Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica juncea (Kai Choy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Digestate and Fertilizer Characterization
2.2. Experimental Growing Conditions
2.3. Experimental Growing Conditions
2.4. Plant Growth and Root Length Analyses
2.5. Plant Nutrient Analyses
2.6. Electrical Conductivity and Biohazard Analysis
2.7. Statistical Analysis
3. Results
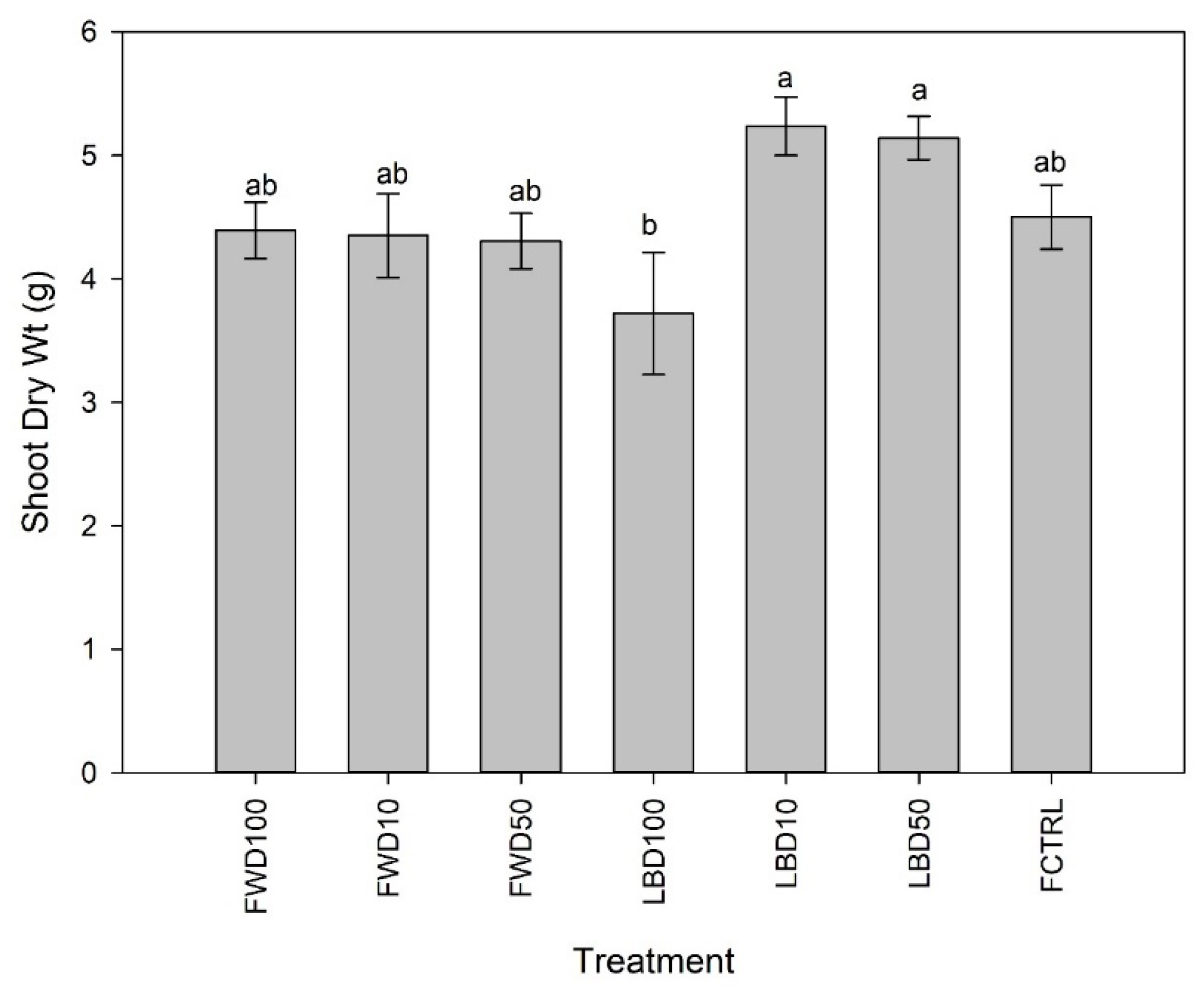
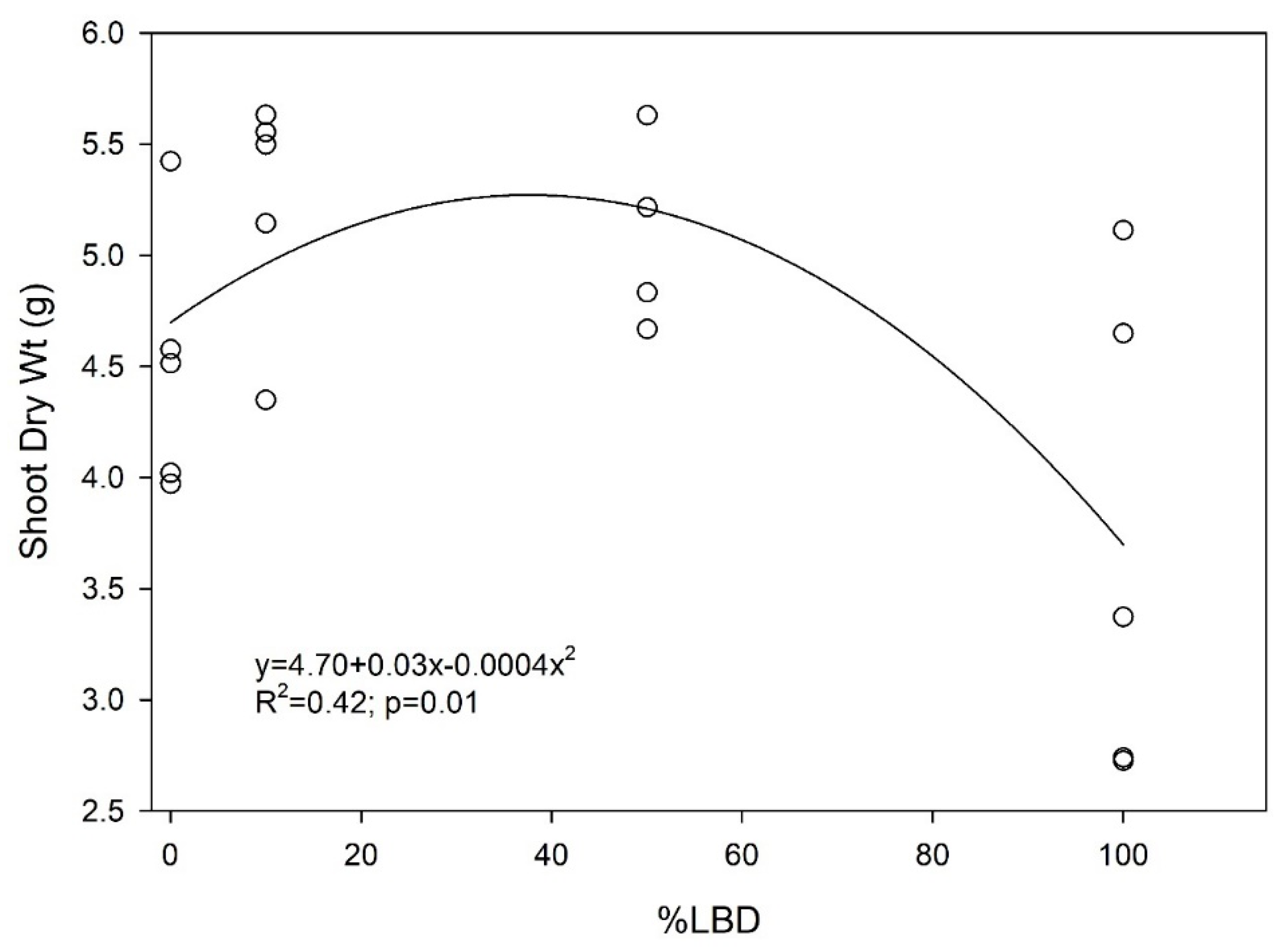
3.1. Digestate Effects on Plant Growth
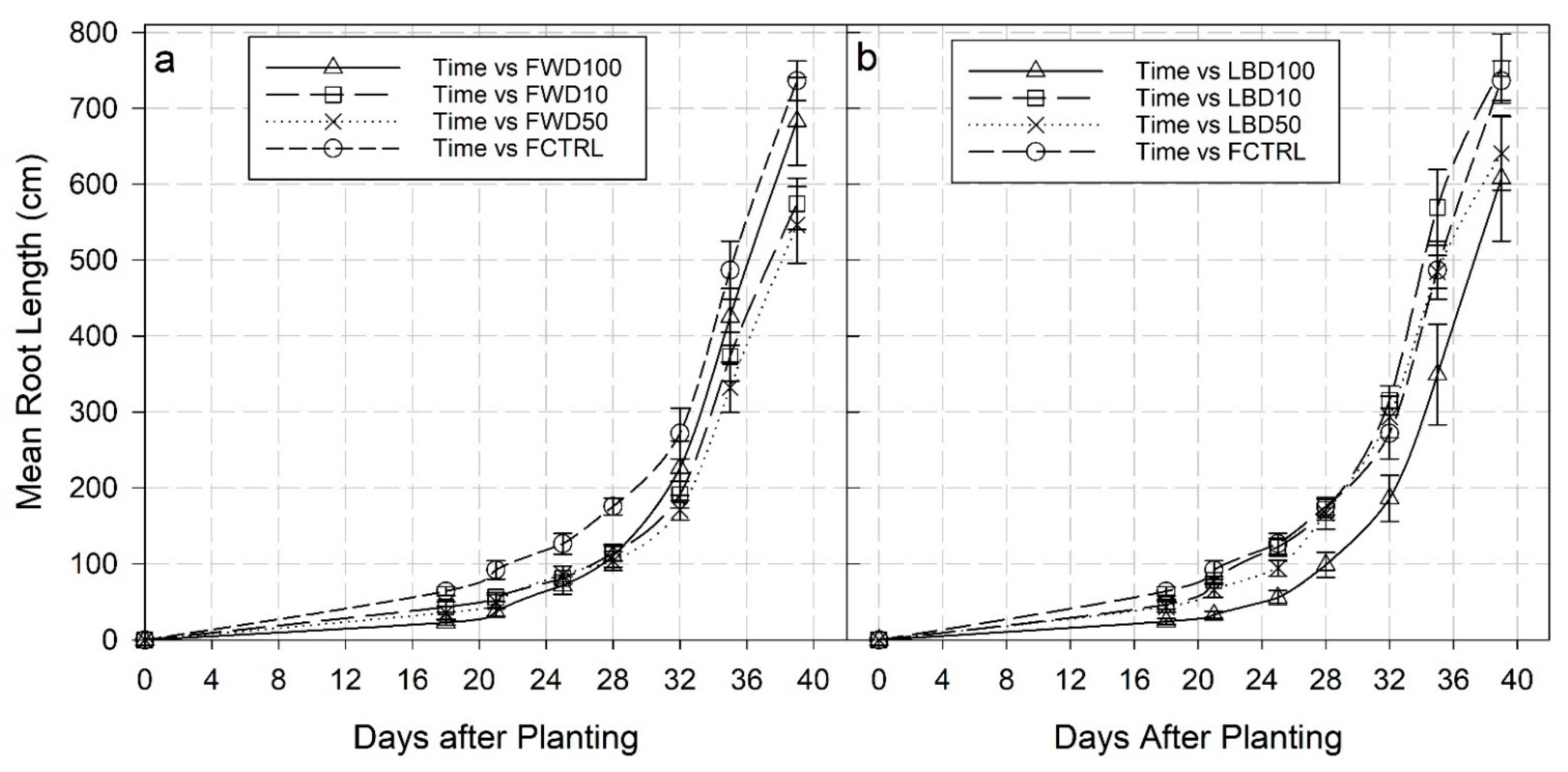
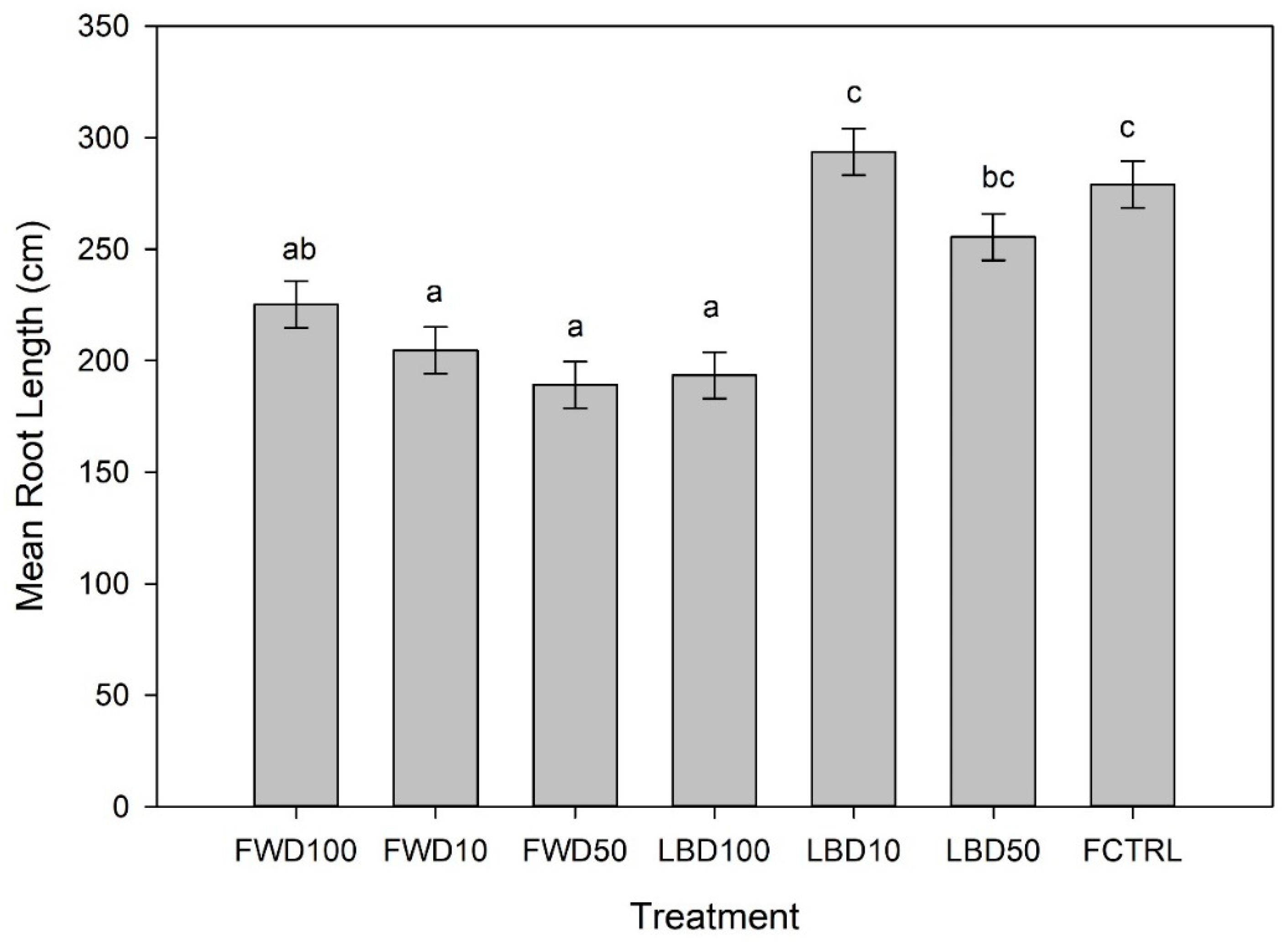
3.2. Root Growth
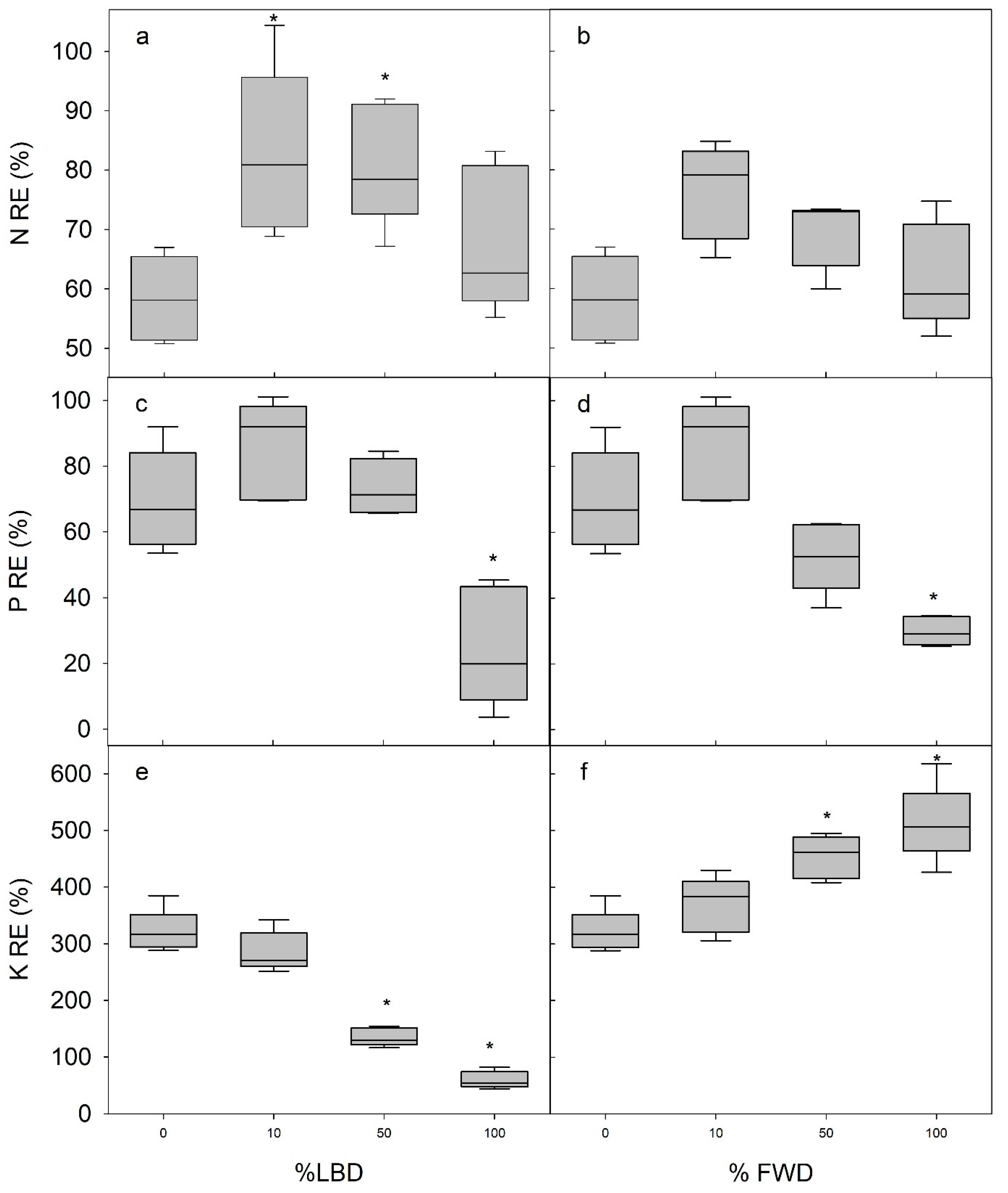
3.3. Nutrient Uptake and Use Efficiency
3.4. Salinity and Fecal Coliforms
4. Discussion
4.1. Plant Growth and Salinity
4.2. Root Growth
4.3. Nutrients and Nutrient Use Efficiency
4.4. Fecal Coliforms
4.5. Implications for Sustainability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Facts and Figures about Materials, Waste and Recycling. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling (accessed on 3 February 2021).
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic characteristics of five different urban waste digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Plaixats, J.; Barcelo, J.; Garcia-Moreno, J. Characterization of the effluent residue from anaerobic digestion of pig excreta for its utilization as fertilizer. Agrochemica 1988, 32, 236–239. [Google Scholar]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldan, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Kostenberg, D.; Marchaim, U.; Watad, A.A.; Epstein, E. Biosynthesis of plant hormones during anaerobic digestion of instant coffee waste. Plant Growth Regul. 1995, 17, 127–132. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.-C.; Du, L. Soilless cultivation for high-quality vegetables with biogas manure in China: Feasibility and benefit analysis. Renew. Agric. Food Syst. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. Evaluation of hormone-like activity of the dissolved organic matter fraction (DOM) of compost and digestate. Sci. Total Environ. 2015, 514, 314–321. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Pang, C.; Dong, R. Anaerobic Digestion and Storage Influence Availability of Plant Hormones in Livestock Slurry. ACS Sustain. Chem. Eng. 2016, 4, 719–727. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Kostenberg, D.; Marchaim, U. Anaerobic digestion and horticultural value of solid waste from manufacture of instant coffee. Environ. Technol. 1993, 14, 973–980. [Google Scholar] [CrossRef]
- Garg, R.N.; Pathak, H.; Das, D.K.; Tomar, R.K. Use of Flyash and Biogas Slurry for Improving Wheat Yield and Physical Properties of Soil. Environ. Monit. Assess. 2005, 107, 1–9. [Google Scholar] [CrossRef]
- Gunnarsson, A.; Bengtsson, F.; Caspersen, S. Use efficiency of nitrogen from biodigested plant material by ryegrass. J. Plant Nutr. Soil Sci. 2010, 173, 113–119. [Google Scholar] [CrossRef]
- Van Eekeren, N.; de Boer, H.; Bloem, J.; Schouten, T.; Rutgers, M.; De Goede, R.; Brussaard, L. Soil biological quality of grassland fertilized with adjusted cattle manure slurries in comparison with organic and inorganic fertilizers. Biol. Fertil. Soils 2009, 45, 595–608. [Google Scholar] [CrossRef]
- Andruschkewitsch, M.; Wachendorf, C.; Wachendorf, M. Effects of digestates from different biogas production systems on above and belowground grass growth and the nitrogen status of the plant-soil-system. Grassl. Sci. 2013, 59, 183–195. [Google Scholar] [CrossRef]
- Wentzel, S.; Joergensen, R.G. Effects of biogas and raw slurries on grass growth and soil microbial indices. J. Plant Nutr. Soil Sci. 2016, 179, 215–222. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient Use Efficiency in Plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Wang, Y.; Thorup-Kristensen, K.; Jensen, L.S.; Magid, J. Vigorous Root Growth Is a Better Indicator of Early Nutrient Uptake than Root Hair Traits in Spring Wheat Grown under Low Fertility. Front. Plant Sci. 2016, 7, 865. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Guo, S.; Wang, Y.; Yi, D.; Wang, J. Poultry biogas slurry can partially substitute for mineral fertilizers in hydroponic lettuce production. Environ. Sci. Pollut. Res. 2019, 26, 659–671. [Google Scholar] [CrossRef]
- Maunuksela, L.; Herranen, M.; Torniainen, M. Quality assessment of biogas plant end products by plant bioassays. Int. J. Environ. Sci. Dev. 2012, 3, 305–310. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Cao, Y.; Liang, P.; Wu, S.; Leung, A.O.W.; Christie, P. Replacement of mineral fertilizers with anaerobically digested pig slurry in paddy fields: Assessment of plant growth and grain quality. Environ. Sci. Pollut. Res. 2017, 24, 8916–8923. [Google Scholar] [CrossRef]
- Tsachidou, B.; Scheuren, M.; Gennen, J.; Debbaut, V.; Toussaint, B.; Hissler, C.; George, I.; Delfosse, P. Biogas residues in substitution for chemical fertilizers: A comparative study on a grassland in the Walloon Region. Sci. Total Environ. 2019, 666, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, G.; Hanlon, E.; Nagata, R.; Snyder, G.; Schueneman, T. Fertilization Recommendations for Crisphead Lettuce Grown on Organic Soils in Florida; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 1994; 10p. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.I. A Method of Estimating the Total Length of Root in a Sample. J. Appl. Ecol. 1966, 3, 139–145. [Google Scholar] [CrossRef]
- Tampio, E.; Ervasti, S.; Rintala, J. Characteristics and agronomic usability of digestates from laboratory digesters treating food waste and autoclaved food waste. J. Clean. Prod. 2015, 94, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rodrígues, A.R.; Carswell, A.M.; Shaw, R.; Hunt, J.; Saunders, K.; Cotton, J.; Chadwick, D.R.; Jones, D.L.; Misselbrook, T.H. Advanced Processing of food waste based digestate for mitigating nitrogen losses in a winter wheat crop. Front. Sustain. Food Syst. 2018, 2. [Google Scholar] [CrossRef]
- Ren, A.T.; Abbott, L.K.; Chen, Y.; Xiong, Y.; Mickan, B.S. Nutrient recovery from anaerobic digestion of food waste: Impacts of digestate on plant growth and rhizosphere bacterial community composition and potential function in ryegrass. Biol. Fertil. Soils 2020, 56, 973–989. [Google Scholar] [CrossRef]
- Fixen, P.; Brenturp, F.; Bruulsema, T.W.; Garcia, F.; Norton, R.; Zingore, S. Nutrient/Fertilizer Use Efficiency: Measurement, Current Situation and Trends. In Managing Water and Fertilizer for Sustainable Agricultural Intensification; International Fertilizer Industry Association (IFA): Paris, France; International Water Management Institute (IWMI): Colombo, Sri Lanka; International Plant Nutrition Institute (IPNI): Peachtree Corners, GA, USA; International Potash Institute (IPI): Horgen, Switzerland, 2015; pp. 8–39. [Google Scholar]
- Sonneveld, C.; Voogt, W. Soil and Substrate Testing to Estimate Nutrient Availability and Salinity Status. In Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Analytical Methods Approved for Compliance Monitoring under the Ground Water Rule. Available online: https://www.epa.gov/sites/production/files/2017-02/documents/gwr_approved_methods.pdf (accessed on 5 February 2021).
- Shannon, M.C.; Grieve, C.M. Tolerance of Vegetable Crops to Salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Toxicity Problems. Available online: http://www.fao.org/3/T0234E/T0234E05.htm (accessed on 23 February 2021).
- Part 503—Standards for the Use or Disposal of Sewage Sludge. Available online: https://www.govinfo.gov/content/pkg/CFR-2018-title40-vol32/xml/CFR-2018-title40-vol32-part503.xml (accessed on 10 February 2021).
- Walsh, J.J.; Jones, D.L.; Edwards-Jones, G.; Williams, A.P. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J. Plant Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Riva, C.; Orzi, V.; Carozzi, M.; Acutis, M.; Boccasile, G.; Lonati, S.; Tambone, F.; D’Imporzano, G.; Adani, F. Short-term experiments using digestate products as substitutes for mineral (N) fertilizer: Agronomic performance, odours, and ammonia emission impacts. Sci. Total Environ. 2016, 547, 206–214. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Febo, P.; La Placa, G.; Mammano, M.M.; Orlando, S. Sustainable valorisation of biowaste for soilless cultivation of Salvia officinalis in a circular bioeconomy. Agronomy 2020, 10, 1158. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of vermicompost, compost and digestate as commercial alternative peat-based substrates on qualitative parameters of Salvia oficinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Shahid, M.; Jaradat, A.A. Barley: A salt tolerant cereal crop. Biosalinity News 2013, 14, 3–4. [Google Scholar]
- De Boer, H.C. Co-digestion of Animal Slurry Can Increase Short-Term Nitrogen Recovery by Crops. J. Environ. Qual. 2008, 37, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Baral, K.R.; Labouriau, R.; Olesen, J.E.; Petersen, S.O. Nitrous oxide emissions and nitrogen use efficiency of manure and digestates applied to spring barley. Agric. Ecosyst. Environ. 2017, 239, 188–198. [Google Scholar] [CrossRef]
- Möller, K.; Stinner, W. Effects of organic wastes digestion for biogas production on mineral nutrient availability of biogas effluents. Nutr. Cycl. Agroecosyst. 2010, 87, 395–413. [Google Scholar] [CrossRef]
- Bachmann, S.; Gropp, M.; Eichler-Löbermann, B. Phosphorus availability and soil microbial activity in a 3 year field experiment amended with digested dairy slurry. Biomass Bioenergy 2014, 70, 429–439. [Google Scholar] [CrossRef]
- Fate of Nutrients and Pathogens During Anaerobic Digestion of Dairy Manure. Available online: https://extension.psu.edu/fate-of-nutrients-and-pathogens-during-anaerobic-digestion-of-dairy-manure (accessed on 3 February 2021).
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Muvhiiwa, R.; Hildebrandt, D.; Chimwani, N.; Ngubevana, L.; Matambo, T. The impact and challenges of sustainable biogas implementation: Moving towards a bio-based economy. Energy Sustain. Soc. 2017, 7, 20. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]

| Parameter | Lignocellulosic-Biomass Feedstock | Food-Waste Feedstock | |
| Total Solids (%) | 91.9, 92.1 | 29.6, 31.8 | |
| Total Nitrogen (mg kg−1) | 7840, 7680 | 30,080, 29,920 | |
| Phosphorus (mg kg−1) | 2300, 2200 | 3600, 3700 | |
| Potassium (mg kg−1) | 14,400, 16,000 | 6000, 6400 | |
| Calcium (mg kg−1) | 1700, 1600 | 1300, 1300 | |
| Magnesium (mg kg−1) | 1800, 2100 | 600, 600 | |
| Sulfur (mg kg−1) | 1200, 1100 | 2400, 2600 | |
| Sodium (mg kg−1) | 180, 150 | 6990, 7470 | |
| Iron (mg kg−1) | 395, 450 | 32, 33 | |
| Zinc (mg kg−1) | 17, 16 | 19, 19 | |
| Copper (mg kg−1) | 3, 4 | 3, 3 | |
| Manganese (mg kg−1) | 27, 24 | 12, 12 | |
| Lignocellulosic-Biomass Digestate (LBD) * | Food-Waste Digestate (FWD) * | FoxFarm GrowBig® | |
| Total Solids (%) | 2.93, 2.81 | 1.21, 1.24 | 0 |
| Electrical Conductivity (EC, mS cm−1) † | 8.34 ± 1.26 | 10.99 ± 0.030 | ** |
| pH † | 8.4 ± 0.102 | 8.6 ± 0.015 | 3.24 |
| Total Nitrogen (mg kg−1) | 740, 680 | 1770, 1800 | 60,000 |
| Ammonium Nitrogen (mg kg−1) | 280, 260 | 1150, 1140 | 29,000 |
| Organic Nitrogen (mg kg−1) | 460, 420 | 620, 660 | 0 |
| Nitrate Nitrogen (mg kg−1) | 0, 0 | 0, 0 | 31,000 |
| Phosphorus (mg kg−1) | 120, 110 | 460, 100 | 17,500 |
| Potassium (mg kg−1) | 910, 920 | 380, 310 | 33,200 |
| Calcium (mg kg−1) | 90, 90 | 250, 60 | 0 |
| Magnesium (mg kg−1) | 110, 110 | 130, 10 | 6000 |
| Sulfur (mg kg−1) | 70, 60 | 410, 40 | ** |
| Sodium (mg kg−1) | 890, 920 | 930, 850 | 230 |
| Chloride (mg kg−1) | 2500, 2500 | 1300, 1200 | 0 |
| Iron (mg kg−1) | 31, 27 | 496, 24 | 1000 |
| Zinc (mg kg−1) | 1.0, 0.9 | 13.1, 0.9 | 500 |
| Copper (mg kg−1) | <1, <1 | 3.7, <1 | 500 |
| Manganese (mg kg−1) | 1, 1 | 1, <1 | 500 |
| Treatment | Description | % N | % N from Digestate | % N from Fertilizer |
|---|---|---|---|---|
| CTRL | No amendments | 0 | 0 | 0 |
| FCTRL | 100% fertilizer control | 100 | 0 | 100 |
| FWD10 | 10% FWD, 90% fertilizer | 100 | 10 | 90 |
| FWD50 | 50% FWD, 50% fertilizer | 100 | 50 | 50 |
| FWD100 | 100% FWD | 100 | 100 | 0 |
| LBD10 | 10% LBD, 90% fertilizer | 100 | 10 | 90 |
| LBD50 | 50% LBD, 50% fertilizer | 100 | 50 | 50 |
| LBD100 | 100% LBD | 100 | 100 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica juncea (Kai Choy). Agronomy 2021, 11, 509. https://doi.org/10.3390/agronomy11030509
Jamison J, Khanal SK, Nguyen NH, Deenik JL. Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica juncea (Kai Choy). Agronomy. 2021; 11(3):509. https://doi.org/10.3390/agronomy11030509
Chicago/Turabian StyleJamison, Jacqueline, Samir Kumar Khanal, Nhu H. Nguyen, and Jonathan L. Deenik. 2021. "Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica juncea (Kai Choy)" Agronomy 11, no. 3: 509. https://doi.org/10.3390/agronomy11030509
APA StyleJamison, J., Khanal, S. K., Nguyen, N. H., & Deenik, J. L. (2021). Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica juncea (Kai Choy). Agronomy, 11(3), 509. https://doi.org/10.3390/agronomy11030509






