Physiological and Yield Responses of Green-Shelled Beans (Phaseolus vulgaris L.) Grown under Restricted Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Open Field Pot Experiment
2.3. Field Experiment
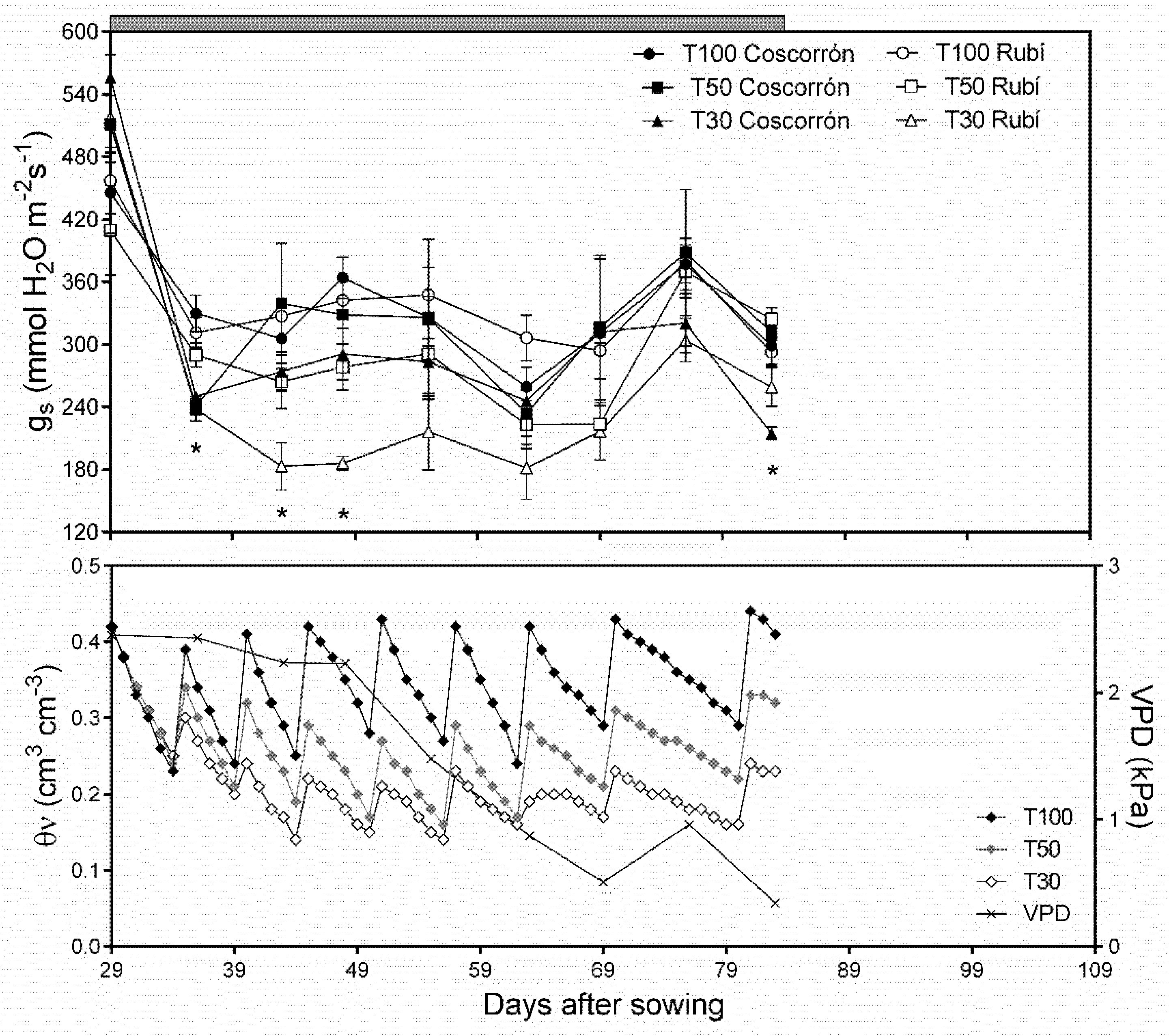
2.4. Evaluated Parameters
2.5. Statistical Analysis
3. Results
3.1. Yield and Yield Components
3.2. Effect of Water Stress on Physiological Parameters
3.3. Water Use Efficiency Estimation for Pot and Field Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwember, A.R.; Schulze, J.; Del Pozo, A.; Cabeza, R.A. Regulation of Symbiotic Nitrogen Fixation in Legume Root Nodules. Plants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bascur, G. Calidad Culinaria del Poroto Verde y Granado: Aspectos relevantes para el desarrollo de variedades mejoradas para el mercado fresco. Tierra Adentro 2011, 95, 31–37. [Google Scholar]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)–model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.; Silva, H.; Ledent, J.; Pinto, M. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Toaldo, D.; De Morais, P.P.P.; Battilana, J.; Coimbra, J.L.M.; Guidolin, A.F. Selection in early generations and the occurrence of heterosis for the character root distribution. Euphytica 2012, 190, 335–344. [Google Scholar] [CrossRef]
- Boutraa, T.; Sanders, F.E. Influence of Water Stress on Grain Yield and Vegetative Growth of Two Cultivars of Bean (Phaseolus vulgaris L.). J. Agron. Crop. Sci. 2001, 187, 251–257. [Google Scholar] [CrossRef]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic Stress Responses in Legumes: Strategies Used to Cope with Environmental Challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Migliorini, P.; Spagnolo, S.; Torri, L.; Arnoulet, M.; Lazzerini, G.; Ceccarelli, S. Agronomic and quality characteristics of old, modern and mixture wheat varieties and landraces for organic bread chain in diverse environments of northern Italy. Eur. J. Agron. 2016, 79, 131–141. [Google Scholar] [CrossRef]
- Ficiciyan, A.; Loos, J.; Sievers-Glotzbach, S.; Tscharntke, T. More than Yield: Ecosystem Services of Traditional versus Modern Crop Varieties Revisited. Sustain. J. Rec. 2018, 10, 2834. [Google Scholar] [CrossRef] [Green Version]
- Tsegaye, B.; Berg, T. Utilization of durum wheat landraces in East Shewa, central Ethiopia: Are home uses an incentive for on-farm conservation? Agric. Hum. Values 2007, 24, 219–230. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Leone, M.; Sunseri, F.; Bacchi, M.; Sorgona, A. Root Phenotyping For Drought Tolerance in Bean Landraces From Calabria (Italy). J. Agron. Crop. Sci. 2015, 202, 1–12. [Google Scholar] [CrossRef]
- Cortés, A.J.; Blair, M.W. Genotyping by Sequencing and Genome–Environment Associations in Wild Common Bean Predict Widespread Divergent Adaptation to Drought. Front. Plant Sci. 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought Stress in Grain Legumes during Reproduction and Grain Filling. J. Agron. Crop. Sci. 2016, 203, 81–102. [Google Scholar] [CrossRef]
- Santos, M.G.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C. Photosynthetic parameters and leaf water potential of five common bean genotypes under mild water deficit. Biol. Plant. 2009, 53, 229–236. [Google Scholar] [CrossRef]
- Lynch, J.; Van Beem, J.J. Growth and Architecture of Seedling Roots of Common Bean Genotypes. Crop. Sci. 1993, 33, 1253–1257. [Google Scholar] [CrossRef]
- Casaroli, D.; Jong van Lier, Q. Critérios para determinação da capacidade de vaso. Rev. Bras. Cienc. Solo 2008, 32, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO irrigation and drainage paper 56; FAO: Rome, Italy, 1998; p. D05109. [Google Scholar]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Rossi, A.M.; Hirmas, D.R.; Graham, R.C.; Sternberg, P.D. Bulk Density Determination by Automated Three-Dimensional Laser Scanning. Soil Sci. Soc. Am. J. 2008, 72, 1591–1593. [Google Scholar] [CrossRef] [Green Version]
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Estimates by Texture and Organic Matter for Hydrologic Solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Faiguenbaum, H. Labranza, Siembra y Producción de los Principales Cultivos de Chile; Facultad de Ciencias Agrícolas y Forestales, Universidad de Chile: Santiago, Chile, 2003; p. 760. [Google Scholar]
- Meyer, W.S.; Reicosky, D. Enclosing leaves for water potential measurement and its effect on interpreting soil-induced water stress. Agric. For. Meteorol. 1985, 35, 187–192. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute Inc. Using JMP 11. 2013. Available online: http://support.sas.com/documentation/onlinedoc/jmp/11/UsingJMP.pdf (accessed on 12 March 2021).
- Beebe, S.; Ramírez, J.; Jarvis, A.; Rao, I.M.; Mosquera, G.; Bueno, J.M.; Blair, M.W. Genetic improvement of common beans and the challenges of climate change. In Crop Adaptation to Climate Change; Yadav, S., Redden, R., Hatfield, J., Lotze-Campen, H., Hall, A., Eds.; John Wiley & Sons. Ltd: Chichester, UK, 2011; pp. 356–369. [Google Scholar]
- Muñoz-Perea, C.G.; Allen, R.G.; Westermann, D.T.; Wright, J.L.; Singh, S.P. Water use efficiency among dry bean landraces and cultivars in drought-stressed and non-stressed environments. Euphytica 2007, 155, 393–402. [Google Scholar] [CrossRef]
- Sezen, S.M.; Yazar, A.; Akyildiz, A.; Dasgan, H.Y.; Gencel, B. Yield and quality response of drip irrigated green beans under full and deficit irrigation. Sci. Hortic. 2008, 117, 95–102. [Google Scholar] [CrossRef]
- Beauzamy, L.; Nakayama, N.; Boudaoud, A. Flowers under pressure: Ins and outs of turgor regulation in development. Ann. Bot. 2014, 114, 1517–1533. [Google Scholar] [CrossRef] [PubMed]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Emam, Y.; Shekoofa, A.; Salehi, F.; Jalali, A.H.; Pessarakli, M. Drought stress effects on two common bean cultivars with contrasting growth habits. Arch. Agron. Soil Sci. 2012, 58, 527–534. [Google Scholar] [CrossRef]
- González, A.M.; Yuste-Lisbona, F.J.; Saburido, S.; Bretones, S.; De Ron, A.M.; Lozano, R.; Santalla, M. Major Contribution of Flowering Time and Vegetative Growth to Plant Production in Common Bean As Deduced from a Comparative Genetic Mapping. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Emam, Y.; Seghatoleslami, M.J. Crop Yield: Physiology and Processes, 1st ed.; Shiraz University Inc.: Shiraz, Iran, 2005; p. 593. [Google Scholar]
- Tardieu, F. Plant tolerance to water deficit: Physical limits and possibilities for progress. C. R. Geosci. 2005, 337, 57–67. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin, Germany, 2001; p. 513. [Google Scholar]
- Nleya, T.M.; Slinkard, A.E.; Vandenberg, A. Differential performance of pinto bean under varying levels of soil moisture. Can. J. Plant Sci. 2001, 81, 233–239. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of Photosynthesis of C3 Plants in Response to Progressive Drought: Stomatal Conductance as a Reference Parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Escalona, J.M.; Galmés, J.; Gulías, J.; Medrano, H. Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Barradas, V.L.; Jones, H.G.; Clark, J.A. Stomatal responses to changing irradiance in Phaseolus vulgaris L. J. Exp. Bot. 1994, 45, 931–936. [Google Scholar] [CrossRef]
- Rosales, M.A.; Ocampo, E.; Rodríguez-Valentín, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological analysis of common bean (Phaseolus vulgaris L.) cultivars uncovers characteristics related to terminal drought resistance. Plant Physiol. Biochem. 2012, 56, 24–34. [Google Scholar] [CrossRef]
- Zlatev, Z.S.; Yordanov, I.T. Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. Bulg. J. Plant Physiol. 2005, 30, 3–18. [Google Scholar]
- Pastenes, C.; Pimentel, P.; Lillo, J. Leaf movements and photoinhibition in relation to water stress in field-grown beans. J. Exp. Bot. 2004, 56, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wentworth, M.; Murchie, E.H.; Gray, J.E.; Villegas, D.; Pastenes, C.; Pinto, M.; Horton, P. Differential adaptation of two varieties of common bean to abiotic stress II. Acclimation of photosynthesis. J. Exp. Bot. 2006, 57, 699–709. [Google Scholar] [CrossRef] [Green Version]
- Webber, H.; Madramootoo, C.; Bourgault, M.; Horst, M.; Stulina, G.; Smith, D. Water use efficiency of common bean and green gram grown using alternate furrow and deficit irrigation. Agric. Water Manag. 2006, 86, 259–268. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Chlorophyll α Fluorescence Parameters as an Indicator to Identify Drought Susceptibility in Common Bush Bean. Agronomy 2019, 9, 526. [Google Scholar] [CrossRef] [Green Version]

| Treatments | Seed Yield (g m−2) | 100-Seed Weight (g) | Biomass (g Plant−1) | Pods Length (cm) | N° (Number) Pods Plant−1 | N° Seeds Pods−1 | Harvest Index (HI) |
|---|---|---|---|---|---|---|---|
| Water stress (WS) | |||||||
| T100 | 647.4 ± 41.9 a | 89.8 ns | 28.5 ± 2.9 a | 10.9 ± 0.1 ns | 18.9 ± 1.2 a | 4.4 ± 0.1 a | 0.50 ± 0.04 a |
| T50 | 409.1 ± 53.9 b | 92.2 ns | 21.5 ± 2.3 a | 10.7 ± 0.1 ns | 14.4 ± 1.3 b | 4.0 ± 0.2 ab | 0.42 ± 0.06 b |
| T30 | 235.9 ± 40.0 c | 92.2 ns | 15.1 ± 2.0 b | 10.6 ± 0.2 ns | 9.2 ± 1.4 c | 3.7 ± 0.1 b | 0.31 ± 0.01 c |
| Cultivar (C) | |||||||
| Cimarrón | 467.3 ± 62.5 ns | 92.7 ns | 24.1 ± 2.8 ns | 10.8 ± 0.1 ns | 15.4 ± 1.6 ns | 4.1 ± 0.1 ns | 0.38 ± 0.25 ns |
| L. Cimarrón | 394.3 ± 51.2 ns | 89.4 ns | 19.2 ± 2.7 ns | 10.7 ± 0.1 ns | 12.9 ± 1.2 ns | 4.0 ± 0.1 ns | 0.43 ± 0.31 ns |
| IR × C | ns | - | ns | - | ns | ns | ns |
| Treatments | Seed Yield (g m−2) | 100-Seed Weight (g) | Biomass (g Plant−1) | Pods Length (cm) | N° Pods Plant−1 | N° Seeds Pods−1 | HI |
|---|---|---|---|---|---|---|---|
| Water stress (WS) | |||||||
| T100 | 398.0 ± 55.9 a | 92.8 ns | 16.6 ± 1.6 a | 11.8 ± 0.1 a | 12.6 ± 1.5 a | 4.1 ± 0.2 a | 0.42 ± 0.23 ns |
| T50 | 223.9 ± 27.8 b | 84.7 ns | 10.3 ± 1.1 b | 11.2 ± 0.1 b | 8.6 ± 0.8 b | 3.7 ± 0.2 ab | 0.39 ± 0.20 ns |
| T30 | 140.3 ± 18.1 c | 79.9 ns | 5.9 ± 0.9 b | 10.8 ± 0.2 b | 6.4 ± 0.8 b | 3.3 ± 0.2 b | 0.37 ± 0.18 ns |
| Cultivar (C) | |||||||
| Coscorrón | 256.4 ± 46.8 ns | 84.0 ns | 11.8 ± 1.7 ns | 11.3 ± 0.1 ns | 9.8 ± 1.2 ns | 3.4 ± 0.2 b | 0.38 ± 0.28 ns |
| Rubí | 251.7 ± 35.6 ns | 85.6 ns | 10.1 ± 1.4 ns | 11.5 ± 0.1 ns | 8.6 ± 1.0 ns | 4.0 ± 0.2 a | 0.41 ± 0.23 ns |
| IR × C | ns | - | ns | ns | ns | ns | - |
| Treatments | Seed Yield (Kg m−2) | 100-Seed Weight (g) | Biomass (g Plant−1) | Pods Length (cm) | N° Pods Plant−1 | N° Seeds Pods−1 | HI |
|---|---|---|---|---|---|---|---|
| Water stress (WS) | |||||||
| I100 | 1.99 ± 0.3 ns | 106.4 a | 369 ± 46.1 a | 13.5 ± 0.1 a | 16.4 ± 2.3 ns | 5.7 ± 0.1 a | 0.38 ± 0.01 ns |
| I40 | 1.64 ± 0.2 ns | 93.5 b | 297 ± 30.2 b | 13.3 ± 0.1 b | 16.6 ± 1.9 ns | 5.4 ± 0.1 b | 0.39 ± 0.02 ns |
| Cultivar (C) | |||||||
| Cimarrón | 1.65 ± 0.1 ns | 88.2 c | 322 ± 7.3 ns | 12.7 ± 0.1 b | 16.6 ± 1.2 ns | 5.6 ± 0.1 ns | 0.36 ± 0.01 ns |
| L. Cimarrón | 1.81 ± 0.4 ns | 97.5 b | 335 ± 63.5 ns | 12.8 ± 0.1 b | 17.9 ± 3.4 ns | 5.7 ± 0.1 ns | 0.38 ± 0.01 ns |
| Coscorrón | 1.91 ± 0.2 ns | 108.3 a | 336 ± 55.3 ns | 14.1 ± 0.1 a | 16.4 ± 1.6 ns | 5.4 ± 0.1 ns | 0.41 ± 0.01 ns |
| Rubí | 1.89 ± 0.3 ns | 106.0 a | 341 ± 55.3 ns | 14.1 ± 0.1 a | 14.9 ± 2.1 ns | 5.5 ± 0.1 ns | 0.40 ± 0.03 ns |
| IR × C | - | ns | ns | ns | - | ns | - |
| Treatments | gs (mmol m−2 s−1) | Fv/Fm |
|---|---|---|
| Water stress (WS) | ||
| I100 | 205.5 ± 10.6 a | 0.72 ± 0.04 a |
| I40 | 161.6 ± 13.3 b | 0.59 ± 0.09 b |
| Cultivar (C) | ||
| Cimarrón | 206.9 ± 12.4 a | 0.63 ± 0.08 ns |
| L. Cimarrón | 181.2 ± 8.7 ab | 0.64 ± 0.09 ns |
| Coscorrón | 166.7 ± 14.0 b | 0.65 ± 0.03 ns a |
| Rubí | 178.8 ± 12.7 b | 0.69 ± 0.06 ns |
| IR × C | ns | ns |
| Cultivar | Irrigation Regime Treatment | Total ETc (mm) | WUEb (g mm−1) | WUEg (g mm−1) |
|---|---|---|---|---|
| Cimarrón | T100 | 418.6 | 0.192 ± 0.022 A a ab | 0.055 ± 0.005 A a |
| T50 | 387.7 | 0.159 ± 0.026 A a | 0.038 ± 0.010 B a | |
| T30 | 341.3 | 0.117 ± 0.031 B a | 0.028 ± 0.008 B a | |
| L. Cimarrón | T100 | 423.7 | 0.168 ± 0.015 A a | 0.049 ± 0.005 A a |
| T50 | 375.8 | 0.128 ± 0.003 A a | 0.034 ± 0.002 B a | |
| T30 | 351.4 | 0.088 ± 0.002 B a | 0.018 ± 0.001 B a | |
| Rubí | T100 | 363.2 | 0.118 ± 0.022 A a | 0.035 ± 0.007 A a |
| T50 | 272.6 | 0.117 ± 0.031 AB a | 0.029 ± 0.002 AB a | |
| T30 | 225.3 | 0.082 ± 0.015 B a | 0.021 ± 0.003 B a | |
| Coscorrón | T100 | 359.2 | 0.142 ± 0.021 A a | 0.039 ± 0.008 A a |
| T50 | 269.8 | 0.110 ± 0.018 AB a | 0.026 ± 0.007 AB a | |
| T30 | 243.6 | 0.083 ± 0.016 B a | 0.019 ± 0.004 B a |
| Cultivar | Irrigation Regime Treatment | Total ETc (mm) | WUEb (g mm−1) | WUEg (g mm−1) |
|---|---|---|---|---|
| Cimarrón | I100 | 191.4 | 16.506 ± 0.650 B a | 5.771 ± 0.495 B a |
| I40 | 76.56 | 32.119 ± 0.230 A a | 12.236 ± 0.245 A a | |
| L. Cimarrón | I100 | 191.4 | 18.916 ± 4.947 B a | 7.383 ± 2.322 B a |
| I40 | 76.56 | 30.260 ± 3.310 A a | 11.540 ± 1.353 A a | |
| Rubí | I100 | 191.4 | 22.122 ± 3.613 B a | 8.700 ± 1.224 B a |
| I40 | 76.56 | 25.632 ± 4.128 A a | 10.239 ± 1.958 A a | |
| Coscorrón | I100 | 191.4 | 21.622 ± 0.801 B a | 8.621 ± 0.307 B a |
| I40 | 76.56 | 25.644 ± 3.968 A a | 10.738 ± 1.883 A a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, K.; Schwember, A.R.; Machado, D.; Ozores-Hampton, M.; Gil, P.M. Physiological and Yield Responses of Green-Shelled Beans (Phaseolus vulgaris L.) Grown under Restricted Irrigation. Agronomy 2021, 11, 562. https://doi.org/10.3390/agronomy11030562
Campos K, Schwember AR, Machado D, Ozores-Hampton M, Gil PM. Physiological and Yield Responses of Green-Shelled Beans (Phaseolus vulgaris L.) Grown under Restricted Irrigation. Agronomy. 2021; 11(3):562. https://doi.org/10.3390/agronomy11030562
Chicago/Turabian StyleCampos, Karen, Andrés R. Schwember, Daniel Machado, Mónica Ozores-Hampton, and Pilar M. Gil. 2021. "Physiological and Yield Responses of Green-Shelled Beans (Phaseolus vulgaris L.) Grown under Restricted Irrigation" Agronomy 11, no. 3: 562. https://doi.org/10.3390/agronomy11030562





