Thermomagnesium: A By-Product of Ni Ore Mining as a Clean Fertilizer Source for Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermomagnesium Processing
2.2. Experimental Conditions
2.3. Experimental Design and Treatments
2.4. Soil–Plant Sampling and Analysis
2.5. Statistical Analysis
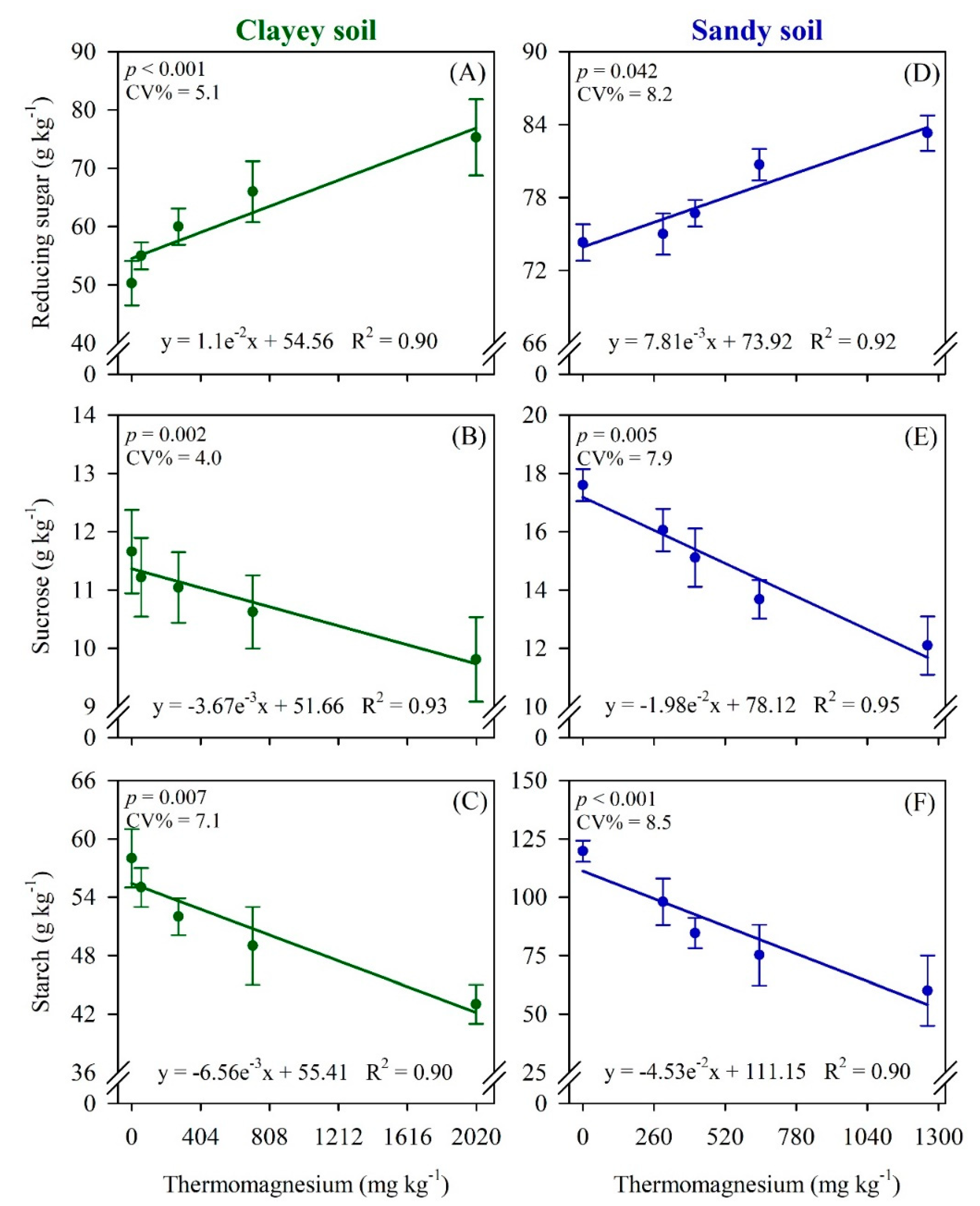
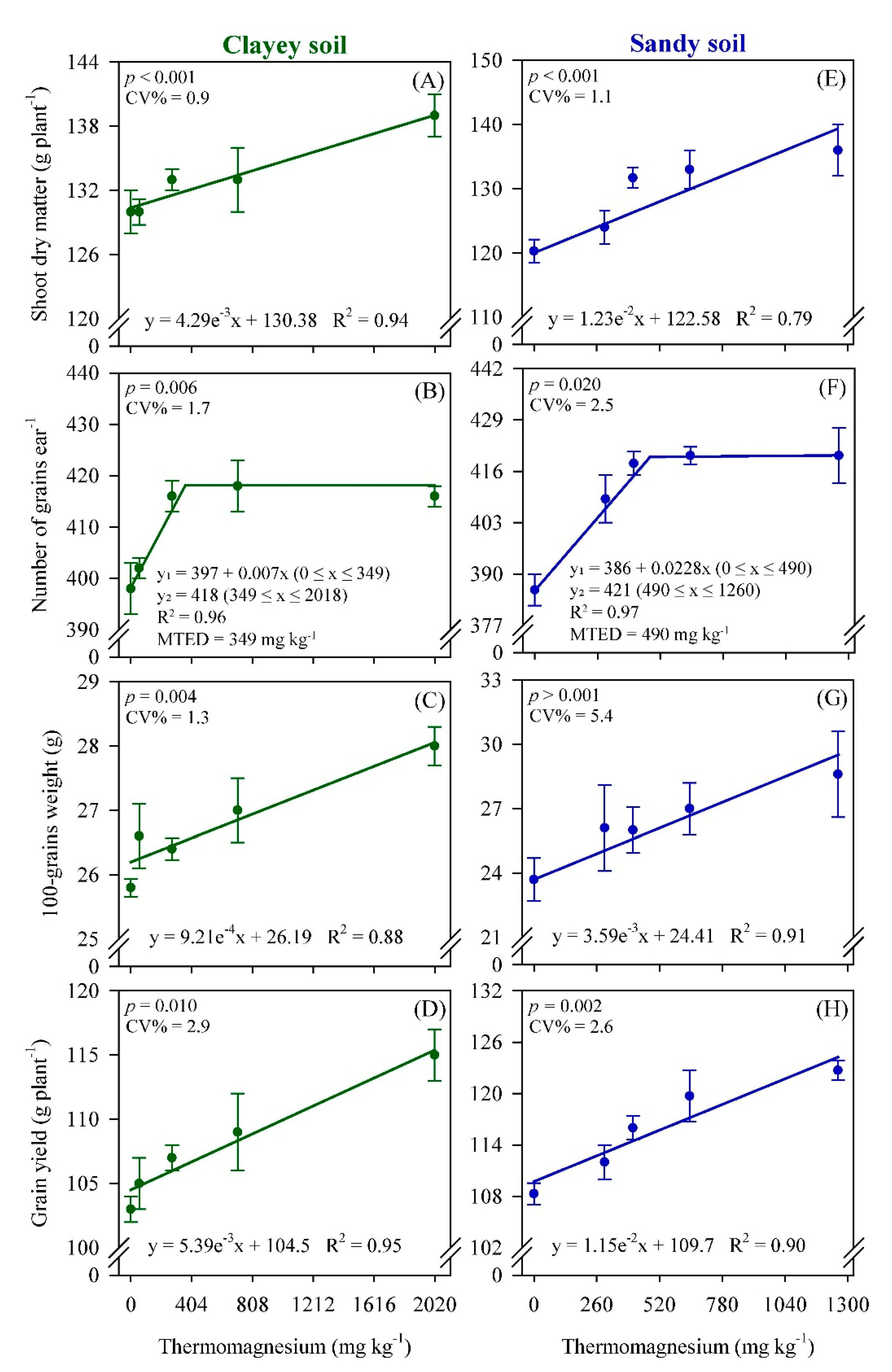
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- FAO. The State of Food Security and Nutrition in the World 2019; FAO: Rome, Italy, 2019. [Google Scholar]
- Sentelhas, P.C.; Battisti, R.; Câmara, G.M.S.; Farias, J.R.B.; Hampf, A.C.; Nendel, C. The soybean yield gap in Brazil-Magnitude, causes and possible solutions for sustainable production. J. Agric. Sci. 2015, 153, 1394–1411. [Google Scholar] [CrossRef]
- Stewart, W.M.; Dibb, D.W.; Johnston, A.E.; Smyth, T.J. The contribution of commercial fertilizer nutrients to food production. Agron. J. 2005, 97, 1–6. [Google Scholar] [CrossRef]
- Rehman, A.; Chandio, A.A.; Hussain, I.; Jingdong, L. Fertilizer consumption, water availability and credit distribution: Major factors affecting agricultural productivity in Pakistan. J. Saudi Soc. Agric. Sci. 2019, 18, 269–274. [Google Scholar] [CrossRef]
- Goulding, K.; Jarvis, S.; Whitmore, A. Optimizing nutrient management for farm systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Heffer, P. Assessment of Fertilizer Use by Crop at the Global Level. Int. Fertil. Ind. Assoc. 2017, 5, 9. [Google Scholar]
- Moretti, L.G.; Lazarini, E.; Bossolani, J.W.; Parente, T.L.; Caioni, S.; Araujo, R.S.; Hungria, M. Can additional inoculations increase soybean nodulation and grain yield? Agron. J. 2018, 110, 715–721. [Google Scholar] [CrossRef]
- Moretti, L.G.; Bossolani, J.W.; Crusciol, C.A.C.; Moreira, A.; Micheri, P.H.; Rossi, R.; Imaizumi, C. Dunite in Agriculture: Physiological Changes, Nutritional Status and Soybean Yield. Commun. Soil Sci. Plant Anal. 2019, 50, 1775–1784. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Moretti, L.G.; Bossolani, J.W.; Moreira, A.; Micheri, P.H.; Rossi, R. Can Dunite Promote Physiological Changes, Magnesium Nutrition and Increased Corn Grain Yield? Commun. Soil Sci. Plant Anal. 2019, 50, 2343–2353. [Google Scholar] [CrossRef]
- Korchagin, J.; Caner, L.; Bortoluzzi, E.C. Variability of amethyst mining waste: A mineralogical and geochemical approach to evaluate the potential use in agriculture. J. Clean. Prod. 2019, 210, 749–758. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Rossi, R.; Moreira, A. Agricultural repurposing of nickel slag residue. J. Plant Nutr. 2020. [Google Scholar] [CrossRef]
- Dalmora, A.C.; Ramos, C.G.; Silva Oliveira, M.L.; Silva Oliveira, L.F.; Homrich Schneider, I.A.; Kautzmann, R.M. Application of andesite rock as a clean source of fertilizer for eucalyptus crop: Evidence of sustainability. J. Clean. Prod. 2020, 256, 120432. [Google Scholar] [CrossRef]
- Das, S.; Galgo, S.J.; Alam, M.A.; Lee, J.G.; Hwang, H.Y.; Lee, C.H.; Kim, P.J. Recycling of ferrous slag in agriculture: Potentials and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 2020, 1–35. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon mechanisms to ameliorate heavy metal stress in plants. Biomed Res. Int. 2018, 2018, 8492898. [Google Scholar] [CrossRef]
- Castro, G.S.A.; Crusciol, C.A.C. Effects of surface application of dolomitic limestone and calcium-magnesium silicate on soybean and maize in rotation with green manure in a tropical region. Bragantia 2015, 74, 311–321. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Garcia, A.; Crusciol, C.A.C.; McCray, J.M.; Nascimento, C.A.C.; Martello, J.M.; de Siqueira, G.F.; Tarumoto, M.B. Magnesium as apromoter of technological quality in sugarcane. J. Soil Sci. Plant Nutr. 2020, 20, 19–30. [Google Scholar] [CrossRef]
- Castro, G.S.A.; Crusciol, C.A.C. Effects of superficial liming and silicate application on soil fertility and crop yield under rotation. Geoderma 2013, 195–196, 234–242. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Tränkner, M.; Jaghdani, S.J. Minimum magnesium concentrations for photosynthetic efficiency in wheat and sunflower seedlings. Plant Physiol. Biochem. 2019, 144, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- Young, R.A. The rietveld method. Int. Union Crystallogr. 1993, 5, 1–38. [Google Scholar]
- Brazil Normative Instruction SDA/MAPA 27/2006. Available online: http://www.mma.gov.br/estruturas/ascom_boletins/_arquivos/03042008_regulamentaprocedimentos.pdf (accessed on 20 June 2020).
- Santos, H.G.; Jacomine, P.T.; Dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System-Portal Embrapa, 5th ed.; Embrapa Solos: Brasília, Brazil, 2018; ISBN 978-85-7035-817-2. [Google Scholar]
- Jahn, R.; Blume, H.P.; Asio, V.B.; Spaargaren, O.; Schad, P. Guidelines for Soil Description; FAO: Rome, Italy, 2006. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Donagema, G.K.; Viana, J.H.M.; Almeida, B.G.; Ruiz, H.A.; Klein, V.A.; Dechen, S.C.F.; Fernandes, R.B.A. Granulometric analysis. In Soil Analysis Methods Manual; Teixeira, P.C., Donagema, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Solos: Brasília, Brazil, 2017; pp. 95–116. [Google Scholar]
- van Raij, B.; Quaggio, J.A.; Cantarella, H.; Abreu, C.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- Cassel, D.K.; Nielsen, D.R. Field Capacity and Available Water Capacity. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Method; Klute, A., Ed.; American Society of Agronomy, Soil Sciense Society of America: Madison, WI, USA, 1986; Volume 2, pp. 901–926. [Google Scholar]
- Moretti, L.G.; Crusciol, C.A.C.; Kuramae, E.E.; Bossolani, J.W.; Moreira, A.; Costa, N.R.; Alves, C.J.; Pascoaloto, I.M.; Rondina, A.B.L.; Hungria, M. Effects of growth-promoting bacteria on soybean root activity, plant development, and yield. Agron. J. 2020, 112, 418–428. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Special Report n. 48. Iowa State University of Science and Technology; Iowa State University: Ames, IA, USA, 1993. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of Nutritional Status of Plants: Principles and Applications, 2nd ed.; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Embrapa. Manual de Métodos de Análise de Solo, 2nd ed.; EMBRAPA-CNPS: Rio de Janeiro, Brazil, 1997; Volume 26. [Google Scholar]
- Korndörfer, G.H.; Pereira, H.S.; Nolla, A. Análise de silício: Solo, planta e fertilizante. In Uberlândia: GPSi-ICIAG-UFU (Boletim técnico, 2); Instituto de Ciências Agrárias, Universidade Federal de Uberlândia: Uberlândia, Brazil, 2004; p. 34. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in …; Olkin, I., Ghurye, S.G., Hoeffding, W., Madow, W.G., Mann, H.B., Eds.; Stanford University Press: Stanford, CA, USA, 1960; Volume 69, pp. 278–292. [Google Scholar]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. London. Ser. A-Math. Phys. Sci. 1937, 160, 268–282. [Google Scholar] [CrossRef]
- SAS Institute Procedure Guide for Personal Computers; Version 9.4; SAS Institute: Cary, NC, USA, 2015.
- Plata, L.G.; Ramos, C.G.; Silva Oliveira, M.L.; Silva Oliveira, L.F. Release kinetics of multi-nutrients from volcanic rock mining by-products: Evidences for their use as a soil remineralizer. J. Clean. Prod. 2021, 279, 123668. [Google Scholar] [CrossRef]
- Aquino, J.M.; Taniguchi, C.A.K.; Magini, C.; Berni, G.V. The potential of alkaline rocks from the Fortaleza volcanic province (Brazil) as natural fertilizers. J. S. Am. Earth Sci. 2020, 103, 102800. [Google Scholar] [CrossRef]
- Dalmora, A.C.; Ramos, C.G.; Plata, L.G.; da Costa, M.L.; Kautzmann, R.M.; Oliveira, L.F.S. Understanding the mobility of potential nutrients in rock mining by-products: An opportunity for more sustainable agriculture and mining. Sci. Total Environ. 2020, 710, 136240. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis from 70 years of research. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Chang, S.X.; Zhang, Q. Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: A global meta-analysis. J. Soils Sediments 2019, 19, 1393–1406. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Lazarini, E.; Santos, F.L.; Sanches, I.R.; Meneghette, H.H.A.; Parra, L.F.; Souza, L.G.M. Surface Reapplication of Lime and Gypsum on Maize Cultivated Sole and Intercropped with Urochloa. Commun. Soil Sci. Plant Anal. 2018, 49, 1855–1868. [Google Scholar] [CrossRef]
- Bossolani, J.W.; dos Santos, F.L.; Meneghette, H.H.A.; Sanches, I.R.; Moretti, L.G.; Parra, L.F.; Lazarini, E. Soybean in Crop Rotation with Maize and Palisade Grass Intercropping Enhances the Long-term Effects of Surface Liming in No-till System. J. Soil Sci. Plant Nutr. 2020, 21, 119–130. [Google Scholar] [CrossRef]
- Jaghdani, S.J.; Jahns, P.; Tränkner, M. Mg deficiency induces photo-oxidative stress primarily by limiting CO2 assimilation and not by limiting photosynthetic light utilization. Plant Sci. 2021, 302, 110751. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany, M.Y.Y.; Al-Qutami, M.A.; Ali, H.M.; Al-Whaibi, M.H.; Al-Wahibi, M.S.; Alharby, H.F. Mitigation of adverse effects of heat stress on Vicia faba by exogenous application of magnesium. Saudi J. Biol. Sci. 2018, 25, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Preston, H.A.F.; Henrique de Sousa Nunes, G.; Preston, W.; Barbosa de Souza, E.; de Lima Ramos Mariano, R.; Datnoff, L.E.; Araújo do Nascimento, C.W. Slag-based silicon fertilizer improves the resistance to bacterial fruit blotch and fruit quality of melon grown under field conditions. Crop Prot. 2020, 105460, in press. [Google Scholar] [CrossRef]
- Laxmanarayanan, M.; Prakash, N.B.; Dhumgond, P.; Ashrit, S. Slag-Based Gypsum as a Source of Sulphur, Calcium and Silicon and Its Effect on Soil Fertility and Yield and Quality of Groundnut in Southern India. J. Soil Sci. Plant Nutr. 2020, 20, 2698–2713. [Google Scholar]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Keeping, M.G. Uptake of silicon by sugarcane from applied sources may not reflect plant-available soil silicon and total silicon content of sources. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef]
- Haynes, R.J. Significance and Role of Si in Crop Production. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 146, pp. 83–166. [Google Scholar]
- Yamaji, N.; Chiba, Y.; Mitani-Ueno, N.; Ma, J.F. Functional characterization of a silicon transporter gene implicated in silicon distribution in barley. Plant Physiol. 2012, 160, 1491–1497. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Deshmukh, R.; Bélanger, R.R. Molecular evolution of aquaporins and silicon influx in plants. Funct. Ecol. 2016, 30, 1277–1285. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Lyons, G.; English, P.; Guppy, C.N. Silicon nutrition of rice is affected by soil pH, weathering and silicon fertilisation. J. Plant Nutr. Soil Sci. 2011, 174, 437–446. [Google Scholar] [CrossRef]
- Alcarde, J.C. Corretivos da Acidez dos Solos: Características e Interpretações Técnicas; Associação Nacional para Difusão de Adubos ANDA: São Paulo, Brazil, 1992. [Google Scholar]
- Crusciol, C.A.C.; Artigiani, A.C.C.A.; Arf, O.; Carmeis Filho, A.C.A.; Soratto, R.P.; Nascente, A.S.; Alvarez, R.C.F. Soil fertility, plant nutrition, and grain yield of upland rice affected by surface application of lime, silicate, and phosphogypsum in a tropical no-till system. Catena 2016, 137, 87–99. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Rossato, O.B.; Foltran, R.; Martello, J.M.; Nascimento, C.A.C. do Soil fertility, sugarcane yield affected by limestone, silicate, and gypsum application. Commun. Soil Sci. Plant Anal. 2017, 48, 2314–2323. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Jalali, M. Effect of water quality on the leaching of potassium from sandy soil. J. Arid Environ. 2007, 68, 624–639. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Crusciol, C.A.C.; Merloti, L.F.; Moretti, L.G.; Costa, N.R.; Tsai, S.M.; Kuramae, E.E. Long-term lime and gypsum amendment increase nitrogen fixation and decrease nitrification and denitrification gene abundances in the rhizosphere and soil in a tropical no-till intercropping system. Geoderma 2020, 375, 114476. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Lindsay, W.L. Inorganic equilibria affecting micronutrients in soils. Micronutr. Agric. 1991, 4, 89–112. [Google Scholar]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Garten, C.T. Correlations between concentrations of elements in plants. Nature 1976, 261, 686–688. [Google Scholar] [CrossRef]
- Jonik, C.; Sonnewald, U.; Hajirezaei, M.; Flügge, U.; Ludewig, F. Simultaneous boosting of source and sink capacities doubles tuber starch yield of potato plants. Plant Biotechnol. J. 2012, 10, 1088–1098. [Google Scholar] [CrossRef]
- Prathap, V.; Ali, K.; Singh, A.; Vishwakarma, C.; Krishnan, V.; Chinnusamy, V.; Tyagi, A. Starch accumulation in rice grains subjected to drought during grain filling stage. Plant Physiol. Biochem. 2019, 142, 440–451. [Google Scholar]
- Li, Q.; Du, L.; Feng, D.; Ren, Y.; Li, Z.; Kong, F.; Yuan, J. Grain-filling characteristics and yield differences of maize cultivars with contrasting nitrogen efficiencies. Crop J. 2020, 8, 990–1001. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Jin, M.; Yu, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J. Cereal Sci. 2012, 55, 331–336. [Google Scholar] [CrossRef]




| Mineral Species | Chemical Formula | Abundance | Unit |
|---|---|---|---|
| Chemical composition | |||
| Arsenic | As3+ | <5 | mg·kg−1 |
| Cadmium | Cd2+ | <5 | mg·kg−1 |
| Lead | Pb2+ | <5 | mg·kg−1 |
| Mercury | Hg2+ | <0.20 | mg·kg−1 |
| Selenium | Se2+ | <0.10 | mg·kg−1 |
| Chromium | Cr3+ | 0.88 | % |
| Nickel | Ni2+ | 0.11 | % |
| Mineralogical phases | |||
| Amorphous phase | - | 74 | % |
| Forsterite | Mg1.8Fe0.2SiO4 | 24 | % |
| Lizardite | Mg3Si2(OH)4O5 | 2 | % |
| Mineral groups | |||
| Magnesium oxide | MgO | 28 | % |
| Silicon dioxide | SiO2 | 48 | % |
| Soil Properties | Unit | Clayey | Sandy | |
|---|---|---|---|---|
| Value | ||||
| Clay | g·kg−1 | 602 | 251 | |
| Silt | g·kg−1 | 281 | 25 | |
| Sand | g·kg−1 | 117 | 724 | |
| Bulk density | g·cm−3 | 1.19 | 1.26 | |
| pH (CaCl2) | – | 5.20 | 5.40 | |
| Soil organic matter | g·kg−1 | 25.0 | 13.0 | |
| Available phosphorus (P resin) | mg·kg−1 | 6.00 | 3.00 | |
| Exchangeable | Calcium (Ca2+ resin) | mmolc·kg−1 | 35.0 | 18.0 |
| Mg (Mg2+ resin) | mmolc·kg−1 | 8.00 | 2.00 | |
| Potassium (K+ resin) | mmolc kg−1 | 0.70 | 0.50 | |
| Aluminum (Al3+ KCl) | mmolc·kg−1 | 0.00 | 0.00 | |
| Potential acidity (H+Al) | mmolc·kg−1 | 29.0 | 18.0 | |
| S–Sulfate (S–SO42− Ca(H2PO4)2) | mg·kg−1 | 9.00 | 6.00 | |
| Boron (B Hot water) | mg·kg−1 | 0.40 | 0.30 | |
| Copper (Cu DTPA-TEA 1) | mg·kg−1 | 4.00 | 1.00 | |
| Iron (Fe DTPA-TEA) | mg·kg−1 | 12.0 | 17.0 | |
| Manganese (Mn DTPA-TEA) | mg·kg−1 | 13.2 | 2.20 | |
| Zinc (Zn DTPA–TEA) | mg·kg−1 | 0.90 | 0.40 | |
| Base saturation (BS) | % | 60.0 | 53.3 | |
| Cation exchange capacity (CEC pH 7.0) | mmolc·kg−1 | 72.7 | 38.5 | |
| Nutrient | Dose | Unit | Source |
|---|---|---|---|
| N | 50 | mg·kg−1 | NH4NO3 |
| 150 1 | mg·kg−1 | NH4NO3 | |
| P | 200 | mg·kg−1 | Ca(H2PO4)2 |
| K | 50 | mg·kg−1 | KCL |
| 100 1 | mg·kg−1 | K2SO4 | |
| Zn | 5.0 | mg·kg−1 | ZnSO4.7H2O |
| Mn | 5.0 | mg·kg−1 | MnSO4.3H2O |
| Fe | 2.5 | mg·kg−1 | FeSO4.2H2O |
| Cu | 1.5 | mg·kg−1 | CuSO4.7H2O |
| B | 0.5 | mg·kg−1 | H3BO3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossolani, J.W.; Moretti, L.G.; Portugal, J.R.; Rossi, R.; Crusciol, C.A.C. Thermomagnesium: A By-Product of Ni Ore Mining as a Clean Fertilizer Source for Maize. Agronomy 2021, 11, 525. https://doi.org/10.3390/agronomy11030525
Bossolani JW, Moretti LG, Portugal JR, Rossi R, Crusciol CAC. Thermomagnesium: A By-Product of Ni Ore Mining as a Clean Fertilizer Source for Maize. Agronomy. 2021; 11(3):525. https://doi.org/10.3390/agronomy11030525
Chicago/Turabian StyleBossolani, João William, Luiz Gustavo Moretti, José Roberto Portugal, Ricardo Rossi, and Carlos Alexandre Costa Crusciol. 2021. "Thermomagnesium: A By-Product of Ni Ore Mining as a Clean Fertilizer Source for Maize" Agronomy 11, no. 3: 525. https://doi.org/10.3390/agronomy11030525
APA StyleBossolani, J. W., Moretti, L. G., Portugal, J. R., Rossi, R., & Crusciol, C. A. C. (2021). Thermomagnesium: A By-Product of Ni Ore Mining as a Clean Fertilizer Source for Maize. Agronomy, 11(3), 525. https://doi.org/10.3390/agronomy11030525






