effects on the Chemical Composition of Temperate Grassland Species under Ambient and Elevated CO2: A Comparison of Fructan and Non-Fructan Accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef]
- Zinta, G.; AbdElgawad, H.; Peshev, D.; Weedon, J.T.; Van den Ende, W.; Nijs, I.; Janssens, I.A.; Beemster, G.T.; Asard, H. Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and ele-vated atmospheric CO2. J. Exp. Bot. 2018, 69, 2159–2170. [Google Scholar] [CrossRef]
- Hassanpour, H.; Khavari-Nejad, R.A.; Niknam, V.; Razavi, K.; Najafi, F. Effect of pen-conazole and drought stress on the essential oil composition and gene expression of Menthapulegium L.(Lamiaceae) at flowering stage. Acta Physiol. Plant. 2014, 36, 1167–1175. [Google Scholar] [CrossRef]
- Hamad, I.; AbdelGawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.T.S.; Hegab, M.; Hagagy, N.; Selim, S.A. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef] [PubMed]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 121–131. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Saleh, A.M.; Habeeb, T.H.; Wadaan, M.A.; AbdelGawad, H. CO2 treatment improves the hypocholesterolemic and antioxidant properties of fenugreek seeds. Food Chem. 2020, 308, 125661. [Google Scholar] [CrossRef]
- Hasan, M.; Hanafiah, M.M.; Taha, Z.A.; Alhilfy, I.H.H.; Said, M.N.M. Laser Irradiation Effects at Different Wavelengths on Phenology and Yield Components of Pretreated Maize Seed. Appl. Sci. 2020, 10, 1189. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Yuan, M.; He, Y.; Li, Y.; Zhang, L. Physiological and transcriptome analysis of He-Ne laser pre-treated wheat seedlings in response to drought stress. Sci. Rep. 2017, 7, 6108. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How Do Sugars Regulate Plant Growth and Development? New Insight into the Role of Trehalose-6-Phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Al Jaouni, S.K.; Al Muhayawi, M.S.; Hussein, A.; Elfiki, I.; Al-Raddadi, R.; Al Muhayawi, S.M.; Harakeh, S. Effects of Honey on Oral Mucositis among Pediatric Cancer Patients Undergoing Chemo/Radiotherapy Treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid. Based Complement. Altern. Med. 2017, 2017, 5861024. [Google Scholar] [CrossRef] [PubMed]
- Ufaz, S.; Galili, G. Improving the Content of Essential Amino Acids in Crop Plants: Goals and Opportunities. Plant Physiol. 2008, 147, 954–961. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Jiang, W. Exogenous Ap-plication of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Soares, J.N.; Reichardt, K.; Neto, D.D. Seed and Foliar Application of Amino Acids Improve Variables of Nitrogen Metabolism and Productivity in Soybean Crop. Front. Plant Sci. 2018, 9, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malundo, T.; Shewfelt, R.; Ware, G.; Baldwin, E. Sugars and Acids Influence Flavor Properties of Mango (Mangifera indica). J. Am. Soc. Hortic. Sci. 2001, 126, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.-X.; Wei, J.-P.; Shen, C.; Wen-Yan, H.; Zhang, L.-P.; Han, W.-Y. Stimulation in primary and secondary metabolism by elevated carbon dioxide alters green tea quality in Camellia sinensis L. Sci. Rep. 2017, 7, 7937. [Google Scholar] [CrossRef] [PubMed]
- Champigny, M.L. Integration of photosynthetic carbon and nitrogen metabolism in higher plants. Photosynth. Res. 1995, 46, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Velez-Ramirez, A.I.; Van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Seibert, J.B.; Rodrigues, I.V.; Carneiro, S.P.; Amparo, T.R.; Lanza, J.S.; Frézard, F.J.G.; Santos, O.D.H.D. Frézard Gustavo Henrique Bianco de Souza Orlando David Henrique dos Santos. Seasonality study of essential oil from leaves of Cymbopogon densiflorus and nanoemulsion development with antioxidant activity. Flavour Fragr. J. 2019, 34, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Bhatta, L.; Kale, R.D. Research articleLemongrass (Cymbopogon Flexuosus Steud.) wats treated textile: A control measure against vector-borne diseases. Heliyon 2019, 5, e02842. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Joubert, E.; Gelderblom, W.; Manley, M. Phenolic Compounds: A Review of Their Possible Role as In Vivo Antioxidants of Wine. S. Afr. J. Enol. Vitic. 2017, 23, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phe-nolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.; Sousa, J.J.; Figueiredo, I.V.; Batista, M.T. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef]
- Figueirinha, A.; Cruz, M.T.; Francisco, V.L.G.; Lopes, M.C.; Batista, M.T. Anti-Inflammatory Activity of Cymbopogon citratus Leaf Infusion in Lipopolysaccharide-Stimulated Dendritic Cells: Contribution of the Polyphenols. J. Med. Food 2010, 13, 681–690. [Google Scholar] [CrossRef]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of laser light treatment on some bio-chemical and physiological processes in seeds and seedlings of white lupine and faba bean. Plant Growth Regul. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- León-López, L.; Escobar-Zúñiga, Y.; Salazar-Salas, N.Y.; Rochín, S.M.; Cuevas-Rodríguez, E.O.; Reyes-Moreno, C.; Milán-Carrillo, J. Improving Polyphenolic Compounds: Antioxidant Activity in Chickpea Sprouts through Elicitation with Hydrogen Peroxide. Foods 2020, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, C.; Chen, K.; Li, X. Flavonoids, Phenolics, and Antioxidant Capacity in the Flower of Eriobotrya japonica Lindl. Int. J. Mol. Sci. 2011, 12, 2935–2945. [Google Scholar] [CrossRef]
- Mohammad, T.; Khudhur, D. Evaluation of the bio-stainging and antioxidant activity of Cymbopogon citratus extraction grass. Asian J. Microbiol. Biotech. Environ. Sci. 2018, 20, 848–856. [Google Scholar]
- Gururaja, G.M.; Mundkinajeddu, D.; Kumar, A.S.; Dethe, S.M.; Allan, J.J.; Agarwal, A. Cite Evaluation of Cholesterol-lowering Activity of Standardized Extract of Mangifera indica in Albino Wistar Rats. Pharmacogn. Res. 2017, 9, 21–26. [Google Scholar]
- Agbafor, K.N.; Akubugwo, E.I. Hypocholesterolaemic effect of ethanolic extract of fresh leaves of Cymbopogon citratus (lemon grass). Afr. J. Biotechnol. 2007, 6, 596–598. [Google Scholar]
- Sosnowska, D.; Podsędek, A.; Redzynia, M.; Kucharska, A.Z. Inhibitory effect of black chokeberry fruit polyphenols on pancreatic lipase—Searching for most active inhibitors. J. Funct. Foods 2018, 49, 196–204. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Akinleye, M.; Omojokun, O.S.; Boligon, A.A.; Athayde, M.L. Starch composition, glycemic indices, phenolic constituents, and antioxidative and antidiabetic properties of some common tropical fruits. J. Ethn. Foods 2015, 2, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Jeong, Y.-K.; Wang, M.-H.; Lee, W.-Y.; Rhee, H.-I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bonesi, M.; Menichini, F.; Tenuta, M.C.; Leporini, M.; Tundis, R. Antioxidant and Carbohydrate-Hydrolysing Enzymes Potential of Sechium edule (Jacq.) Swartz (Cucurbitaceae) Peel, Leaves and Pulp Fresh and Processed. Plant Foods Hum. Nutr. 2016, 71, 381–387. [Google Scholar] [CrossRef]
- Wongsa, P.; Chaiwarit, J.; Zamaludien, A. In vitro screening of phenolic compounds, potential inhibition against α-amylase and α-glucosidase of culinary herbs in Thailand. Food Chem. 2012, 131, 964–971. [Google Scholar] [CrossRef]
- Smalley, P.J. Laser safety: Risks, hazards, and control measures. Laser Ther. 2011, 20, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Time (min) | He-Ne_Laser | He-Ca_Laser | Ar_Laser |
|---|---|---|---|
| FW (g/sprout) | |||
| 0 | 1.4 ± 0.12 a | 1.4 ± 0.11 a | 1.4 ± 0.12 a |
| 2 | 1.8 ± 0.06 b | 1.4 ± 0.12 a | 1.7 ± 0.34 |
| 5 | 2.1 ± 0.05 b | 1.5 ± 0.1 a | 1.7 ± 0.11 a |
| 10 | 2.1 ± 0.06 b | 1.8 ± 0.13 b | 1.8 ± 0.1 b |
| FRAP (μmol trolox/gFW) | |||
| 0 | 13.1 ± 0.5 a | 13.1 ± 0.5 a | 13.1 ± 0.5 a |
| 2 | 17.5 ± 0.17 b | 14.1 ± 0.19 a | 13.9 ± 0.74 a |
| 5 | 20.9 ± 1.1 b | 18.3 ± 0.25 b | 15.7 ± 0.87 a |
| 10 | 19.8 ± 2.74 b | 17.3 ± 0.9 ab | 17.8 ± 0.4 b |
| Control | Laser | |
|---|---|---|
| FW (g/sprout) | 1.45 ± 0.2 a | 2.08 ± 0.26 b |
| DW (g/sprout) | 0.15 ± 0.05 a | 0.25 ± 0.03 b |
| Photosynthesis (μmol CO2 m−2 s−1) | 2.9 ± 0.35 a | 4.08 ± 0.15 b |
| Respiration (μmol CO2 m−2 s−1) | 0.018 ± 0.003 a | 0.025 ± 0.005 b |
| Parameters | Seed-Control | Sprout-Control | Sprout-Laser |
|---|---|---|---|
| Sugars | |||
| Glucose | 1.2 ± 0.1 a | 1.3 ± 0 a | 2.2 ± 0.23 b |
| Fructose | 0.32 ± 0.04 a | 0.49 ± 0.05 b | 0.9 ± 0.01 c |
| Sucrose | 1.1 ± 0.06 a | 1.9 ± 0.06 b | 2.7 ± 0.09 b |
| Soluble sugars | 4.9 ± 0.7 a | 5.4 ± 0.2 a | 10.3 ± 2.6 a |
| Starch | 48.7 ± 5.3 a | 52.7 ± 2.4 a | 49.3 ± 5.7 a |
| Total Carbohydrates | 83.7 ± 3.8 a | 92.7 ± 2.1 b | 137 ± 9.7 c |
| Amino Acids | |||
| Lysine | 2.1 ± 0.1 a | 3.9 ± 0.2 b | 4.9 ± 0.03 c |
| Histidine | 2.1 ± 0.12 a | 2.8 ± 0.22 a | 2.7 ± 0.17 a |
| Alanine | 16.9 ± 2.1 a | 17.3 ± 1 a | 18.9 ± 1.7 a |
| Arginine | 1.1 ± 0.61 a | 2 ± 0.01 b | 2.3 ± 0.1 b |
| Isoleucine | 0.02 ± 0.0 a | 0.2 ± 0.01 b | 0.3 ± 0.0 c |
| Asparagine | 0.4 ± 0.06 a | 1 ± 0.06 b | 1.8 ± 0.1 c |
| Ornithine | 0.15 ± 0.01 a | 0.2 ± 0.03 a | 0.2 ± 0.02 a |
| Glycine | 1.1 ± 0.1 a | 1.2 ± 0.07 a | 2 ± 0.13 b |
| Phenylalanine | 0.1 ± 0.01 a | 0.3 ± 0.02 b | 0.7 ± 0 c |
| Serine | 0.15 ± 0.01 a | 0.3 ± 0.02 b | 0.6 ± 0.04 b |
| Proline | 1.1 ± 0.1 a | 1.1 ± 0.07 a | 2.4 ± 0.1 c |
| Valine | 0.5 ± 0.1 a | 0.5 ± 0.03 a | 0.6 ± 0.04 a |
| Aspartate | 0.01 ± 0 a | 0.03 ± 0 b | 0.05 ± 0 c |
| Cystine | 0.02 ± 0 a | 0.03 ± 0 a | 0.2 ± 0.01 b |
| Leucine | 0.03 ± 0 a | 0.02 ± 0 a | 0.03 ± 0.1 a |
| Methionine | 0.01 ± 0 a | 0.02 ± 0 b | 0.02 ± 0 b |
| Threonine | 0.1 ± 0.0 a | 0.1 ± 0.01 a | 0.2 ± 0.01 b |
| Tyrosine | 0.34 ± 0.0 a | 1 ± 0.06 b | 1.3 ± 0.1 b |
| Glutamine | 71.1 ± 1.8 a | 91.7 ± 5.6 b | 106 ± 6.6 c |
| Glutamic acid | 54 ± 3.1 a | 67 ± 4.2 b | 90 ± 8.9 c |
| Organic Acids | |||
| Oxalic | 2.11 ± 0.2 a | 2.84 ± 0.2 a | 3.04 ± 0.1 a |
| Malic | 2.08 ± 0.2 a | 3.08 ± 0.19 b | 4.72 ± 1.5 c |
| Isobutyric | 3.1 ± 0.1 a | 3.2 ± 0.2 a | 4.1 ± 1.37 a |
| Fumaric | 0.73 ± 0.01 a | 0.93 ± 0.01 a | 0.95 ± 0.3 a |
| Succinic | 1.24 ± 0.5 a | 3.24 ± 0.33 b | 4.15 ± 1.1 c |
| Citric | 2.1 ± 0.2 a | 3.5 ± 0.3 b | 5.4 ± 1.8 c |
| Essential Oils (mg/gFW) | Seed-Control | Sprout-Control | Sprout-Laser |
|---|---|---|---|
| α-Pinene | 0.5 ± 0.2 a | 0.8 ± 0.17 b | 1.3 ± 0.3 b |
| α-Eudesmol | 0.2 ± 0.0 a | 0.3 ± 0.05 b | 0.5 ± 0.01 c |
| Elemol | 7.4 ± 0.2 a | 9.4 ± 0.71 b | 14.2 ± 0.4 c |
| α-Terpinene | 1 ± 0.1 a | 1 ± 0.15 a | 1.6 ± 0.2 a |
| p-Cymene | 3.4 ± 0.6 a | 5.6 ± 0.64 b | 9.2 ± 0.1 c |
| 1,8-Cineole | 2.5 ± 0.09 a | 2.2 ± 0.1 a | 3.7 ± 0.3 b |
| Piperitone | 37.6 ± 2.1 a | 45.6 ± 1.4 b | 64 ± 2.9 c |
| (E)-β-Ocimene | 0.7 ± 0.03 a | 1.28 ± 0.2 b | 1.9 ± 0.3 c |
| γ-Terpinene | 0.3 ± 0.04 a | 0.3 ± 0.04 a | 0.47 ± 0.07 b |
| α-Terpinolene | 0.01 ± 0 a | 0.01 ± 0 a | 0.01 ± 0 a |
| Linalool | 1.1 ± 0.2 a | 1 ± 0.15 a | 1.6 ± 0.25 a |
| Linalyl acetate | 0.07 ± 0.0 a | 0.14 ± 0.03 b | 0.23 ± 0.05 c |
| β-Bourbobene | 0.24 ± 0.01 a | 0.3 ± 0.02 a | 0.49 ± 0.04 b |
| β-Elemene | 0.32 ± 0.0 a | 0.62 ± 0.06 b | 1.0 ± 0.1 b |
| γ-Eudesmol | 0.10 ± 0.0 a | 0.18 ± 0.03 a | 0.31 ± 0.04 b |
| β-Eudesmol | 0.11 ± 0.0 a | 0.28 ± 0.05 b | 0.47 ± 0.08 c |
| (Z)-β-Ocimene | 0.22 ± 0.01 a | 0.64 ± 0.06 b | 1.06 ± 0.11 c |
| δ-3-Carene | 0.4 ± 0.02 a | 0.5 ± 0.01 a | 0.83 ± 0.01 b |
| Phenolics and Flavonoids (mg/gFW) | Seed-Control | Sprout-Control | Sprout-Laser |
|---|---|---|---|
| Gallic acid | 0.21 ± 0.03 a | 0.29 ± 0.02 a | 0.5 ± 0.05 b |
| Caffeic acid | 0.22 ± 0.0 a | 0.42 ± 0.03 b | 0.74 ± 0.1 c |
| p-Coumaric acid | 1.10 ± 0.1 a | 2.27 ± 0.1 b | 2.3 ± 0.16 b |
| Chicoric acid | 1.1 ± 0.04 a | 1.1 ± 0.04 a | 1.2 ± 0.04 a |
| Rosmarinic acid | 1.0 ± 0.07a | 1.0 ± 0.07 a | 1.7 ± 0.05 b |
| Protocatechuic acid | 1.1 ± 0.01 a | 3.1 ± 0.06 b | 4.9 ± 0.3 b |
| Quercetin | 0.053 ± 0.01 a | 0.055 ± 0.0 a | 0.057 ± 0.0 a |
| Isoquercetrin | 0.026 ± 0.0 a | 0.045 ± 0.0 b | 0.046 ± 0.0 b |
| Kaempferol | 0.042 ± 0.0 a | 0.06 ± 0.0 b | 0.07 ± 0.0 b |
| Luteolin | 0.06 ± 0.01 a | 0.06 ± 0.0 a | 0.08 ± 0.0 b |
| Apigenin | 0.03 ± 0.02 a | 0.06 ± 0.02 b | 0.09 ± 0.0 c |
| Naringenin | 0.004 ± 0.0 a | 0.01 ± 0.0 b | 0.023 ± 0.01 b |
| Rutin | 0.001 ± 0.0 a | 0.007 ± 0.0 b | 0.021 ± 0.0 c |
| Chlorogenic acid | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.017 ± 0.0 b |
| Total phenols | 6.43 ± 0.4 a | 9.43 ± 0.1 b | 11.87 ± 0.3 c |
| Total Flavonoids | 1.45 ± 0.0 a | 2.05 ± 0.07 b | 2.91 ± 0.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okla, M.K.; El-Tayeb, M.A.; Qahtan, A.A.; Abdel-Maksoud, M.A.; Elbadawi, Y.B.; Alaskary, M.K.; Balkhyour, M.A.; Hassan, A.H.A.; AbdElgawad, H. Laser Light Treatment of Seeds for Improving the Biomass Photosynthesis, Chemical Composition and Biological Activities of Lemongrass Sprouts. Agronomy 2021, 11, 478. https://doi.org/10.3390/agronomy11030478
Okla MK, El-Tayeb MA, Qahtan AA, Abdel-Maksoud MA, Elbadawi YB, Alaskary MK, Balkhyour MA, Hassan AHA, AbdElgawad H. Laser Light Treatment of Seeds for Improving the Biomass Photosynthesis, Chemical Composition and Biological Activities of Lemongrass Sprouts. Agronomy. 2021; 11(3):478. https://doi.org/10.3390/agronomy11030478
Chicago/Turabian StyleOkla, Mohammad K., Mohamed A. El-Tayeb, Ahmed Ali Qahtan, Mostafa A. Abdel-Maksoud, Yahya B. Elbadawi, Mohamed Khamis Alaskary, Mansour A. Balkhyour, Abdelrahim H. A. Hassan, and Hamada AbdElgawad. 2021. "Laser Light Treatment of Seeds for Improving the Biomass Photosynthesis, Chemical Composition and Biological Activities of Lemongrass Sprouts" Agronomy 11, no. 3: 478. https://doi.org/10.3390/agronomy11030478








