Abstract
Black rot disease, caused by the bacterium Xanthomonas campestris pv. campestris (Pammel) Dowson (Xcc), causes important yield losses in Brassica oleracea L. crops worldwide. In temperate areas, yield losses are mostly due to the discarding of those plants showing chlorotic and necrotic lesions, since they may be unmarketable. However, the biomass loss caused by the diversion of resources from the primary to the secondary defense metabolism could also affect the final crop yield. In this work, we have focused on studying the impact of Xcc race 1 invasion on the biomass production of young and adult B. oleracea plants. The results have shown that Xcc infection reduces biomass and photosynthesis in the aerial parts of seedlings and modifies their water percentage in a time-dependent manner. When adult plants were inoculated in the field, no effect was detected on the leaves or the biomass of marketable products. This was probably due to a better immune response when compared to seedlings. Since the first developmental stages of B. oleracea crops are especially vulnerable to Xcc, plant disease control should be increased in order to avoid yield losses of marketable products at the adult stage.
1. Introduction
The genus Brassica belongs to Brassicaceae, family [1,2] which includes a wide range of horticultural crops, many of them having economic significance and being extensively consumed as commodities worldwide [3]. Consumed parts of Brassica crops include the root, stems, leaves, and terminal and axillary buds [4]. According to Food and Agriculture Organization of the United Nations (FAO) [5], during 2018 alone, it was estimated that 100 million tons of Brassica crops were produced worldwide. The main vegetable species of this genus is Brassica oleracea L., which includes many common vegetables with a long consumption tradition in Europe [6], such as the cabbage (B. oleracea var. capitata L.), cauliflower (B. oleracea var. botrytis L.), broccoli (B. oleracea L. var. italic Plenck), kale (B. oleracea var. acephala DC.), Brussels sprout (B. oleracea var. gemmifera DC.), and kohlrabi (B. oleracea var. gongylodes L.) [7]. These vegetables are highly appreciated for human health because they are rich in various secondary metabolites that can be effective in the prevention and treatment of certain types of cancer, cardiovascular health problems, and neurodegenerative diseases [6].
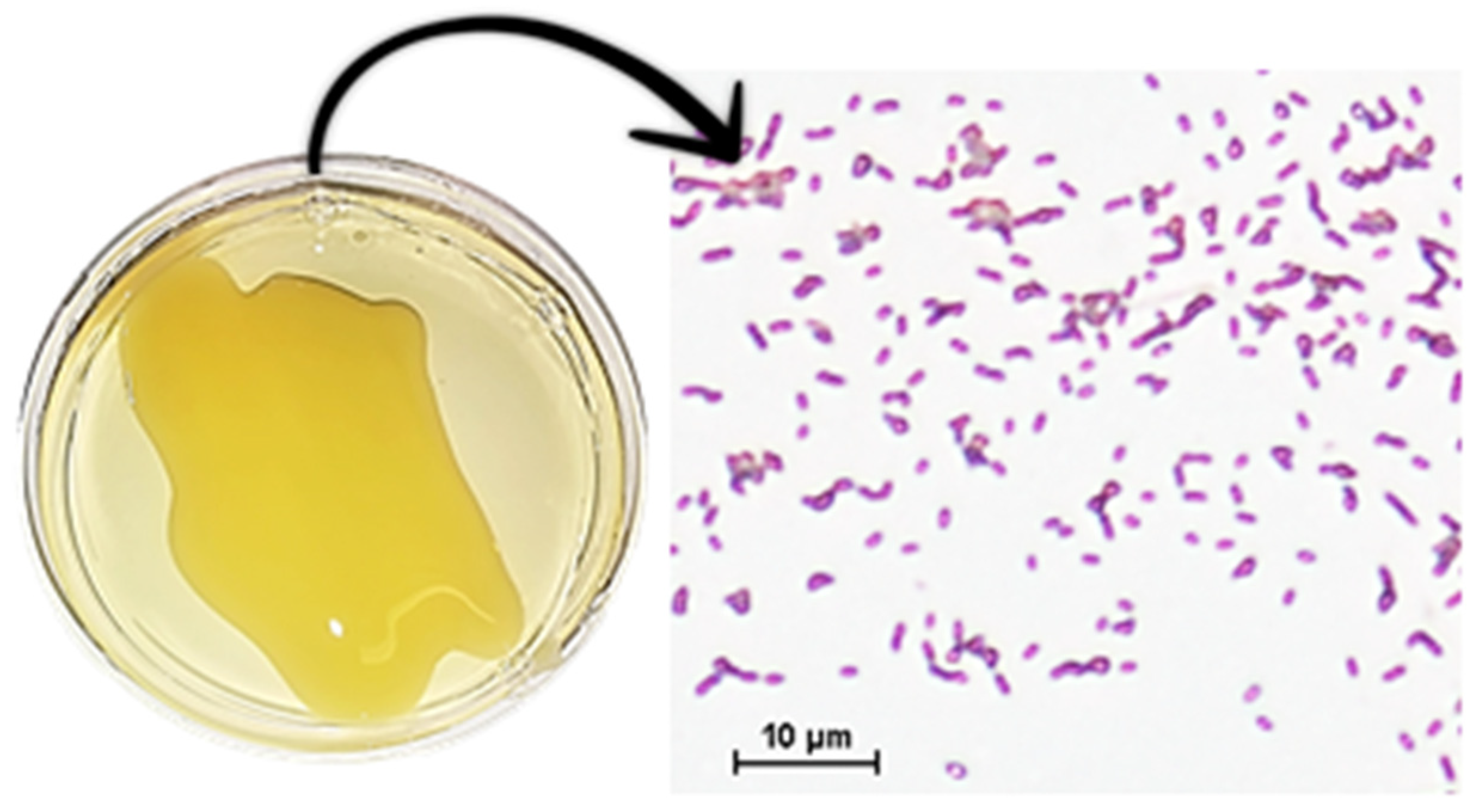
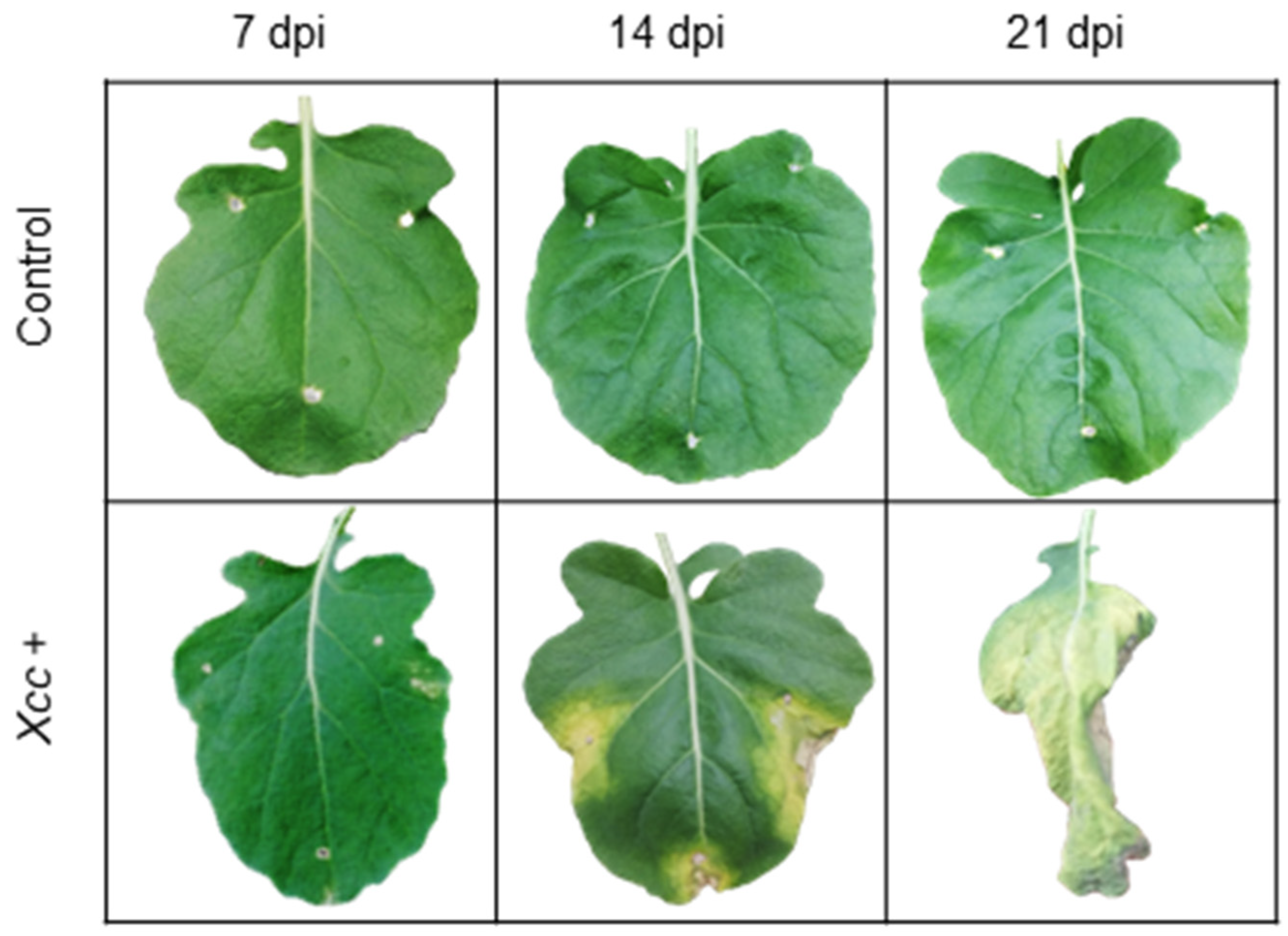
The productivity and quality of B. oleracea crops are seriously affected by infections, which result in substantial economic losses for agricultural producers every year [8]. Nowadays, the bacterial disease black rot, caused by Xanthomonas campestris pv. campestris (Pammel) Dowson (Xcc), is one of the most significant for B. oleracea crops, because of its rapid propagation, the resulting management difficulties, the ineffective and polluting chemical control methods used, and the consequent crop yield losses [9,10]. Xcc is a Gram-negative aerobic bacillus. It produces large amounts of the polysaccharide known as “xanthan,” which gives a slimy appearance to colonies of plate-grown bacteria (Figure 1) [11]. Xcc enters into the plant through wounds and hydathodes, and colonizes the mesophyll first and the plant vascular system later. The infection is very distinctive because of the appearance of V-shaped necrotic lesions in the affected plants [2,11] (Figure 2). Up to now, eleven races of Xcc have been identified [12], races 1 and 4 being the most aggressive and widespread [13]. In the case of B. oleracea, race 1 is the most virulent [14]. Xcc needs living tissues for growth and reproduction, but tissues may die eventually and feed the pathogen; thus it behaves as a biotrophic and necrotrophic pathogen accordingly. In fact, it has been reported that infected plants show gene upregulation related to the synthesis and signaling of phytohormones, salicylic acid (SA), and jasmonic acid (JA) against both types of pathogens [15,16].
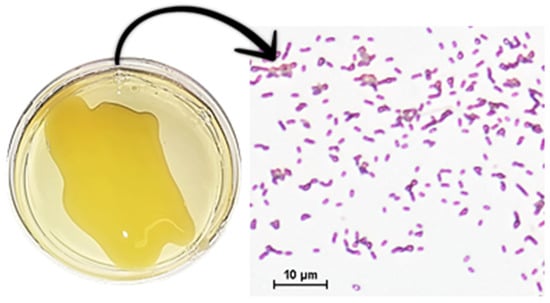
Figure 1.
Xanthomonas campestris pv. campestris grown in potato dextrose agar (PDA) medium and an observation of the plate by a light microscope (Nikon eclipse E2000, Nikon® Instruments Inc., Melville, NY, USA) with 100× magnification, after Gram staining.
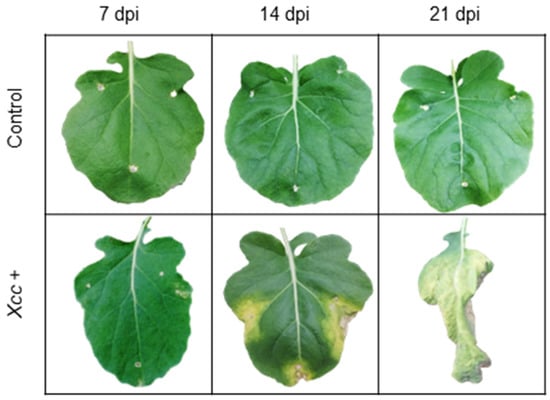
Figure 2.
V-shaped necrotic lesions appeared in Brassica oleracea leaves when infected with bacterium Xanthomonas campestris pv. campestris. The picture shows a leaf’s appearance at 7, 14, and 21 days post inoculation (dpi), and a control leaf on the same days.
B. oleracea’s immune system comprises multiple defense strategies against Xcc invasion. The first layer of basal defense response is conferred by a non-host resistance mechanism. In susceptible plants, Xcc can overcome the first barrier by delivering effector proteins into plant cells to suppress the host’s basal defense. The second layer of defense termed the effector triggered immunity (ETI) is mediated by the intracellular receptors encoded by a family of plant disease resistance genes (R genes) [16]. R genes recognize the pathogen invasion by binding to pathogen effector proteins and activating downstream responses in order to inhibit bacteria development [2,11].
After recognition of the infection by the immune system, the downstream responses influence plant physiology, growth, and metabolism. In Brassica crops, examples of defense-induced secondary metabolites triggered after pathogen infection, such as Xcc, are glucosinolates (GSLs) [17]. These are constitutively synthetized and stored in plant cells as phytoanticipins, but in response to stimuli such as Xcc, de novo synthesis is induced to interfere with the infection [5,18,19]. As a counterpart, the regulation of secondary metabolism to enhance defense responses after Xcc infection probably requires resources from primary metabolism [20].
In general, Xcc is more serious in tropical, subtropical, and humid continental regions, where temperatures exceed 25 °C [11]. Yield loss caused by Xcc in cultivated B. oleracea crops in temperate areas is mostly a consequence of neglecting those plants showing symptoms (for example, cabbages with chlorotic and necrotic lesions) because they are unmarketable [21,22,23]. However, biomass loss caused by the derivation of resources from primary to secondary metabolism could also be translated into yield loss of the crops´ final products [21], even when the damage is no so evident. To our knowledge, the effect of Xcc infection in plant biomass and its relationships with physiological parameters have been poorly studied. In this work, we focused on studying the effect of Xcc race 1 invasion on biomass in young and adult B. oleracea plants, and we discuss the implications of our results for the cultivation of this species.
2. Material and Methods
2.1. Inoculation under Greenhouse Conditions
Four B. oleracea crops: broccoli (B. oleracea var. italica), cauliflower (B. oleracea var. botrytis), cabbage (B. oleracea var. capitata) and kale (B. oleracea var. acephala) were tested in a controlled greenhouse study. Broccoli and cabbage seeds were purchased in Batlle Seeds (Spain), whereas cauliflower seeds were purchased in Rocalba Seeds (Spain). The kale variety (MBGBRS103) was obtained from the Gene Bank, at Misión Biológica de Galicia (CSIC, Spain). Plants were grown in pots containing 2.5 L of peat (Gramoflor GmbH and Co. KG Produktion, Vechta, Germany). Conditions inside the greenhouse were a 14 h photoperiod and a day/night mean temperature of 24/18 °C. Plants were planted in a split-plot design, where B. oleracea inoculated and control plants were the main plots. Within each plot, genotypes were randomized in three repetitions, giving sixteen plants by repetition. Plants of each repetition were split into four sets of four plants each. Each one of the sets was used to take measurements at different time points 7, 14, 21, and 28 days post-inoculation (dpi).
Six weeks after sowing, the second youngest leaf of each plant (counting from the apex) was inoculated with Xcc race 1. The isolate of Xcc (strain HRI3811, synonymous with PHW1205 collected from B. oleracea in the USA, Joana Vicente personal communication) was provided by Joana Vicente (Warwick HRI, Wellesbourne, UK). Fresh bacterial colonies of Xcc were sub-cultured on petri dishes containing potato dextrose agar (PDA) and incubated at 32 °C for 24 h in the dark. For inoculum preparation, a loop of bacteria was transferred to nutrient broth and shaken overnight at 150 rpm and 30 °C in the dark. The culture was diluted in sterile tap water to a concentration of 5 × 108 cfu·mL−1, which corresponds to an absorbance of 0.51 at a wavelength of 600 nm, measured using a spectrophotometer (Spectra MR; Dynex Technologies, Chantilly, VA, USA). The inoculum was injected into three different points of each leaf, by puncturing the main veins using mouse-tooth forceps wrapped in cotton soaked in the bacterial suspension. After inoculation, conditions inside the greenhouse were set to a day/night mean temperature of 24/28 °C, a 14 h photoperiod, and a relative humidity of 90 to 100%.
Different measurements were taken at 7, 14, 21, and 28 dpi. Fluorescence was measured with a portable fluorometer (OS-30p Chlorophyll Fluorometer, OptiScience, Inc., Hudson, NH, USA). Fluorescent transience was induced by red light of 3000 μmoL m−2 s −1 provided by an array of 3 light-emitting diodes (peak at 660 nm) using plants dark adapted for 20 minutes. In order to check the damage caused by Xcc 7 and 14 dpi, subjective scores were assessed on a visual 1–9 rating scale based on the relative lesion size, where 1 = no visible symptoms and 9 = severely diseased with typical V-shaped chlorotic leaf edge lesions presenting blackened veins areas [19]. Pictures of inoculated leaves (Figure 2) were taken and then we estimated the areas of the chlorotic lesions and of the inoculated leaves using ImageJ 1.50i (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). A percentage of chlorotic area was calculated per leaf. Lastly, fresh and dry weights of the aerial parts of seedlings were measured in inoculated and control plants. Based on these parameters, the water percentage was calculated.
All measurements carried out during this work are routinely used in our group and no novel method was employed.
Control plants did not show symptoms (chlorotic lesions 0% and rating scale 1). Infected plants showed the first chlorotic lesions (1.5%), corresponding to 2 in the rating scale at 7 dpi. The symptoms became more severe (chlorotic lesions 20% and rating scale 5) at 14 dpi. Finally, the infected leaves showed symptoms of senescence at 21 dpi (chlorotic lesions 100% and rating scale 9).
2.2. Inoculation under Field Conditions
Two crops, namely, cabbage and kale, were assayed in the field in a split-plot design in Pontevedra (42°24′ N, 8° 38′ W, 20 m.a.s.l), where the crops were the main plot. Crops were planted in multipot trays and seedlings were transplanted into the field at the five–six leaf stage. Transplant date was 22 June 2020 and plants were spaced 0.80 m apart. Plants were subjected to three different randomized treatments in each block: inoculation 1, inoculation 2, and control, with three repetitions per treatment and 20 plants per repetition. Cultural operations, fertilization, and weed control were done according to local practices. For pest control, Force® was added at the time of transplantation to combat soil insects. After six or nine weeks, two leaves per plant were inoculated with Xcc (inoculation 1 and inoculation 2, respectively) following the procedure described above. Twenty-one days after inoculation 2, three bulks containing the leaves of seven plants each (the third leaf when counting from the apex) were taken from inoculated and control plants in each repetition in order to measure fresh and dry weights and calculate the water percentage. Besides, three other bulks were taken from each of the three treatments, frozen in dry ice, and transferred to the laboratory and stored at −80 °C for GSL extraction. Extraction was done by following the methodology of [24]. Identification and quantification of compounds was performed by using ultra-high performance liquid chromatography (UHPLC) according to [25]. GSL concentration was measured in µmoL g−1 dry weight. Finally, the fresh weights of cabbage heads and kale apex leaves were measured for each treatment.
2.3. Statistical Analysis
Analyses of variance were performed using the GLM procedure of Statistical Analysis Software (SAS, Institute Inc. Cary, NC, USA) [26]. In the greenhouse trial, ANOVA was performed based on time. Cultivars were grouped for each time period, and comparisons of means between treatments (control and inoculated) were done with a Student’s t test at the 0.05 level of probability. In the field trial, ANOVA was by cultivar. Means comparisons among treatments (control, inoculation1, and inoculation 2) were done by means of Fisher’s least significant difference (LSD) at the 0.05 level of probability. In both trials, cultivars and treatments were considered fixed factors, whereas replications were considered random factors.
3. Results
3.1. Greenhouse-Grown Seedlings Inoculated with Xcc Lose Biomass and Water Percentage over Infection Time
Young broccoli, cauliflower, cabbage, and kale plants were inoculated with Xcc under greenhouse conditions. After infection, resistance parameters were measured in inoculated plants at 7 and 14 dpi. Chlorophyll fluorescence, fresh weight, dry weight, and water percentage were monitored at 7, 14, 21, and 28 dpi.
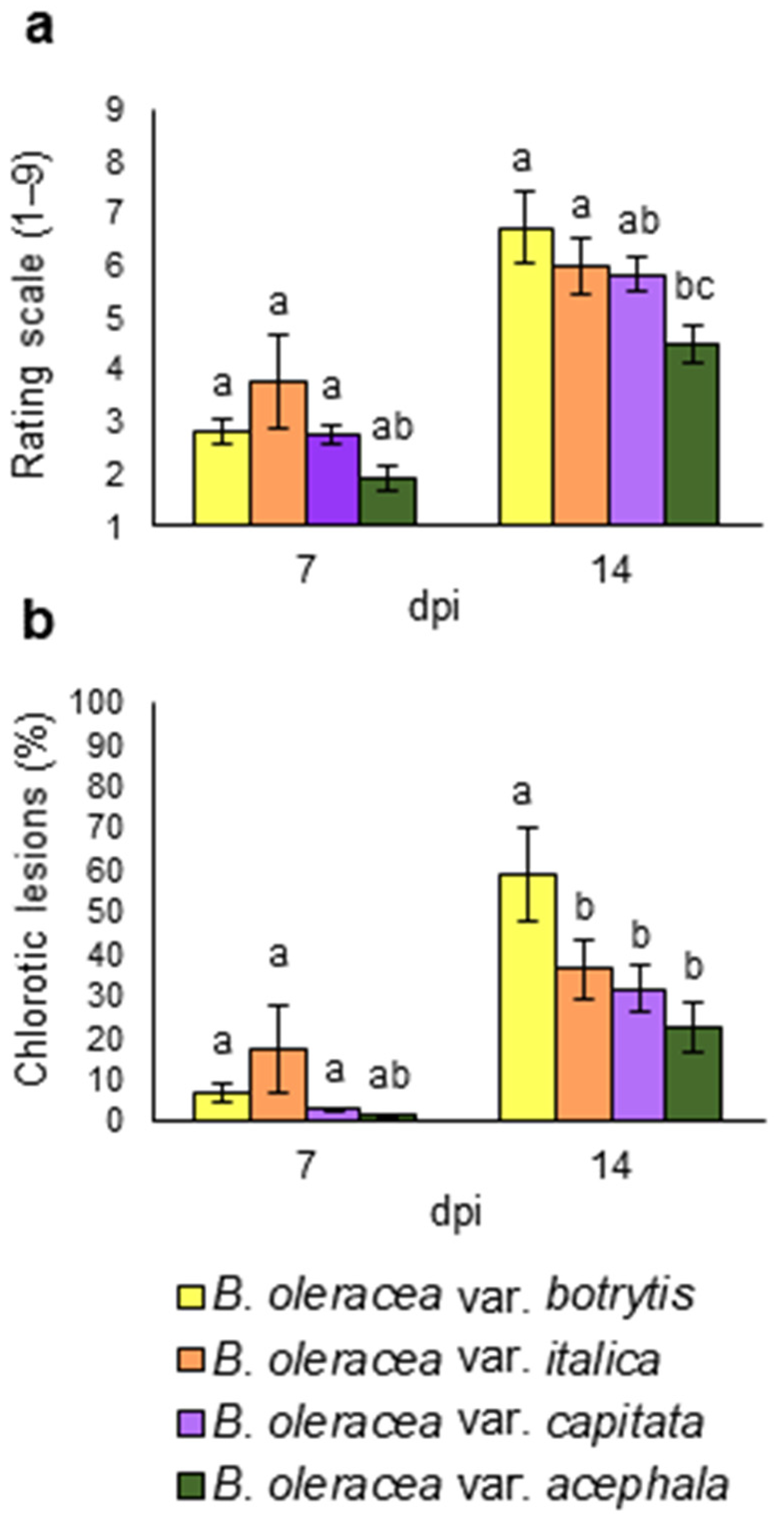
Damage level (Figure 3a) and lesion percentage (Figure 3b) were perceptible 7 dpi and had increased 14 dpi for all crops. Differences were only significant at 14 dpi. All crops were susceptible to the disease, although quantitative differences were found. B. oleracea var. acephala showed the lowest rating scale, although it did not differ from B. oleracea var. italica. B. oleracea var. botrytis showed a higher percentage of chlorotic lesions than the other crops. Although there were quantitative differences in terms of susceptibility, the four crops showed similar performances for the biomass-related parameters during the Xcc infection. There was no interaction between genotypes and treatments in ANOVA (data not shown). Therefore, crops were combined in a second analysis (Figure 4).
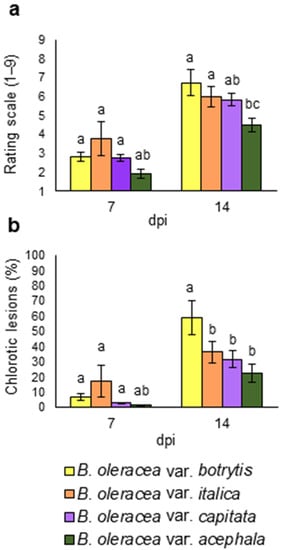
Figure 3.
Resistance parameters from four different Brassica oleracea crops: B. oleracea var. botrytis, B. oleracea var. italica, B. oleracea var. capitata, and B. oleracea var. acephala, inoculated with Xanthomonas campestris pv. campestris under greenhouse conditions, at 7 and 14 days post inoculation (dpi). (a) Damage score and (b) lesion percentage. Data represent the averages of 3 repetitions. Different letters indicate significant differences at p < 0.05. Error bars indicate the standard errors.
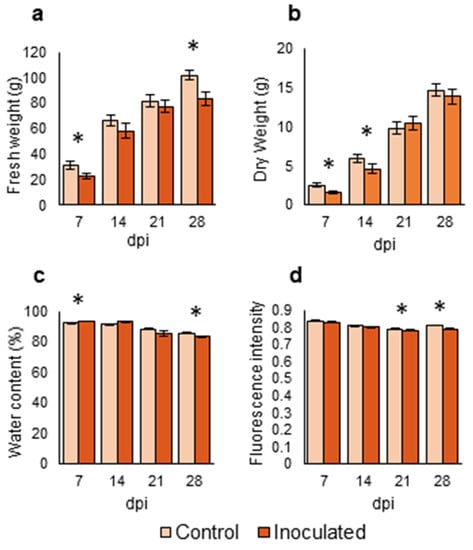
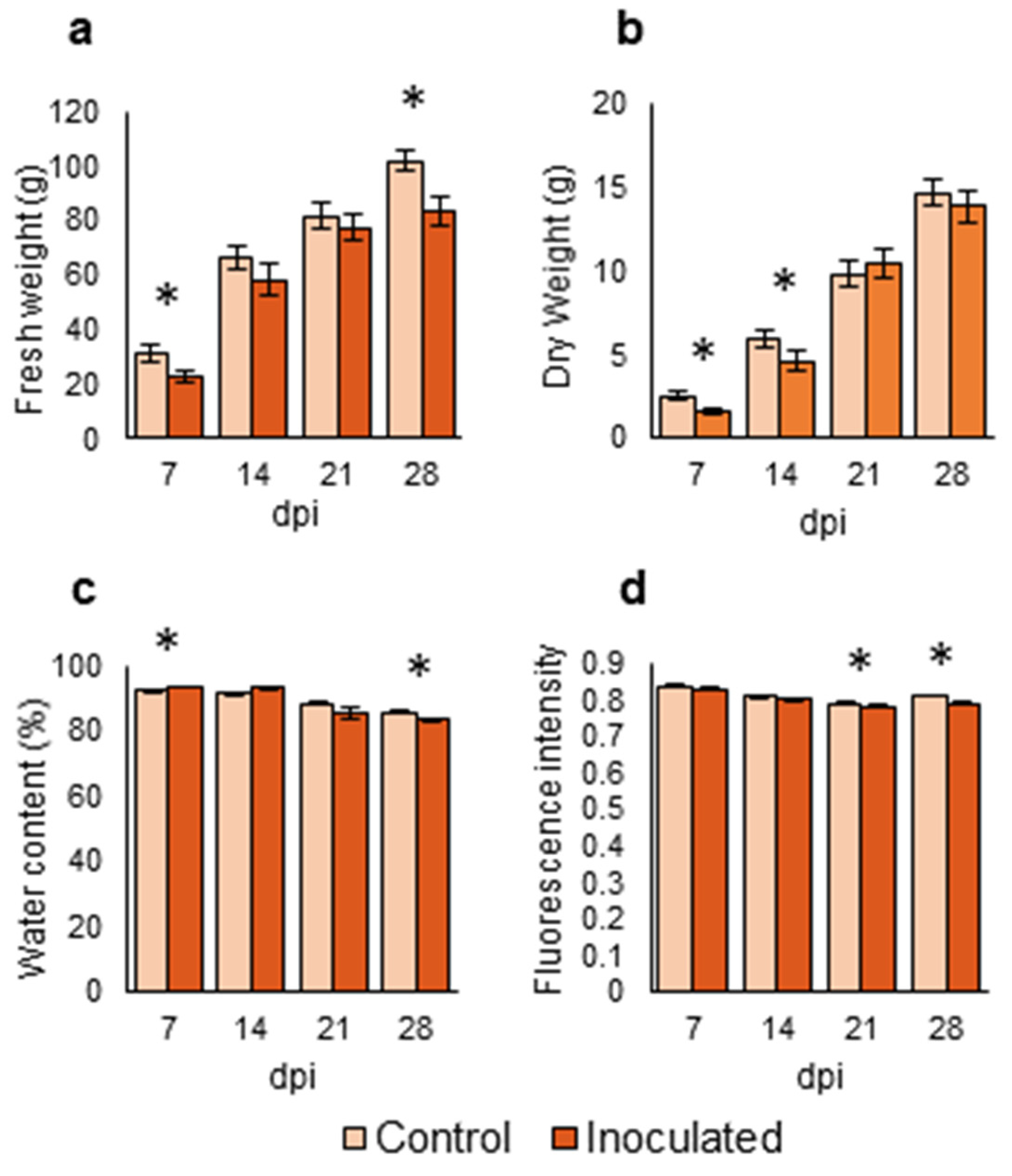
Figure 4.
Parameters related to biomass and physiology of Brassica oleracea seedlings inoculated with Xanthomonas campestris pv. campestris under greenhouse conditions. (a) Fresh weight (b). Dry weight (c). Water percentage (d). Levels of fluorescence. Measurements were done at 7, 14, 21 and 28 days post inoculation (dpi). Data represent the averages of 12 repetitions, representing four crops (B. oleracea var. botrytis, B. oleracea var. italica, B. oleracea var. capitata, and B. oleracea var. acephala). Asterisks indicate significant differences at p < 0.05. Bars represent the standard errors.
Inoculated seedlings had lower fresh weight than the control ones at all analyzed times (Figure 4a), but this difference was only significant at 7 and 28 dpi. Besides, inoculated plants had significantly lower dry weights than control plants at 7 and 14 dpi (Figure 4b). Water percentage was significantly higher in infected plants than in control plants at 7 dpi. However, the water percentage in control plants was significantly higher than that of inoculated plants at 28 dpi (Figure 4c). Fluorescence was slightly lower in the inoculated plants compared to control plants at all analyzed times, being statistically significant at 21 and 28 dpi (Figure 4d).
3.2. No Effect on Biomass, Water Percentage or GSLs Levels was Detected in Adult B. oleracea Plants after Inoculation with Xcc under Field Conditions
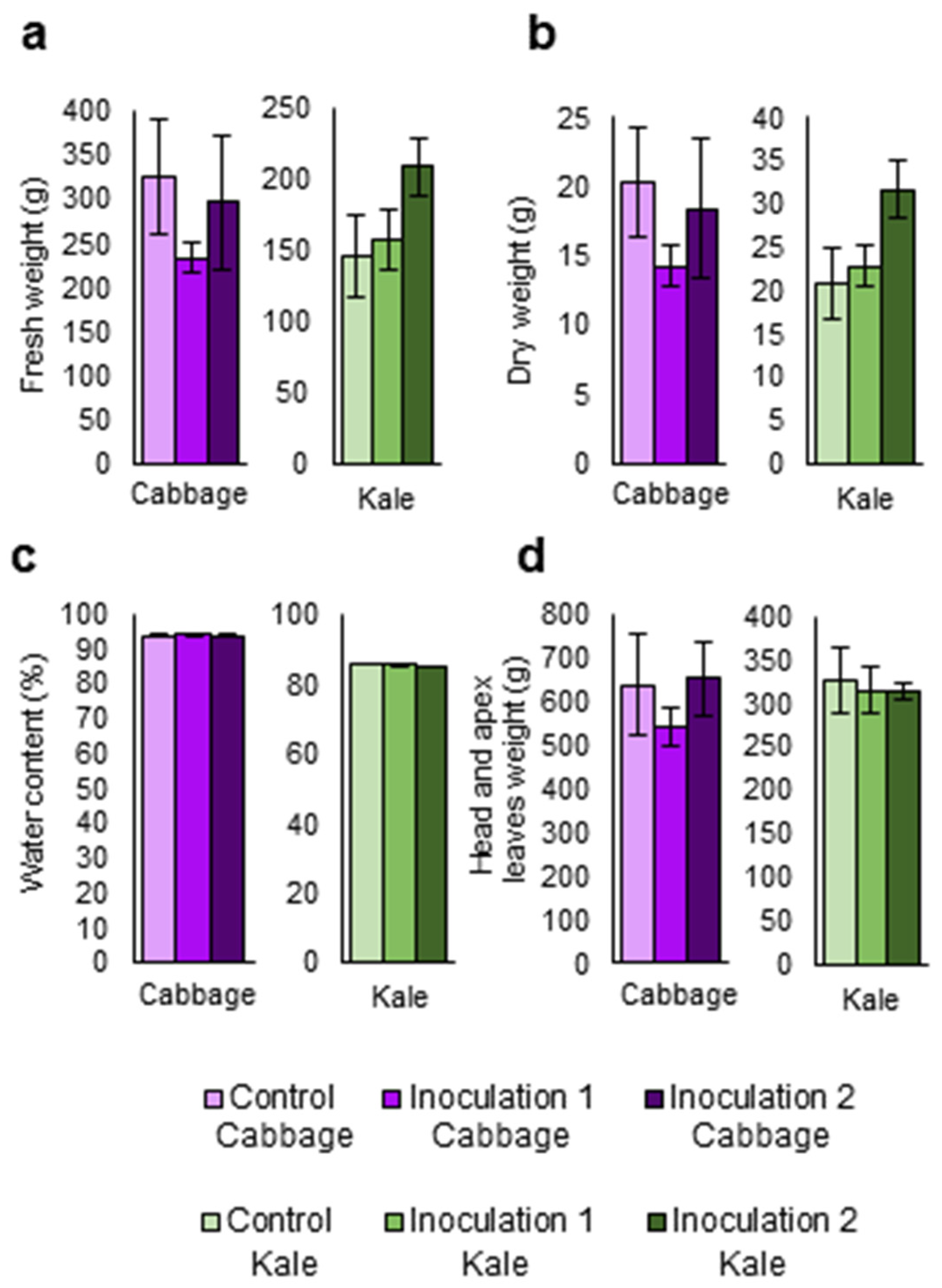
Kale and cabbage plants were inoculated with Xcc under field conditions at two different times. One set of plants was inoculated one month after transplant (inoculation 1) and a second set of plants was inoculated three weeks later (inoculation 2). Measurements were performed, and mean comparisons were done among the three treatments 21 days after the last inoculation. No significant differences were found among treatments (inoculation 1, inoculation 2, and control) for weights of fresh and dry leaves (Figure 5a,b), water percentage (Figure 5c), cabbage head weight, or kale apex leaf weight (Figure 5d).
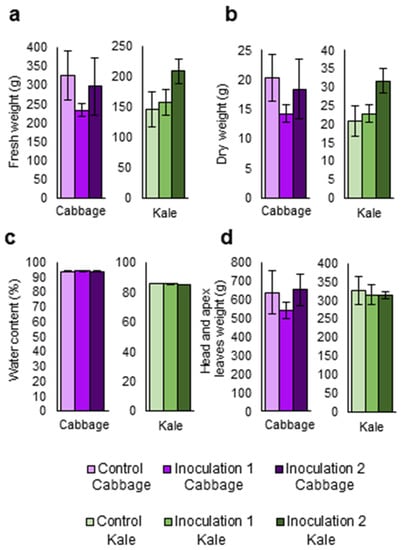
Figure 5.
Parameters related to the biomass and physiology of two Brassica oleracea crops inoculated with Xanthomonas campestris pv. campestris in a field trial. (a) Weight of fresh leaves. (b) Weight of dry leaves. (c) Water percentage of leaves. (d) Cabbage head weight and wight of kale apex leaves. Data represent the averages of 3 repetitions for each crop: B. oleracea var. capitata (cabbage) and B. oleracea var. acephala (kale). Bars represent the standard error.
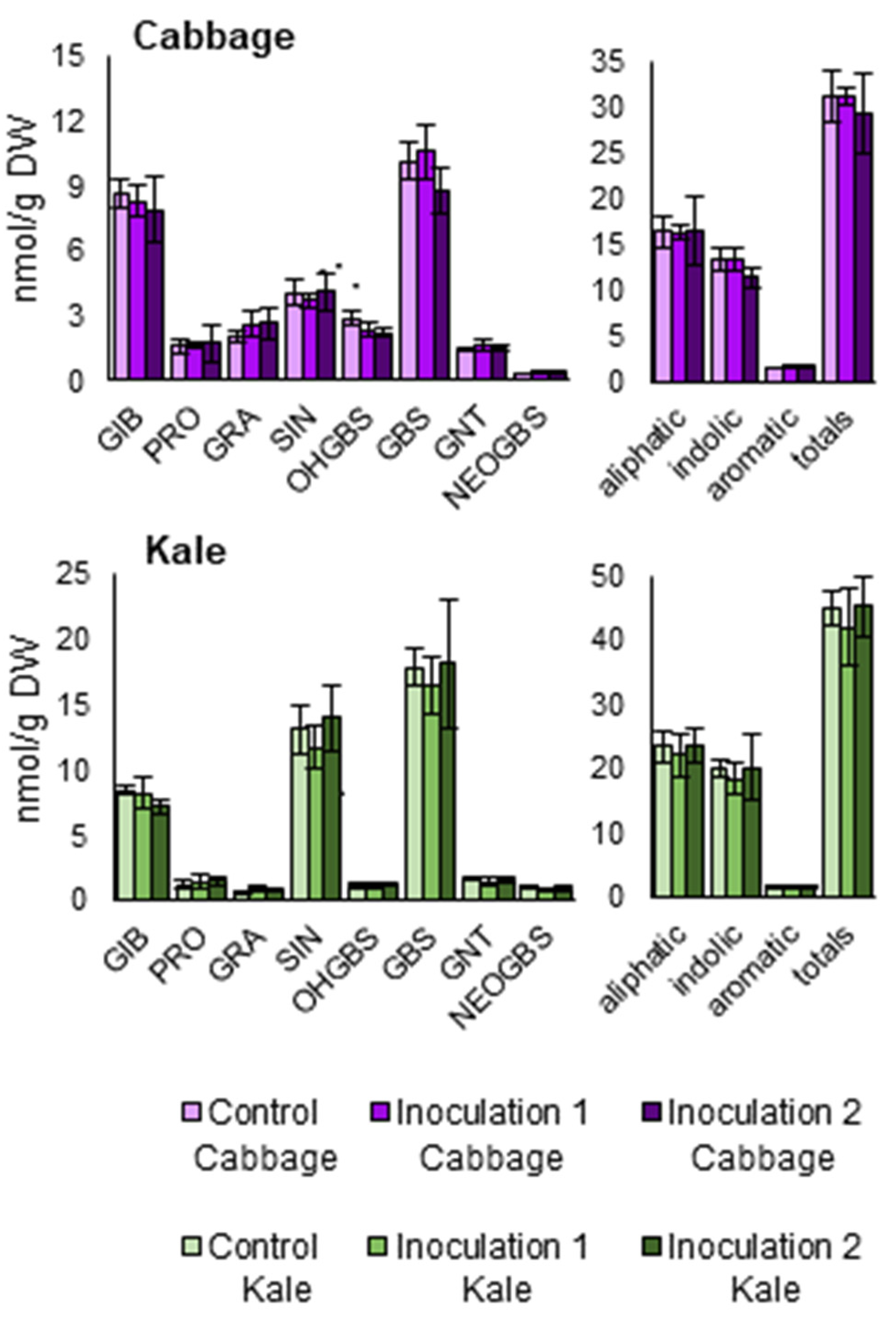
Individual and total GSLs (aliphatics, indolics, aromatics, and total) were measured in inoculated and control plants. The same GSLs profile was found among treatments: we could identify the aliphatic GSLs glucoiberin, progoitrin, glucoraphanin, and sinigrin; the aromatic GSL gluconasturtiin; and the indolic GSLs 4-hydroxyglucobrassicin, glucobrassicin, and neoglucobrassicin, in all samples. With regard to the chemical class, aliphatics were the most prevalent, followed by indolics and aromatics GSLs (Figure 6). No significant differences were found between treatments.
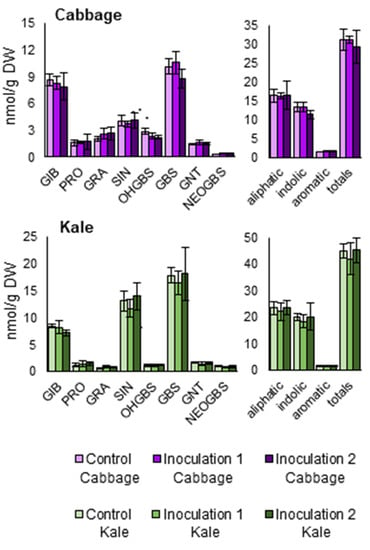
Figure 6.
Glucosinolate contents of two Brassica oleracea crops inoculated with Xanthomonas campestris pv. campestris in a field trial. Glucosinolate concentration of leaves (GIB = glucoiberin; PRO = progoitrin; GRA = glucoraphanin; SIN = sinigrin; OHGBS = 4-hydroxyglucobrassicin; GBS = glucobrassicin; GNT = gluconasturtiin; NEOGBS = neoglucobrassicin; aliphatic = GIB + PRO + GRA + SIN; indolic = OHGBS + GBS + NEOGBS; aromatic = GNT; totals = total glucosinolates). Data represent the averages of 3 replicates for each crop: B. oleracea var. capitata (cabbage) and B. oleracea var. acephala (kale). Bars represent the standard errors.
4. Discussion
The effects of a Xcc race 1 infection on biomass production and related parameters from B. oleracea were evaluated in seedlings, under greenhouse conditions, and in adult plants in the field. In the greenhouse, assay parameters were analyzed at different times post-infection in order to get estimates of how the infection interferes with plant development. A field trial was performed with plants infected in the adult stage, at two different times, with the aim of discerning whether or not the alleged post-infection alterations subside over time.
Damage levels and lesion percentages of plants in the greenhouse assay (Figure 3) indicated that the infection was carried out satisfactorily. Although quantitative differences were found among genotypes, all of them were susceptible. Regarding biomass-related parameters, no interaction between genotypes and treatments was found in ANOVA. These facts suggest that the development of the infection caused by Xcc is independent upon the B. oleracea crop.
It was remarkable that inoculated seedlings increased their water percentage at 7 and 14 dpi under greenhouse conditions (Figure 4c). This agrees with biochemical studies about stomatal closure in the presence of phytopathogenic bacterium Xcc [27]. When brassicas are infected with Xcc, pathogenicity factors, elicitors, and virulence proteins are delivered to the plant cell [2]. Reactive oxygen species (ROS) increased after inoculating of the cruciferous Arabidopsis with Xcc [15,28]. The abscisic acid (ABA) signaling pathway is manipulated by a type III effector (AvrXccC8004) of Xcc, thereby increasing ABA levels [27] and proteins responsive to ABA [29] after infection. ABA, ROS, and elicitors of plant defense stimulate Ca2+ influx current in Arabidopsis [15,30], which increases Ca2+ in guard cells, providing stomatal closure [30]. In that way, stomata work as part of the plant’s innate immune response by closing to prevent the entry of the pathogen [31,32].
Interestingly enough, the effect seemed to be reversed and the water percentage decreased in infected plants at 21 and 28 dpi (Figure 4c). A factor secreted by Xcc is capable of interfering with stomatal closure, reversing it by means of virulence factors, as occurs in Arabidopsis after infection [32]. Xcc can penetrate the plant using hydathodes, wounds, and through stomata [32]. Therefore, this could be an evolutionary advantage for the bacterium to interfere with the closure of stomata.
Xcc decreases B. oleracea seedlings’ biomass, at least 28 days after infection (Figure 4a). This effect was observed before in rice infected with another bacterium from the genus Xanthomonas, X. oryzae (Uyeda et Ishiyama) Dowson pv. oryzae [33], and in broccoli cultivars infected by Paraburkholderia (Sawana et al. 2015) [34]. Xcc displays the defining characteristics of a biotrophic and necrotrophic pathogen. A common feature of both types of pathogens is that, as well as changing secondary metabolism, they promote changes in primary metabolism to induce defense programs that affect growth and development. Changes include photosynthetic efficiency [35], by down-regulating proteins involved in photosynthesis [29,36], such as those of photosystem II (PSII) [37]. Consequently, there is a loss of effective PSII quantum yield [38]. This is supported by the fact that, in this work, PSII chlorophyll fluorescence dropped when plants were infected (Figure 4d). Fluorescence is a very sensitive marker for the efficiency of photosynthesis [38].
Previous studies proposed that infected plants switch off photosynthesis to initiate respiration and other processes required for defense [39]. Additionally, Xcc can affect photosynthetic tissues directly, being able to kill them [15].
Interestingly, biomass and fluorescence reductions corresponding to 7 and 14 dpi coincided with the moment when the water percentage was the highest. Accordingly, the disruption of gas exchange due to stomatal closure could cause the plant to incorporate less CO2 from the atmosphere during photosynthesis. Hence, stomatal closure would be another factor to influence the yield reduction, during the first dpi.
Definitely, Xcc may influence growth-related processes, water content, and ultimately, the plant physiology of B. oleracea seedlings. In order to measure the impact that these processes may have in an agricultural environment, we performed a field trial, testing adult B. oleracea plants.
Three-month old kale and cabbage plants were inoculated in the field with Xcc. Three weeks later, another set of plants was inoculated. The reason to perform two inoculations staggered in time was to test whether there were temporary effects of the infection on physiological traits. However, we did not find significant differences between plants from inoculation 1 compared to inoculation 2 for any trait, which indicates that they were at a similar developmental stage. Both crops showed a similar performance, like in the greenhouse trial.
Inoculated plants were compared to control plants twenty-one days after the second inoculation. No significant differences were found in ANOVA among treatments (inoculation 1, inoculation 2, and control) for fresh and dry leaf weights (Figure 5a,b). Besides, there were no differences among treatments for water percentage (Figure 5c). Therefore, when adult plants are inoculated with Xcc, there is no evident effect of the disease on factors related to leaf biomass. To test the effect of Xcc in the final weight of the marketable products, we measured the cabbage heads’ weight and the kale apex leaves’ weight (Figure 5d). We did not find any evidence of a loss in weight for marketable products.
Taking into account that two leaves per plant were inoculated (instead of one leaf in the greenhouse) and that all plants showed symptoms, adult plants may have overcome the effect of Xcc invasion by having more defenses than seedlings [40]. Young and adult plants may differ depending on their resistance levels [40,41,42,43], and these differences can be attributed to a differential gene expression [41,42]. Differences in cytosine methylation between seedlings and adult plants from rice cultivar Wase Aikoku 3 could be related to their differential resistance to the bacterium X. oryzae pv. Oryzae [41]. Expression of Pp523 in adult broccoli plants is related to their increased resistance to Xcc compared to seedlings [42]. Another important aspect to keep in mind is that the greater resistance in adult plants could also have been due to a reduced ability of the pathogen to enter through thicker, tougher cell walls [44]. Accordingly, B. oleraceae may increase its resistance against the attack of Xcc during development, possibly due to changes in gene expression and lignification.
Differences in resistance between young and adult plants may be due to the accumulation of defensive secondary metabolites, such as GSLs [17]. The contents of these compounds increase throughout the plant’s development [45,46]. Besides, their synthesis may be induced after a pathogenic attack [17,18]. GSL content was measured in inoculated and control plants. No differences in GSL content were found between them (Figure 6), which indicates that there was no induction of GSL synthesis or that we did not detect it, because induction normally occurs right after a pathogenic attack [18]. In addition, we measured GSLs three and six weeks after the inoculations (from second and first inoculations, respectively) in field experiments. Induction of GSL synthesis upon pathogenic attack has been proven under controlled conditions, normally in greenhouse assays [17,18,45,46]. Induction of GSLs in adult plants in the field may be a much more complex matter, influenced by the attack of pests and other pathogens present in field trials, and by the changing environmental conditions. Therefore, it could cause a higher experimental error.
Xcc invasion in B. oleracea seedlings provokes a biomass loss in the aerial parts of the plants over time, associated with a drop in the photosynthetic efficiency and with changes in water content. This effect is not evident when adult plants were inoculated in the field, probably due to an increase in the defenses of mature plants. Therefore, the first developmental stages of B. oleracea crops are more susceptible to the disease. Hence, control measurements should be increased to avoid the contact of the plants with bacteria, and also to prevent biomass loss that could be translated into yield loss of marketable products.
Author Contributions
Design of experiments, P.S.; experimentation and data analysis, P.S., M.F., and C.V.-Á.; writing, reviewing, and editing, P.S., M.F., and C.V.-Á., All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Science and Innovation, of the Government of Spain, through the research project RTI2018-096591-B-I00. Carmen Vega-Álvarez acknowledges a PFI fellowship from the Spanish Ministry of Science and Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors want to thank Berea Rodríguez and Lara Álvarez for their support in the greenhouse assay, Juan Carlos Fernández and Rosaura Abilleira for their support in the laboratory analysis, and Elena Zubiaurre for her support with the microscopic photography.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Rakow, G. Species origin and economic importance of brassica. In Brassica; Pua, E.-C., Douglas, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 54, pp. 3–11. [Google Scholar]
- Singh, S.; Dey, S.S.; Bhatia, R.; Batley, J.; Kumar, R. Molecular breeding for resistance to black rot [Xanthomonas campestris pv. campestris (Pammel) Dowson] in Brassicas: Recent advances. Euphytica 2018, 214, 1–17. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Cheng, F.; Liu, B.; Hu, Z.; Chen, S.; Pental, D.; Ju, Y.; et al. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.C.; Löptien, H.; Röbbelen, G. Breeding: An Overview; Gómez-Campo, C., Ed.; Biology of Brassica Coenospecies; Elsevier Science B.V.: Amsterdam, The Netherlands, 1999; Volume 4, pp. 413–460. [Google Scholar]
- Fao.org FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 19 November 2020).
- Kapusta-Duch, J.; Kopeć, A.; Piatkowska, E.; Borczak, B.; Leszczyńska, T. The beneficial effects of Brassica vegetables on human health. Rocz. Państwowego Zakładu Hig. 2012, 63, 389–395. [Google Scholar] [PubMed]
- Golicz, A.A.; Bayer, P.E.; Barker, G.C.; Edger, P.P.; Kim, H.R.; Martinez, P.A.; Chan, C.K.K.; Severn-Ellis, A.; McCombie, W.R.; Parkin, I.A.P.; et al. The pangenome of an agronomically important crop plant Brassica oleracea. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Marin, V.R.; Ferrarezi, J.H.; Vieira, G.; Sass, D.C. Recent advances in the biocontrol of Xanthomonas spp. World J. Microbiol. Biotechnol. 2019, 35, 1–11. [Google Scholar] [CrossRef]
- Vicente, J.G.; Holub, E.B. Xanthomonas campestris pv. Campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol. Plant Pathol. 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Tang, J.L.; Tang, D.; Dubrow, Z.E.; Bogdanove, A.; An, S.Q. Xanthomonas campestris Pathovars. Trends Microbiol. 2021, 2, 182–183. [Google Scholar] [CrossRef]
- Williams, P.H. Black Rot: A Continuing. Plant Dis. 1980, 64, 736. [Google Scholar] [CrossRef]
- Cruz, J.; Tenreiro, R.; Cruz, L. Assessment of diversity of Xanthomonas campestris pathovars affecting cruciferous plants in Portugal and disclosure of two novel X. campestris pv. campestris races. J. Plant Pathol. 2017, 99, 403–414. [Google Scholar] [CrossRef]
- Lema, M.; Cartea, M.E.; Sotelo, T.; Velasco, P.; Soengas, P. Discrimination of Xanthomonas campestris pv. campestris races among strains from northwestern Spain by Brassica spp. genotypes and rep-PCR. Eur. J. Plant Pathol. 2012, 133, 159–169. [Google Scholar] [CrossRef]
- Iglesias-Bernabé, L.; Madloo, P.; Rodríguez, V.M.; Francisco, M.; Soengas, P. Dissecting quantitative resistance to Xanthomonas campestris pv. campestris in leaves of Brassica oleracea by QTL analysis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Tortosa, M.; Cartea, M.E.; Velasco, P.; Soengas, P.; Rodriguez, V.M. Calcium-signaling proteins mediate the plant transcriptomic response during a well-established Xanthomonas campestris pv. campestris infection. Hortic. Res. 2019, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.; Islam, T.; Lee, B.; La, V.H.; Bae, D. Genotypic Variation in Resistance Gene-Mediated Calcium Signaling and Hormonal Signaling Involved in Effector-Triggered Immunity or Disease Susceptibility in the Xanthomonas campestris pv. Campestris–Brassica napus Pathosystem. Plants 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Rubel, M.H.; Abuyusuf, M.; Nath, U.K.; Robin, A.H.K.; Jung, H.J.; Kim, H.T.; Park, J.I.; Nou, I.S. Glucosinolate profile and glucosinolate biosynthesis and breakdown gene expression manifested by black rot disease infection in cabbage. Plants 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Velasco, P.; Lema, M.; Francisco, M.; Soengas, P.; Elena Cartea, M. In Vivo and in vitro effects of secondary metabolites against Xanthomonas campestris pv. campestris. Molecules 2013, 18, 11131–11143. [Google Scholar] [CrossRef]
- Madloo, P.; Lema, M.; Francisco, M.; Soengas, P. Role of Major Glucosinolates in the Defense of Kale against Sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris. Phytopathology 2019, 109, 1246–1256. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- da Silva, A.L.B.R.; Candian, J.S.; Do Rego, E.R.; Coolong, T.; Dutta, B. Screening cabbage cultivars for resistance to black rot under field conditions. Horttechnology 2020, 30, 448–455. [Google Scholar] [CrossRef]
- Mandiriza, G.; Kritzinger, Q.; Aveling, T.A.S. The evaluation of plant extracts, biocontrol agents and hot water as seed treatments to control black rot of rape in South Africa. Crop Prot. 2018, 114, 129–136. [Google Scholar] [CrossRef]
- Chidamba, L.; Bezuidenhout, C.C. Characterisation of Xanthomonas campestris pv. campestris isolates from South Africa using genomic DNA fingerprinting and pathogenicity tests. Eur. J. Plant Pathol. 2012, 133, 811–818. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Lambrix, V.M.; Reichelt, M.; Gershenzon, J.; Mitchell-Olds, T. Gene duplication in the diversification of secondary metabolism: Tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 2001, 13, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, T.; Soengas, P.; Velasco, P.; Rodriguez, V.M.; Cartea, M.E. Identification of metabolic QTLs and candidate genes for glucosinolate synthesis in Brassica oleracea leaves, seeds and flower buds. PLoS ONE 2014, 9, e91428. [Google Scholar] [CrossRef]
- SAS institute Statistical Analysis Software© (SAS). IntrNet Software; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Ho, Y.P.; Tan, C.M.; Li, M.Y.; Lin, H.; Deng, W.L.; Yang, J.Y. The AvrB-AvrC domain of AvrXccC of Xanthomonas campestris pv. campestris is required to elicit plant defense responses and manipulate ABA homeostasis. Mol. Plant Microbe Interact. 2013, 26, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Tinte, M.M.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Lipopolysaccharide perception in Arabidopsis thaliana: Diverse LPS chemotypes from Burkholderia cepacia, Pseudomonas syringae and Xanthomonas campestris trigger differential defence-related perturbations in the metabolome. Plant Physiol. Biochem. 2020, 156, 267–277. [Google Scholar] [CrossRef]
- Santos, C.; Nogueira, F.C.S.; Domont, G.B.; Fontes, W.; Prado, G.S.; Habibi, P.; Santos, V.O.; Oliveira-Neto, O.B.; Grossi-de-Sá, M.F.; Jorrín-Novo, J.V.; et al. Proteomic analysis and functional validation of a Brassica oleracea endochitinase involved in resistance to Xanthomonas campestris. Front. Plant Sci. 2019, 10, 414. [Google Scholar] [CrossRef]
- Klüsener, B.; Young, J.J.; Murata, Y.; Allen, G.J.; Mori, I.C.; Hugouvieux, V.; Schroeder, J.I. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 2002, 130, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Adie, B.A.T.; Pérez-Pérez, J.; Pérez-Pérez, M.M.; Godoy, M.; Sánchez-Serrano, J.J.; Schmelz, E.A.; Solano, R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef] [PubMed]
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol. 2009, 149, 1017–1027. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Sengupta, D.; Das, S.N.; Pandey, M.K.; Bohra, A.; Sharma, N.K.; Sinha, P.; Sk, H.; Ghazi, I.A.; et al. Deployment of genetic and genomic tools toward gaining a better understanding of rice-Xanthomonas oryzae pv. oryzae interactions for development of durable bacterial blight resistant rice. Front. Plant Sci. 2020, 11, 1–23. [Google Scholar] [CrossRef]
- Jeon, J.-S.; Carreno-Quintero, N.; van Eekelen, H.D.L.M.; De Vos, R.C.H.; Raaijmakers, J.M.; Etalo, D.W. Impact of root—Associated strains of three Paraburkholderia species on primary and secondary metabolism of Brassica oleracea. Sci. Rep. 2021, 11, 2781. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, E.; Liu, Y.; Xu, Z.; Hui, M.; Zhang, X.; Cai, M. Transcriptome analysis of two lines of Brassica oleracea in response to early infection with Xanthomonas campestris pv. campestris. Can. J. Plant Pathol. 2020, 43, 127–139. [Google Scholar] [CrossRef]
- Ribeiro, D.G.; da Cunha, G.C.R.; dos Santos, C.; Silva, L.P.; de Oliveira Neto, O.B.; Labuto, L.B.D.; Mehta, A. Brassica oleracea resistance-related proteins identified at an early stage of black rot disease. Physiol. Mol. Plant Pathol. 2018, 104, 9–14. [Google Scholar] [CrossRef]
- Islam, M.T.; Lee, B.; La, V.H.; Bae, D.; Jung, W.; Kim, T. Label-free quantitative proteomics analysis in susceptible and resistant Brassica napus cultivars infected with Xanthomonas campestris pv. campestris. Microorganisms 2021, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, M.; Cartea, M.E.; Rodríguez, V.M.; Velasco, P. Unraveling the metabolic response of Brassica oleracea exposed to Xanthomonas campestris pv. campestris. J. Sci. Food Agric. 2018, 98, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Mew, T.W. Adult-plant resistance of rice cultivars to bacterial blight. Plant Dis. 1985, 69, 896–898. [Google Scholar]
- Sha, A.H.; Lin, X.H.; Huang, J.B.; Zhang, D.P. Analysis of DNA methylation related to rice adult plant resistance to bacterial blight based on methylation-sensitive AFLP (MSAP) analysis. Mol. Genet. Genom. 2005, 273, 484–490. [Google Scholar] [CrossRef]
- Farinhó, M.; Coelho, P.; Carlier, J.; Svetleva, D.; Monteiro, A.; Leitão, J. Mapping of a locus for adult plant resistance to downy mildew in broccoli (Brassica oleracea convar. italica). Theor. Appl. Genet. 2004, 109, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Minaas, E.S.; Walid, M.E.O.; Reda, I.O. Seedling and adult plant resistance to leaf rust in some Egyptian wheat genotypes. Afr. J. Agric. Res. 2016, 11, 247–258. [Google Scholar] [CrossRef][Green Version]
- Guest, D.; Brown, J. Plant defence against pathogens. In Plant Pathogens and Plant Diseases; Brown, J.F., Ogle, H.J., Eds.; Rockvale Publications for the Division of Botany, Rockvale Publications for the Division of Botany, School of Rural Science and Natural Resources, University of New England: Armidale New South Wales, UK, 1997. [Google Scholar]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Bonnema, G.; Lee, J.G.; Shuhang, W.; Lagarrigue, D.; Bucher, J.; Wehrens, R.; De Vos, R.; Beekwilder, J. Glucosinolate variability between turnip organs during development. PLoS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).