Polarity-Based Sequential Extraction as a Simple Tool to Reveal the Structural Complexity of Humic Acids
Abstract
1. Introduction
2. Materials and Methods
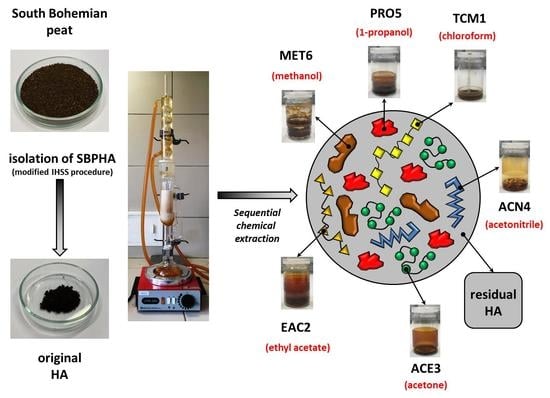
2.1. Origin and Isolation of Humic Acids
2.2. Sequential Chemical Extraction
2.3. Organic Fractions Analysis
2.3.1. Elemental Composition
2.3.2. UV/Vis Spectrometry
2.3.3. ATR-FTIR Spectrometry
2.3.4. Fluorescence Spectrometry
2.3.5. Liquid-state 13C NMR Spectroscopy
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Yields, Ash Contents, and Elemental Analysis
3.2. UV/Vis Spectrometry
3.3. ATR-FTIR Spectrometry
3.4. Fluorescence Spectrometry
3.5. Liquid-State 13C NMR Spectrometry
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleber, M.; Lehmann, J. Humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems. J. Environ. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Myneni, S.C.B. Chemistry of Natural Organic Matter—The Next Step: Commentary on a humic substances debate. J. Environ. Qual. 2019, 48, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zheng, Q.; Noll, L.; Zhang, S.; Wanek, W. Direct measurement of the in situ decomposition of microbial-derived soil organic matter. Soil Biol. Biochem. 2020, 141, 107660. [Google Scholar] [CrossRef]
- Hayes, M.H.B. Solvent systems for the isolation of organic components from soils. Soil Sci. Soc. Am. J. 2006, 70, 986–994. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Scott, R.L. Regular Solutions, 1st ed.; Prentice-Hall, Inc.: Englewood Cliffe, NJ, USA, 1962; p. 180. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 544. [Google Scholar]
- Senesi, N.; Testini, C.; Polemio, M. Chemical and spectroscopic characterization of soil organic matter fractions isolated by sequential extraction procedure. J. Soil Sci. 1983, 34, 801–813. [Google Scholar] [CrossRef]
- Piccolo, A. Characteristic of soil humic extracts obtained by some organic and inorganic solvents and purified by HCl-HF treatment. Soil Sci. 1988, 146, 418–426. [Google Scholar] [CrossRef]
- Fujitake, N.; Kusumato, A.; Tsukamato, M.; Kawahigashi, M.; Suzuki, T.; Otsuka, H. Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs. I. Yield and particle size distribution. Soil Sci. Plant Nutr. 1998, 44, 253–260. [Google Scholar] [CrossRef]
- Li, L.; Huang, W.; Peng, P.; Sheng, G.; Fu, J. Chemical and molecular heterogeneity of humic acids repetitively extracted from a peat. Soil Sci. Soc. Am. J. 2003, 67, 740–746. [Google Scholar] [CrossRef]
- Olk, D.C. A Chemical fractionation for structure-function relations of soil organic matter in nutrient cycling. Soil Sci. Soc. Am. J. 2006, 70, 1013–1022. [Google Scholar] [CrossRef]
- Shirshova, L.T.; Ghabbour, E.A.; Davies, G. Spectroscopic characterization of humic acid fractions isolated from soil using different extraction procedures. Geoderma 2006, 133, 204–216. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Basis of a humeomics science: Chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromolecules 2011, 12, 1187–1199. [Google Scholar] [CrossRef]
- Vinci, G.; Mazzei, P.; Drosos, M.; Zaccone, C.; Piccolo, A. Molecular characterization of ombrotrophic peats by humeomics. Chem. Biol. Technol. Agric. 2020, 7, 18. [Google Scholar] [CrossRef]
- Drosos, M.; Nebbioso, A.; Mazzei, P.; Vinci, G.; Spaccini, R.; Piccolo, A. A molecular zoom into soil Humeome by a direct sequential chemical fractionation of soil. Sci. Total Environ. 2017, 586, 807–816. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; WILEY-VCH: Weinheim, Germany, 2010; p. 718. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Baltimore, MD, USA, 2006; p. 954. [Google Scholar]
- Novák, F.; Šestauberová, M.; Hrabal, R. Structural features of lignohumic acids. J. Mol. Struct. 2015, 1093, 179–185. [Google Scholar] [CrossRef]
- Thomas, J.D.R. Chemistry of peat bitumen: Fractionation and infra-red studies. J. Appl. Chem. 2007, 12, 289–294. [Google Scholar] [CrossRef]
- Doskočil, L.; Enev, V.; Pekař, M.; Wasserbauer, J. The spectrometric characterization of lipids extracted from lignite samples from various coal basins. Org. Geochem. 2016, 95, 34–40. [Google Scholar] [CrossRef]
- Martins, T.; Saab, S.D.C.; Milori, D.M.B.P.; Brinatti, A.M.; Rosa, J.A.; Cassaro, F.A.M.; Pires, L.F. Soil organic matter humification under different tillage managements evaluated by Laser Induced Fluorescence (LIF) and C/N ratio. Soil Tillage Res. 2011, 111, 231–235. [Google Scholar] [CrossRef]
- Ma, X.; Green, S.A. Fractionation and spectroscopic properties of fulvic acid and its extract. Chemosphere 2008, 72, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Korshin, G.V.; Li, C.-W.; Benjamin, M.M. Monitoring the properties of natural organic matter through UV spectroscopy: A consistent theory. Water Res. 1997, 31, 1787–1795. [Google Scholar] [CrossRef]
- del Vecchio, R.; Blough, N.V. On the origin of the optical properties of humic substances. Environ. Sci. Technol. 2004, 38, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Baigorri, R.; Fuentes, M.; González-Gaitano, G.; García-Mina, J.M. Analysis of molecular aggregation in humic substances in solution. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 301–306. [Google Scholar] [CrossRef]
- Song, J.; Jin, X.; Wang, X.C.; Jin, P. Preferential binding properties of carboxyl and hydroxyl groups with aluminium salts for humic acid removal. Chemosphere 2019, 234, 478–487. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Zinc binding to fulvic acids: Assessing the impact of pH, metal concentrations and chemical properties of fulvic acids on the mechanism and stability of formed soluble complexes. Molecules 2020, 25, 1297. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical characterization and antioxidant activity of humic acids isolated from peat of various origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and 1H NMR techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; LeBooeuf, E.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Maeng, S.K.; Timmes, T.C.; Kim, H.-C. Characterization of EfOM fraction responsible for short-term fouling in ultrafiltration. Sep. Sci. Technol. 2015, 50, 2697–2707. [Google Scholar] [CrossRef]
- Tinoco, P.; Almendros, G.; González-Vila, F.J.; Sanz, J.; González-Pérez, J.A. Revisiting molecular characteristics responsive for the aromaticity of soil humic acids. J. Soils Sediments 2015, 15, 781–791. [Google Scholar] [CrossRef]
- Yakimenko, O.; Khundzhua, D.; Izosimov, A.; Yuzhakov, V.; Patsaeva, S. Source indicator of commercial humic products: UV-Vis and fluorescence proxies. J. Soils Sediments 2018, 18, 1279–1291. [Google Scholar] [CrossRef]
- Kumada, K.; Sato, O. Characteristics of the green fraction of P type humic acid. J. Soil Sci. Plant Nutr. 1980, 26, 309–316. [Google Scholar] [CrossRef]
- Davis, W.M.; Erickson, C.L.; Johnston, C.T.; Delfino, J.J.; Porter, J.E. Quantitative fourier transform infrared spectroscopic investigation humic substance functional group composition. Chemosphere 1999, 38, 2913–2928. [Google Scholar] [CrossRef]
- Senesi, N.; Sipos, S. Molecular weight distribution, analytical and spectroscopic characterization of humic fractions sequentially isolated by organic solvents from a brown coal humic acid. Org. Geochem. 1985, 8, 157–162. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 244. [Google Scholar]
- Baes, A.U.; Bloom, P.R. Diffuse reflectance and transmission fourier transform infrared (DRIFT) spectroscopy of humic and fulvic acids. Soil Sci. Soc. Am. J. 1989, 53, 695–700. [Google Scholar] [CrossRef]
- Terkhi, M.C.; Taleb, F.; Gossart, P.; Semmoud, A.; Addou, A. Fourier transform infrared study of mercury interaction with carboxyl groups in humic acids. J. Photochem. Photobiol. A 2008, 198, 205–214. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Hanc, A.; Enev, V.; Hrebeckova, T.; Klucakova, M.; Pekar, M. Characterization of humic acids in a continuous-feeding vermicomposting system with horse manure. Waste Manag. 2019, 99. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N.; Miano, T.M.; Provenzano, M.R.; Brunetti, G. Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy. Soil Sci. 1991, 152, 259–271. [Google Scholar] [CrossRef]
- Enev, V.; Pospíšilová, Ľ.; Klučáková, M.; Liptaj, T.; Doskočil, L. Spectral characterization of selected humic substances. Soil Water Res. 2014, 9, 9–17. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Katsoyiannis, I.A.; Ernst, M.; Zouboulis, A.I. Impact of O3 or O3/H2O2 treatment via a membrane contacting system on the composition and characteristics of the natural organic matter of surface waters. Environ. Sci. Pollut. Res. 2018, 25, 12246–12255. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-F.; Dou, S.; Wang, Z.-G. Structural analysis of humic acid in soil at different corn straw returning modes through fluorescence spectroscopy and infrared spectroscopy. Int. J. Anal. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Sierra, M.M.D.; Giovanela, M.; Parlanti, E.; Soriano-Sierra, E.J. Fluorescence fingerprint of fulvic and humic acids from varied origins as viewed by single-scan and excitation/emission matrix techniques. Chemosphere 2005, 58, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Benedetti, M.F.; Han, W.; Korshin, G.V. Comparison of the properties of standard soil and aquatic fulvic and humic acids based on the data of differential absorbance and fluorescence spectroscopy. Chemosphere 2020, 261, 128189. [Google Scholar] [CrossRef]
- Alberts, J.J.; Takács, M. Total luminescence spectra of IHSS standard and reference fulvic acids, humic acids and natural organic matter: Comparison of aquatic and terrestrial source terms. Org. Geochem. 2004, 35, 243–256. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. A comprehensive structural evaluation of humic substances using several fluorescence techniques before and after ozonation. Part I: Structural characterization of humic substances. Sci. Total Environ. 2014, 476–477, 718–730. [Google Scholar] [CrossRef]
- Doskočil, L.; Burdíková-Szewieczková, J.; Enev, V.; Kalina, L.; Wasserbauer, J. Spectral characterization and comparison of humic acids isolated from some European lignites. Fuel 2018, 213, 123–132. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Yang, L.; Hur, J.; Zhuang, W. Occurrence and behaviors of fluorescence EEM-PARAFAC components in drinking water and wastewater treatment systems and their applications: A review. Environ. Sci. Pollut. Res. 2015, 22, 6500–6510. [Google Scholar] [CrossRef]
- Yu, M.-D.; Xi, B.-D.; Zhu, Z.-Q.; Zhang, L.; Yang, C.; Geng, C.-M.; He, X.-S. Fate and removal of aromatic organic matter upon a combined leachate treatment process. Chem. Eng. J. 2020, 401, 126157. [Google Scholar] [CrossRef]
- Zhou, Y.; Martin, P.; Müller, M. Composition and cycling of dissolved organic matter from tropical peatlands of coastal Sarawak, Borneo, revealed by fluorescence spectroscopy and parallel factor analysis. Biogeosciences 2019, 16, 2733–2749. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Sun, J.; Guo, L.; Li, Q.; Zhao, Y.; Gao, M.; She, Z.; Jin, C. Three-dimensional fluorescence excitation-emission matrix (EEM) spectroscopy with regional integration analysis for assessing waste sludge hydrolysis at different pretreated temperatures. Environ. Sci. Pollut. Res. 2016, 23, 24061–24067. [Google Scholar] [CrossRef] [PubMed]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV-Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, J. Reducing capacities in continuously released low molecular weight fractions from bulk humic acids. J. Environ. Manag. 2019, 244, 172–179. [Google Scholar] [CrossRef]
- Coble, P.G.; Lead, J.; Baker, A.; Reynolds, D.M.; Spencer, R.G.M. Aquatic Organic Matter Fluorescence, 1st ed.; Cambridge University Press: New York, NY, USA, 2014; p. 418. [Google Scholar]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Boyle, E.S.; Guerriero, N.; Thiallet, A.; del Vecchio, R.; Blough, N.V. Optical properties of humic substances and CDOM: Relation to structure. Environ. Sci. Technol. 2009, 43, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Boguta, P.; D’Orazio, V.; Sokołowska, Z.; Senesi, N. Effects of selected chemical and physicochemical properties of humic acids from peat soils on their interaction mechanisms with copper ions at various pHs. J. Geochem. Explor. 2016, 168, 119–126. [Google Scholar] [CrossRef]
- Conte, P.; Piccolo, A.; van Lagen, B.; Buurman, P.; de Jager, P.A. Quantitative differences in evaluating soil humic substances by liquid- and solid-state 13C-NMR spectroscopy. Geoderma 1997, 80, 339–352. [Google Scholar] [CrossRef]
- Khatami, S.; Deng, Y.; Tien, M.; Hatcher, P.G. Formation of water-soluble organic matter through fungal degradation of lignin. Org. Geochem. 2019, 135, 64–70. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Waggoner, D.; Chen, H. Evidence for the existence of humic acids in peat soils based on solid-state 13C NMR. J. Environ. Qual. 2019, 48, 1571–1577. [Google Scholar] [CrossRef]
- Schnitzer, M. Soil organic matter—The next 75 years. Soil Sci. 1991, 151, 41–58. [Google Scholar] [CrossRef]



| Yield | Ash | C | H | N | O | H/C | O/C | N/C | |
|---|---|---|---|---|---|---|---|---|---|
| (wt.%) 1 | (wt.%) 1 | (at.%) 2 | |||||||
| SBPHA | - | 0.7 | 35.00 ± 0.24 | 49.84 ± 0.16 | 1.12 ± 0.09 | 14.03 | 1.42 | 0.40 | 0.032 |
| TCM1 | 3.76 | 0.07 ± 0.02 | 30.83 ± 0.11 | 67.10 ± 0.32 | 0.15 ± 0.02 | 1.92 | 2.18 | 0.06 | 0.005 |
| EAC2 | 1.10 | 1.81 ± 0.09 | 33.30 ± 0.16 | 65.65 ± 0.28 | 0.63 ± 0.05 | 0.42 | 1.97 | 0.01 | 0.019 |
| ACE3 | 13.00 | 0.34 ± 0.04 | 43.78 ± 0.20 | 34.62 ± 0.13 | 0.82 ± 0.05 | 20.77 | 0.79 | 0.47 | 0.019 |
| ACN4 | 7.83 | 4.10 ± 0.08 | 30.52 ± 0.34 | 49.87 ± 0.19 | 3.30 ± 0.10 | 16.31 | 1.63 | 0.53 | 0.108 |
| PRO5 | 19.74 | 0.83 ± 0.17 | 37.22 ± 0.21 | 44.23 ± 0.36 | 0.94 ± 0.07 | 17.61 | 1.19 | 0.47 | 0.025 |
| MET6 | 11.91 | 2.99 ± 0.12 | 34.62 ± 0.37 | 46.18 ± 0.22 | 1.43 ± 0.12 | 17.77 | 1.33 | 0.51 | 0.041 |
| UV/Vis Ratios | UV Parameter | FTIR Intensity Ratios | |||
|---|---|---|---|---|---|
| EET/EBz | E2/E4 | SUVA254 1 | IAr/ICOOH2 | ICH2/ICH33 | |
| SBPHA | 0.74 ± 0.06 | 5.53 ± 0.12 | 5.68 | 1.26 | 1.06 |
| TCM1 | 0.51 ± 0.02 | 11.87 ± 0.15 | 0.24 | 0.22 | 1.81 |
| EAC2 | 0.70 ± 0.02 | 12.00 ± 0.06 | 0.66 | 0.34 | 1.87 |
| ACE3 | 0.74 ± 0.01 | 9.77 ± 0.11 | 5.89 | 0.80 | 1.11 |
| ACN4 | 0.66 ± 0.00 | 9.53 ± 0.10 | 2.03 | 1.44 | 1.11 |
| PRO5 | 0.74 ± 0.04 | 7.64 ± 0.14 | 6.40 | 1.06 | 0.98 |
| MET6 | 0.83 ± 0.02 | 7.18 ± 0.22 | 5.79 | 1.21 | 1.04 |
| Fluorescence Peak Region | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | C | V | B | H | ||||||
| Ex/Em (nm) | IF (CPS) 1 | Ex/Em (nm) | IF (CPS) 1 | Ex/Em (nm) | IF (CPS) 1 | Ex/Em (nm) | IF (CPS) 1 | Ex/Em (nm) | IF (CPS) 1 | |
| SBPHA | 255/495 | 1.77 | ||||||||
| TCM1 | 270/305 | 6.86 | 255/355 | 3.23 | ||||||
| EAC2 2 | 250/415 | 4.36 | 270/305 | 0.99 | ||||||
| ACE3 | 255/465 | 4.22 | ||||||||
| ACN4 | 255/445 | 4.49 | 300/435 | 2.74 | 270/315 | 0.98 | ||||
| PRO5 | 265/500 | 2.76 | 445/530 | 0.80 | ||||||
| MET6 | 260/505 | 2.57 | 435/525 | 0.72 | 250/300 | 0.99 | ||||
| Organic Fractions | Average Distribution of Individual Carbon Types (%) | (fa) 1 | |||||
|---|---|---|---|---|---|---|---|
| 0–45 ppm | 45–106 ppm | 106–145 ppm | 145–165 ppm | 165–190 ppm | 190–220 ppm | ||
| TCM1 | 78 | 8 | 8 | 2 | 3 | 1 | 0.10 |
| ACE3 | 23 | 19 | 36 | 11 | 9 | 2 | 0.53 |
| PRO5 | 24 | 22 | 33 | 9 | 10 | 2 | 0.48 |
| MET6 | 16 | 30 | 31 | 8 | 13 | 2 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enev, V.; Sedláček, P.; Kubíková, L.; Sovová, Š.; Doskočil, L.; Klučáková, M.; Pekař, M. Polarity-Based Sequential Extraction as a Simple Tool to Reveal the Structural Complexity of Humic Acids. Agronomy 2021, 11, 587. https://doi.org/10.3390/agronomy11030587
Enev V, Sedláček P, Kubíková L, Sovová Š, Doskočil L, Klučáková M, Pekař M. Polarity-Based Sequential Extraction as a Simple Tool to Reveal the Structural Complexity of Humic Acids. Agronomy. 2021; 11(3):587. https://doi.org/10.3390/agronomy11030587
Chicago/Turabian StyleEnev, Vojtěch, Petr Sedláček, Leona Kubíková, Šárka Sovová, Leoš Doskočil, Martina Klučáková, and Miloslav Pekař. 2021. "Polarity-Based Sequential Extraction as a Simple Tool to Reveal the Structural Complexity of Humic Acids" Agronomy 11, no. 3: 587. https://doi.org/10.3390/agronomy11030587
APA StyleEnev, V., Sedláček, P., Kubíková, L., Sovová, Š., Doskočil, L., Klučáková, M., & Pekař, M. (2021). Polarity-Based Sequential Extraction as a Simple Tool to Reveal the Structural Complexity of Humic Acids. Agronomy, 11(3), 587. https://doi.org/10.3390/agronomy11030587