A Mixture of Green Waste Compost and Biomass Combustion Ash for Recycled Nutrient Delivery to Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Physicochemical Characterization
2.2. BA and GWC Mixtures for Composting
2.3. Field Experiment Description and Experimental Design
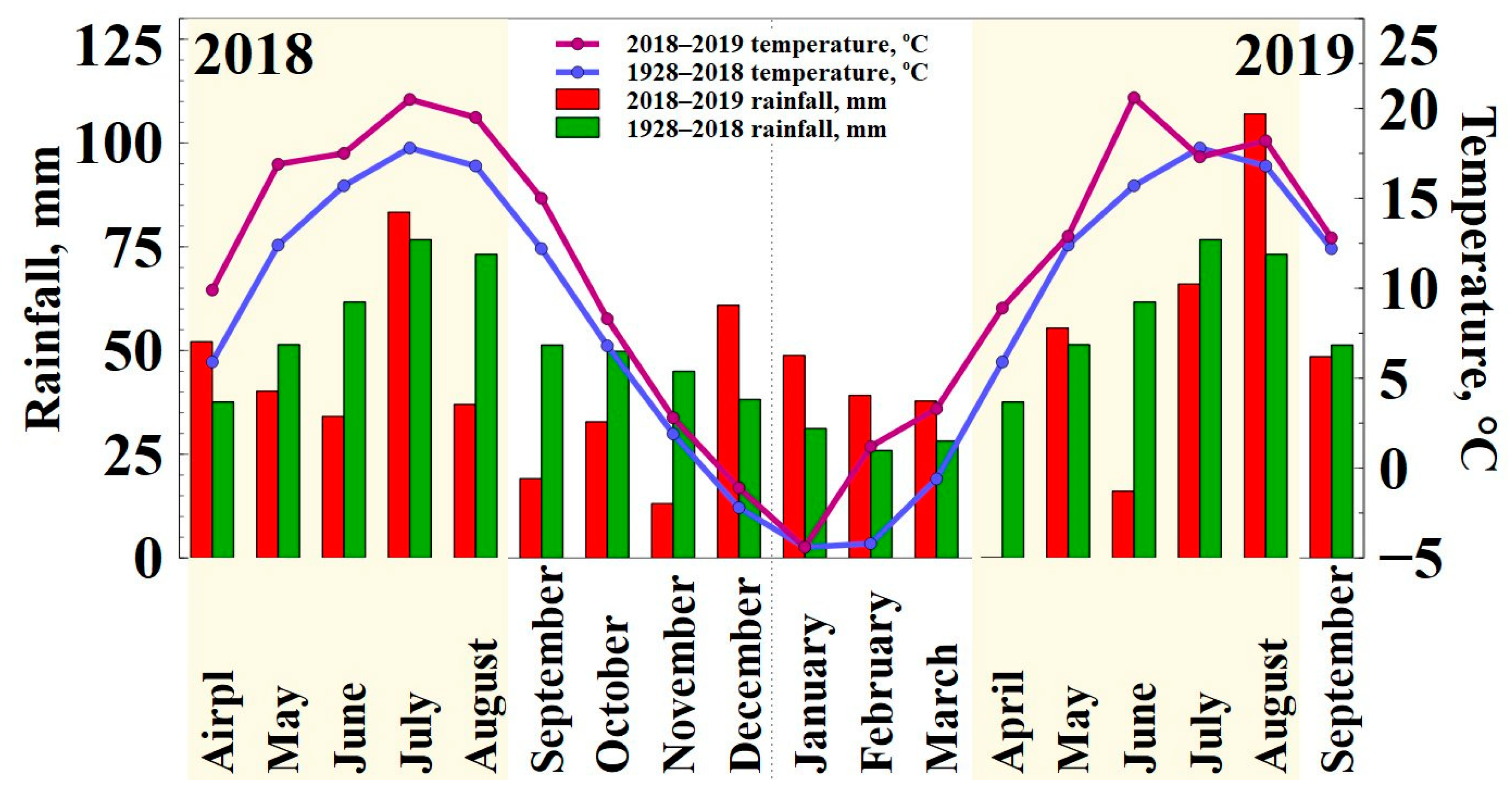
2.4. Meteorological Conditions
2.5. Statistical Analysis
3. Results and Discussion
3.1. Nutrient Composition of the BA, GWC, and GWC + BA Mixtures
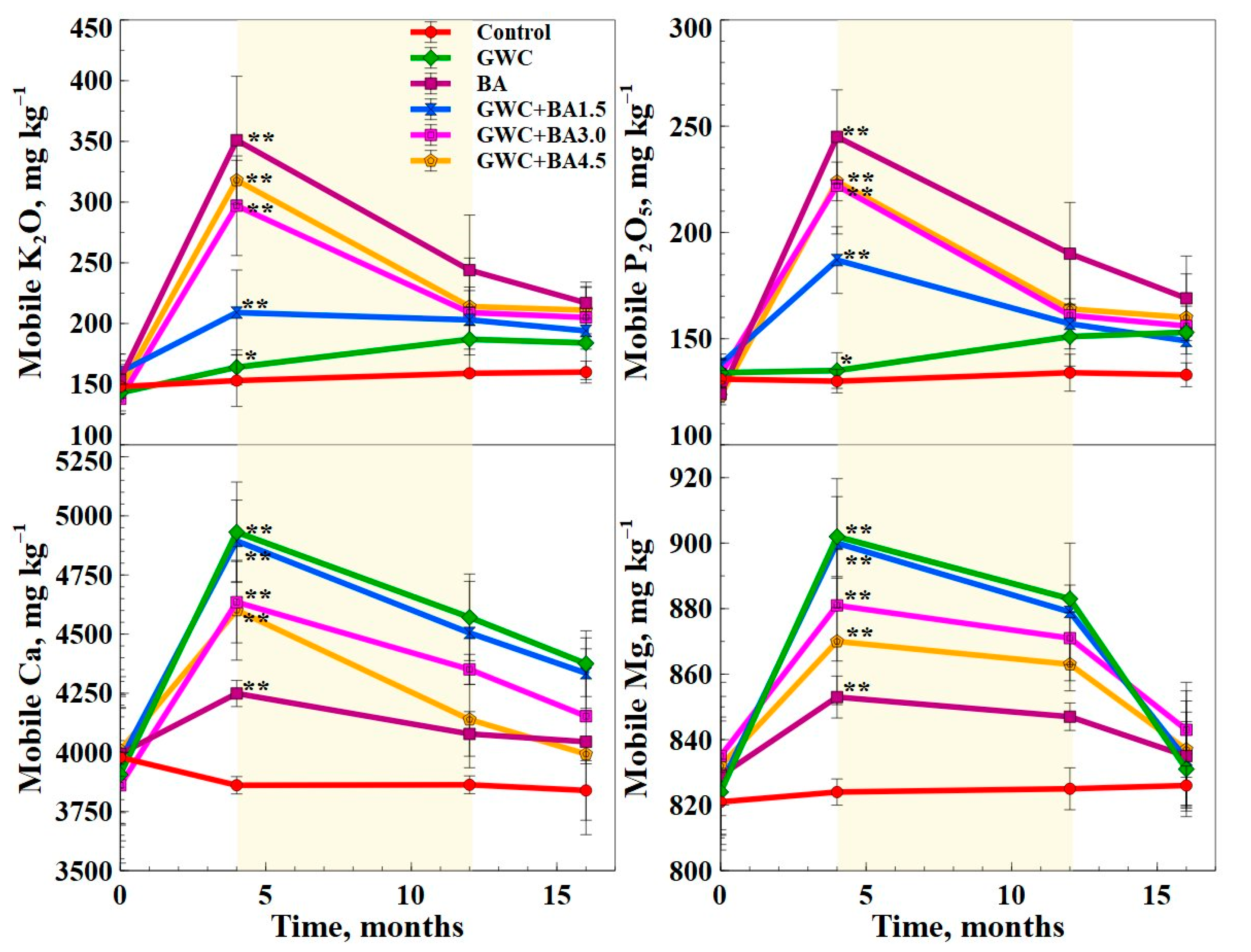
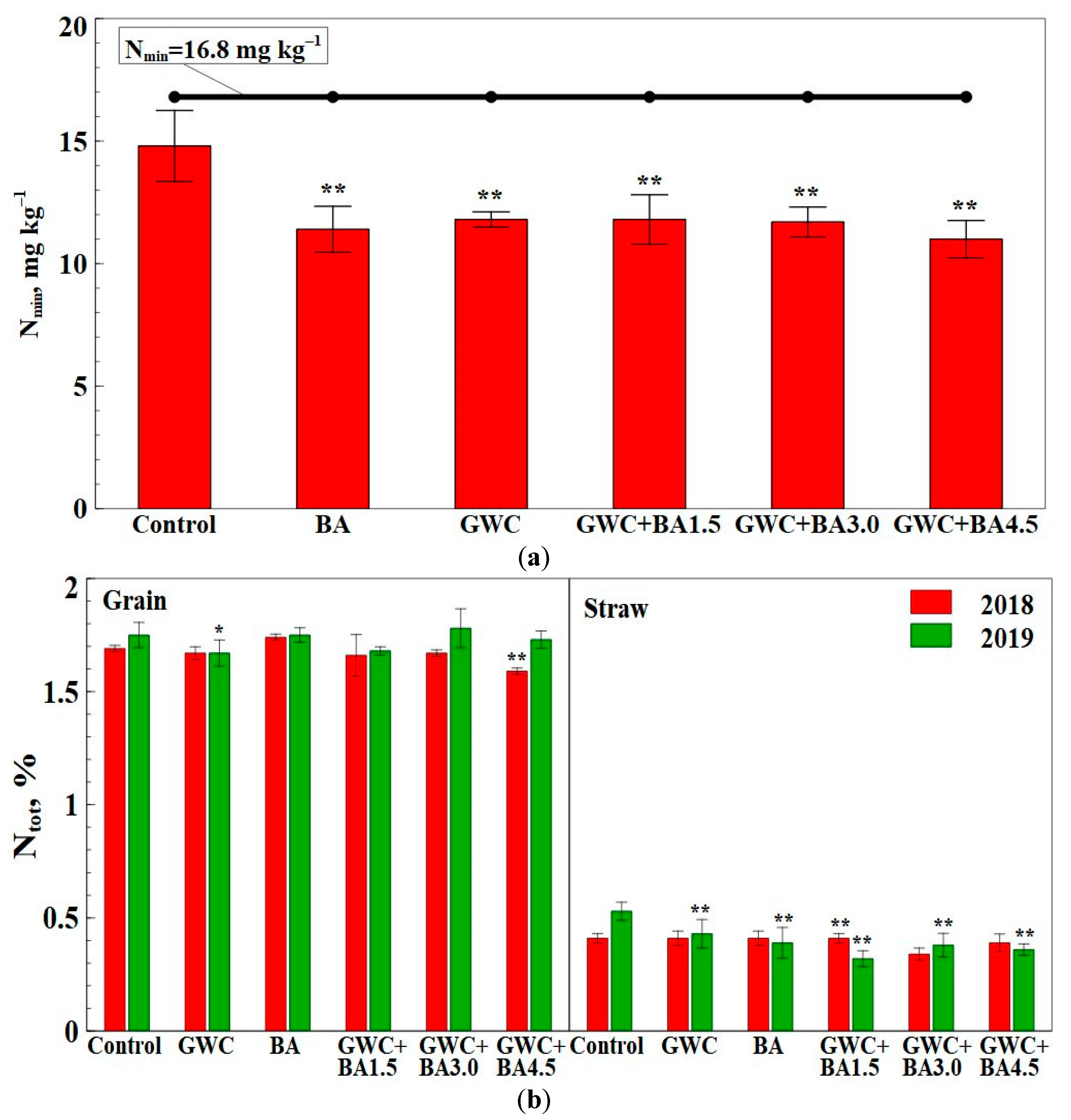
3.2. Nutrient and Heavy Metal Release from GWC + BA Mixtures as Measured via Chemical Composition of the Soil
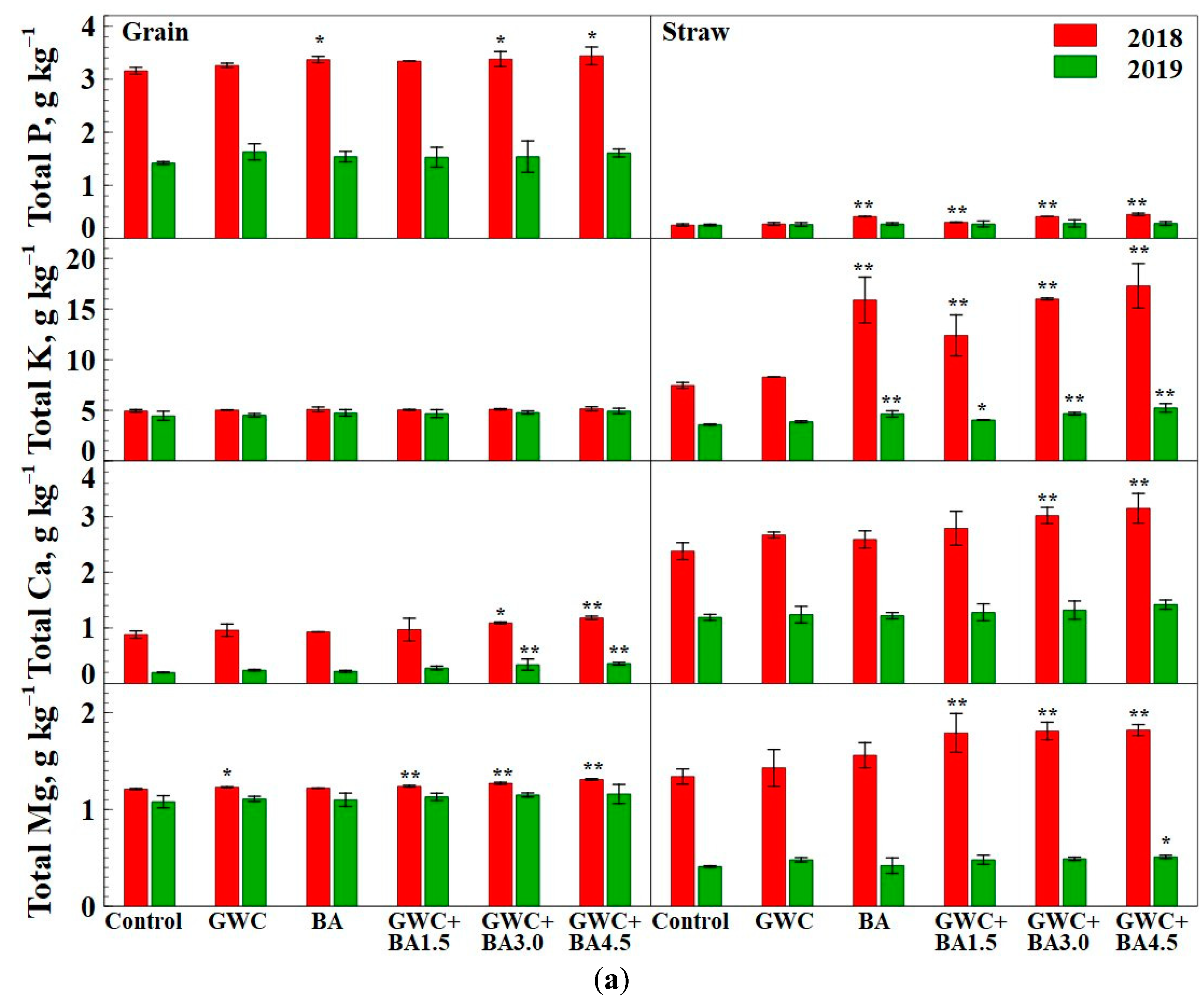
3.3. Chemical Composition of Major Nutrients and Heavy Metals in Spring Wheat and Spring Triticale Grain and Straw
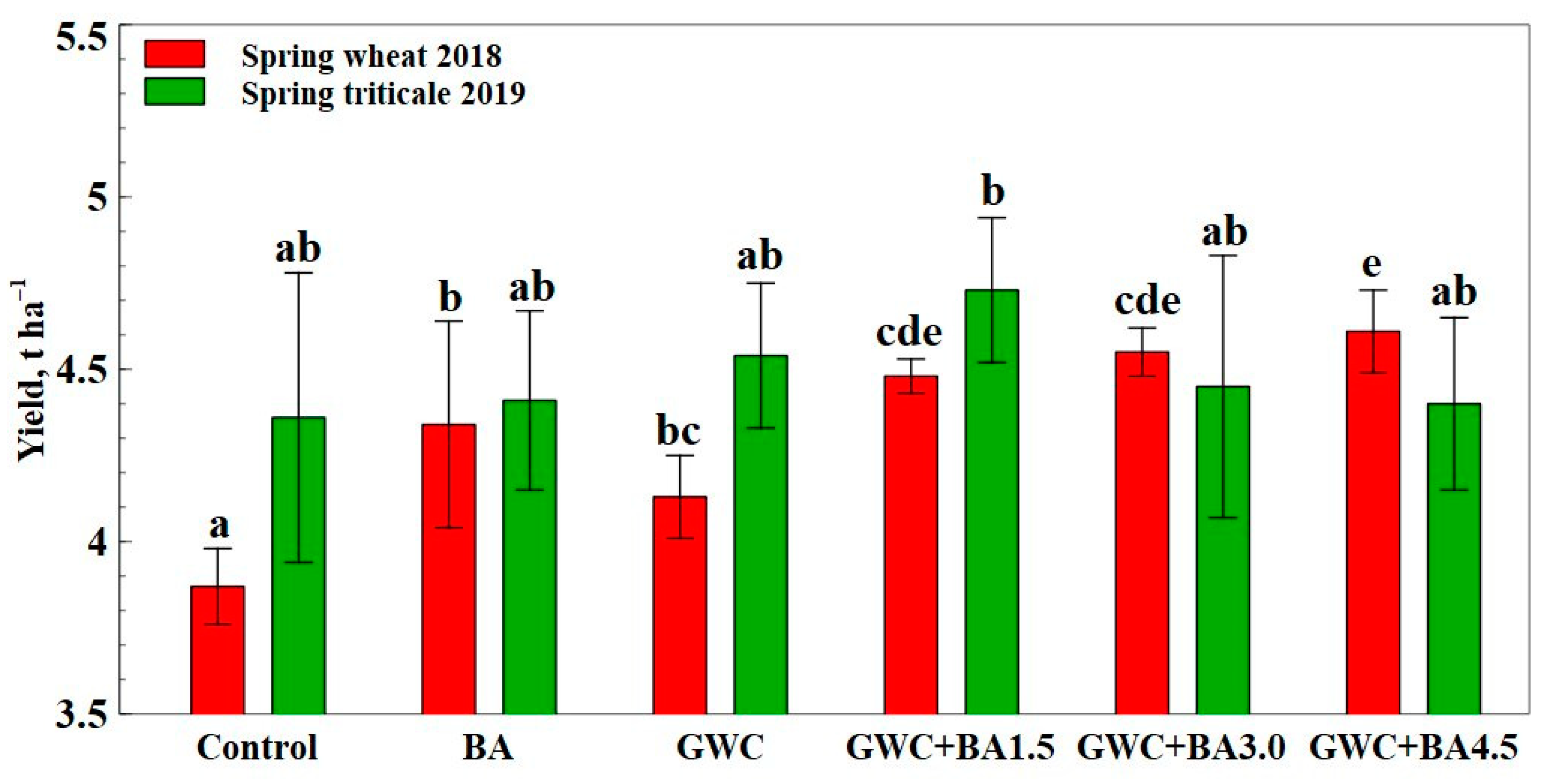
3.4. Quantifying Spring Wheat and Spring Triticale Grain Yields
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karak, T.; Bhagat, R.M.; Bhattacharyya, P. Municipal solid waste generation, composition, and management: The world scenario. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1509–1630. [Google Scholar] [CrossRef]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of incineration MSW ash: A review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Brannvall, E.; Nilsson, M.; Sjoblom, R.; Skoglund, N.; Kumpiene, J. Effect of residue combinations on plant uptake of nutrients and potentially toxic elements. J. Environ. Manag. 2014, 132, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Ronn, R.; Kjoller, R.; Hansen, H.C.B.; Jacobsen, C.S. Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Bougnom, B.P.; Insam, H. Ash additives to compost affect soil microbial communities and apple seedling growth. Die Bodenkult. 2009, 60, 5–15. [Google Scholar]
- Fernandez-Delgado Juarez, M.; Prahauser, B.; Walter, A.; Insam, H.; Franke-Whittle, I.H. Co-composting of biowaste and wood ash, influence on a microbially driven-process. Waste Manag. 2015, 46, 155–164. [Google Scholar] [CrossRef]
- Karnchanawong, S.; Najarut, Y. Effects of composts from co-composting of tree pruning waste, cow dung, and biomass fly ash on soil acidity neutralization and growth of chinese cabbage. Adv. Mater. Res. 2014, 954, 168–172. [Google Scholar] [CrossRef]
- Huotari, N.; Tillman-Sutela, E.; Moilanen, M.; Laiho, R. Recycling of ash—For the good of the environment? For. Ecol. Manag. 2015, 348, 226–240. [Google Scholar] [CrossRef]
- Yao, Q.; Samad, N.B.; Keller, B.; Seah, X.S.; Huang, L.; Lau, R. Mobility of heavy metals and rare earth elements in incineration bottom ash through particle size reduction. Chem. Eng. Sci. 2014, 118, 214–220. [Google Scholar] [CrossRef]
- Kuba, T.; Tscholl, A.; Partl, C.; Meyer, K.; Insam, H. Wood ash admixture to organic wastes improves compost and its performance. Agric. Ecosyst. Environ. 2008, 127, 43–49. [Google Scholar] [CrossRef]
- Jokinen, H.K.; Kiikkila, O.; Fritze, H. Exploring the mechanisms behind elevated microbial activity after wood ash application. Soil Biol. Biochem. 2006, 38, 2285–2291. [Google Scholar] [CrossRef]
- Koivula, N.; Raikkonen, T.; Urpilainen, S.; Ranta, J.; Hanninen, K. Ash in composting of source-separated catering waste. Bioresour. Technol. 2004, 93, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Buneviciene, K.; Drapanauskaite, D.; Mazeika, R.; Tilvikiene, V.; Baltrusaitis, J. Granulated biofuel ash as a sustainable source of plant nutrients. Waste Manag. Res. 2020, in press. [Google Scholar] [CrossRef]
- Buneviciene, K.; Drapanauskaite, D.; Mazeika, R.; Tilvikiene, V. Biofuel ash granules as a source of soil and plant nutrients. Zemdirb. Agric. 2021, 108, 19–26. [Google Scholar] [CrossRef]
- Višniauskė, I.; Barčauskaitė, K.; Bakšienė, E.; Mažeika, R. Evaluation of contamination levels of different types of composts and their suitability for usage in agriculture. Zemdirb. Agric. 2018, 105, 211–220. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Mair, J.; Etoa, F.X.; Insam, H. Composts with wood ash addition: A risk or a chance for ameliorating acid tropical soils? Geoderma 2009, 153, 402–407. [Google Scholar] [CrossRef]
- The Council of European Communities. Council directive concerning the protection of waters against pollution caused by nitrates from agricultural sources (91/676/EEC). Off. J. Eur. Commun. 1991, L 375, 1–8.
- Asquer, C.; Cappai, G.; De Gioannis, G.; Muntoni, A.; Piredda, M.; Spiga, D. Biomass ash reutilisation as an additive in the composting process of organic fraction of municipal solid waste. Waste Manag. 2017, 69, 127–135. [Google Scholar] [CrossRef]
- WBR. World Reference base for Soil Resources; WBR: New York, NY, USA, 2014; Volume 43, pp. 187–189. [Google Scholar] [CrossRef]
- Oreshkin, N.G. Extraction of mobile forms of phosphorus and potassium by the Egner-Riehm-Domingo method. Agrokhimiia 1980, 8, 135–138. [Google Scholar]
- SAS Institute. The SAS System for Windows Version 9.3; SAS Institute: Cary, NC, USA, 2011. [Google Scholar]
- Abou-el-Seoud, I.I.; Abdel-Megeed, A. Impact of rock materials and biofertilizations on P and K availability for maize (Zea Maize) under calcareous soil conditions. Saudi J. Biol. Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef]
- Barčauskaitė, K.; Žydelis, R.; Mažeika, R. Screening of chemical composition and risk index of different origin composts produced in Lithuania. Environ. Sci. Pollut. Res. 2020, 27, 24480–24494. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.M.M.; Sarkar, G.; Alamgir, M.; Bari, Q.H.; Haedrich, G. Study on the quality and stability of compost through a demo compost plant. Waste Manag. 2012, 32, 2046–2055. [Google Scholar] [CrossRef]
- Barrena, R.; Font, X.; Gabarrell, X.; Sanchez, A. Home composting versus industrial composting: Influence of composting system on compost quality with focus on compost stability. Waste Manag. 2014, 34, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Bera, R.; Datta, A.; Bose, S.; Dolui, A.K.; Chatterjee, A.K.; Dey, G.C.; Barik, A.K.; Sarkar, R.K.; Majumdar, D.; Seal, A. Comparative evaluation of compost quality, process convenience and cost under different composting methods to assess their large scale adoptability potential as also complemented by compost quality index. Int. J. Sci. Res. Publ. 2013, 3, 1–11. [Google Scholar]
- Suhu, S.K.; Pattanayak, S.K.; Mahananda, M.R. Compost quality assessment for successful organic waste recycling. An Int. Q. J. Environ. Sci. 2013, 3, 199–203. [Google Scholar] [CrossRef]
- Karltun, E.; Saarsalmi, A.; Ingerslev, M.; Mandre, M.; Andersson, S.; Gaitnieks, T.; Ozolinčius, R.; Varnagiryte-Kabasinskiene, I. Wood ash recycling—Possibilities and risks. In Sustainable Use of Forest Biomass for Energy; Springer: Dordrecht, The Netherlands, 2008; pp. 79–108. [Google Scholar] [CrossRef]
- Knapp, B.A.; Insam, H. Current technologies and future research needs. In Recycling of Biomass Ashes; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–16. [Google Scholar] [CrossRef]
- Pesonen, J.; Kuokkanen, V.; Kuokkanen, T.; Illikainen, M. Co-granulation of bio-ash with sewage sludge and lime for fertilizer use. J. Environ. Chem. Eng. 2016, 4, 4817–4821. [Google Scholar] [CrossRef]
- Mainardis, M.; Flaibani, S.; Mazzolini, F.; Peressotti, A.; Goi, D. Techno-economic analysis of anaerobic digestion implementation in small Italian breweries and evaluation of biochar and granular activated carbon addition effect on methane yield. J. Environ. Chem. Eng. 2019, 7, 103184. [Google Scholar] [CrossRef]
- Aissaoui, H.; Barkat, D. Physico-chemical characterizations and impact of organic matter on the dynamics of heavy metals (Cu, and Zn) in some soils of Biskra (Algeria). J. King Saud Univ. Sci. 2018, 32, 307–311. [Google Scholar] [CrossRef]
- Yliniemi, J.; Pesonen, J.; Tiainen, M.; Illikainen, M. Alkali activation of recovered fuel-biofuel fly ash from fluidised-bed combustion: Stabilisation/solidification of heavy metals. Waste Manag. 2015, 43, 273–282. [Google Scholar] [CrossRef]
- Lietuvos Respublikos Aplinkos Ministerija. Dėl Biologiškai Skaidžių Atliekų Kompostavimo, Anaerobinio Apdorojimo Aplinkosauginių Reikalavimų Patvirtinimo. 2007, p. D1-57. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.292658/asr (accessed on 25 March 2021).
- United States Environmental Protection Agency. Background Report on Fertilizer Use, Contaminants and Regulations; United States Environmental Protection Agency: Washington, DC, USA, 1999; pp. 1–395.
- Morais, S.; e Costa, F.G.; de Lourdes Pereir, M. Heavy metals and human health. Environ. Health Emerg. Issues Pract. 2012, 10, 227–246. [Google Scholar] [CrossRef]
- Moreno-Jimenez, E.; Fernandez, J.M.; Puschenreiter, M.; Williams, P.N.; Plaza, C. Availability and transfer to grain of As, Cd, Cu, Ni, Pb and Zn in a barley agri-system: Impact of biochar, organic and mineral fertilizers. Agric. Ecosyst. Environ. 2016, 219, 171–178. [Google Scholar] [CrossRef]
- European Commission. Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Commun. 1986, 4, 6–12. [Google Scholar]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [PubMed]
- Brod, E.; Haraldsen, T.K.; Breland, T.A. Fertilization effects of organic waste resources and bottom wood ash: Results from a pot experiment. Agric. Food Sci. 2012, 21, 332–347. [Google Scholar] [CrossRef][Green Version]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; p. 12. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2001; pp. 1–86. [Google Scholar]
- Kim, R.-Y.; Yoon, J.-K.; Kim, T.-S.; Yang, J.E.; Owens, G.; Kim, K.-R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of Setting Maximum Levels for Certain Contaminants in Foodstuffs; Cambridge University Press: Cambridge, UK, 2006; pp. 1–29. [Google Scholar] [CrossRef]
- European Commission. Applying Compost Benefits and Needs; European Commission: Brussels, Belgium, 2001; pp. 1–282. [Google Scholar]
- Adugna, G. A review on impact of compost on soil properties, water use and crop productivity. Acad. Res. J. Agric. Sci. Res. 2016, 4, 93–104. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: Mechanisms, efficacy, problems, and strategies. Water Air. Soil Pollut. 2016, 359, 1–18. [Google Scholar] [CrossRef]


| Sample | Abbreviation |
|---|---|
| Control (without any fertilizer material) data | Control |
| Biofuel ash (3.0 t h−1) | BA |
| Green waste compost (20.0 t h−1) | GWC |
| Green waste compost (20.0 t h−1) mixed with biofuel ash (1.5 t h−1) | GWC + BA1.5 |
| Green waste compost (20.0 t h−1) mixed with biofuel ash (3.0 t h−1) | GWC + BA3.0 |
| Green waste compost (20.0 t h−1) mixed with biofuel ash (4.5 t h−1) | GWC + BA4.5 |
| Nutrients | BA | GWC | GWC + BA1.5 | GWC + BA3.0 | GWC + BA4.5 |
|---|---|---|---|---|---|
| % | |||||
| Total P | 3.64 d | 0.52 a | 0.74 a,b | 0.93 b,c | 1.08 c |
| Total K | 16.9 d | 0.69 a | 1.82 b | 2.80 c | 3.61 c |
| Total Ca | 20.9 d | 3.08 a | 4.33 a,b | 5.40 b,c | 6.29 c |
| Total Mg | 3.15 e | 0.51 a | 0.70 b | 0.85 c | 0.99 d |
| Heavy Metals | BA | GWC | GWC + BA1.5 | GWC + BA3.0 | GWC + BA4.5 | Max Allowable Limits |
|---|---|---|---|---|---|---|
| mg kg−1 | ||||||
| Ni | 11.3 c | 8.44 a | 8.64 a,b | 8.81 b | 8.95 b | ≤50 |
| Cr | 43.7 c | 15.3 a | 31.1 a,b | 44.6 a,b | 55.8 b | ≤70 |
| Cd | 75.8 d | 0.60 a | 5.86 a,b | 10.4 b,c | 14.1 c | ≤2 |
| Pb | 216 d | 15.9 a | 29.9 b | 41.9 c | 51.9 c | ≤120 |
| Cu | 240 d | 21.5 a | 35.1 b | 46.8 b,c | 56.5 c | ≤300 |
| Zn | 9028 b | 128 a | 751 a | 1285 a | 1730 a | ≤800 |
| Heavy Metals | BA | GWC | GWC + BA1.5 | GWC + BA3.0 | GWC + BA4.5 | Max Allowable Limits |
|---|---|---|---|---|---|---|
| kg ha−1 | ||||||
| Ni | 0.03 | 0.17 | 0.19 | 0.20 | 0.22 | 36 |
| Cr | 0.72 | 0.31 | 0.67 | 1.03 | 1.39 | - |
| Cd | 0.23 | 0.01 | 0.13 | 0.24 | 0.35 | 4 |
| Pb | 0.65 | 0.32 | 0.64 | 0.97 | 1.29 | 100 |
| Cu | 0.65 | 0.43 | 0.75 | 1.08 | 1.40 | - |
| Zn | 27.1 | 2.56 | 16.1 | 29.6 | 43.2 | 370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buneviciene, K.; Drapanauskaite, D.; Mazeika, R.; Baltrusaitis, J. A Mixture of Green Waste Compost and Biomass Combustion Ash for Recycled Nutrient Delivery to Soil. Agronomy 2021, 11, 641. https://doi.org/10.3390/agronomy11040641
Buneviciene K, Drapanauskaite D, Mazeika R, Baltrusaitis J. A Mixture of Green Waste Compost and Biomass Combustion Ash for Recycled Nutrient Delivery to Soil. Agronomy. 2021; 11(4):641. https://doi.org/10.3390/agronomy11040641
Chicago/Turabian StyleBuneviciene, Kristina, Donata Drapanauskaite, Romas Mazeika, and Jonas Baltrusaitis. 2021. "A Mixture of Green Waste Compost and Biomass Combustion Ash for Recycled Nutrient Delivery to Soil" Agronomy 11, no. 4: 641. https://doi.org/10.3390/agronomy11040641
APA StyleBuneviciene, K., Drapanauskaite, D., Mazeika, R., & Baltrusaitis, J. (2021). A Mixture of Green Waste Compost and Biomass Combustion Ash for Recycled Nutrient Delivery to Soil. Agronomy, 11(4), 641. https://doi.org/10.3390/agronomy11040641





