Nosema locustae (Protozoa, Microsporidia), a Biological Agent for Locust and Grasshopper Control
Abstract
:1. Introduction
2. Host Spectrum
3. Pathogenicity
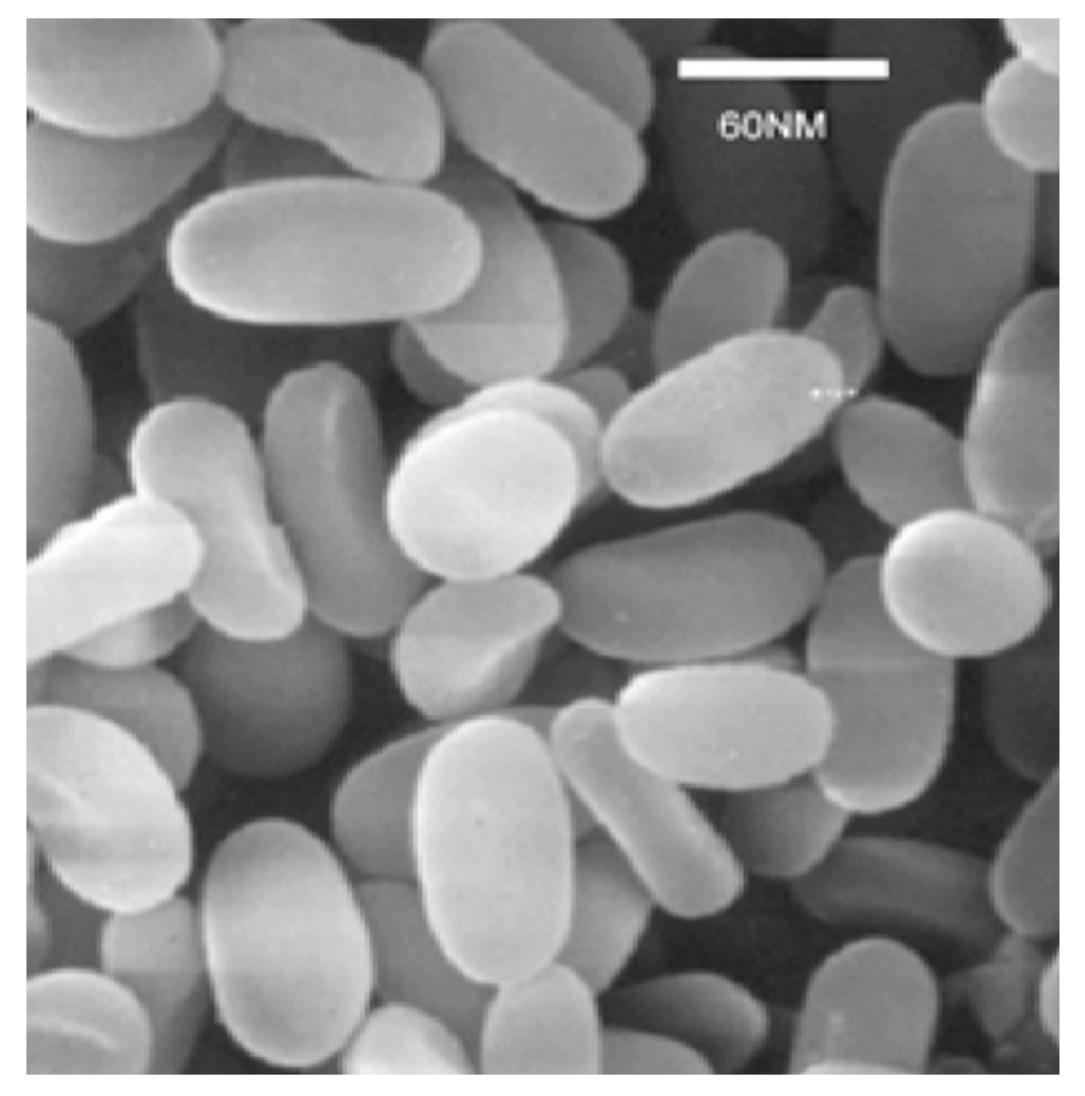
4. Genomics and Molecular Biology
5. Mass Production and Products
6. Application and Epizootics
7. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Greathead, D.J. Natural Enemies of Tropical Locusts and Grasshoppers: Their Impact and Potential as Biological Control Agents. In Biological Control of Locusts and Grasshoppers; Lomer, C.J., Prior, C., Eds.; CAB International: Wallingford, UK, 1992; pp. 105–122. [Google Scholar]
- Lomer, C.J.; Prior, C. Biological Control of Locusts and Grasshoppers; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Goettel, M.S.; Johnson, D.L. (Eds.) Microbial Control of Grasshoppers and Locusts. Mem. Entomol. Soc. Can. 1997, 129, 400. [Google Scholar] [CrossRef]
- Solter, L.F.; Becnel, J.J.; Oi, D.H. Microsporidian Entomopathogens. In Insect Pathology, 2nd ed.; Vega, F.E., Vega, H.K., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 221–263. [Google Scholar] [CrossRef]
- Keeling, P.J.; Fast, N.M. Microsporidia: Biology and Evolution of Highly Reduced Intracellular Parasites. Annu. Rev. Microbiol. 2002, 56, 93–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeling, P.J.; McFadden, G.I. Origins of Microsporidia. Trends Microbiol. 1998, 6, 19–23. [Google Scholar] [CrossRef]
- Canning, E.U. The Life Cycle of Nosema locustae Canning in Locusta migratoria migratorioides (R. & F.) and its Infectivity to other Hosts. J. Inver. Pathol. 1962, 4, 237–247. [Google Scholar]
- Canning, E.U. The Pathologenicity of Nosema locustae Canning. J. Insect Pathol. 1962, 4, 248–256. [Google Scholar]
- Henry, J.; Tiahrt, K.; Oma, E. Importance of Timing, Spore Concentrations, and Levels of Spore Carrier in Applications of Nosema locustae (Microsporida: Nosematidae) for Control of Grasshoppers. J. Invertebr. Pathol. 1973, 21, 263–272. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Ewen, A.B. Biological Control of Rangeland Grasshoppers and Locusts. In The Bionomics of Grasshoppers, Katydids and their Kin; Gangwere, S.K., Muralirangan, M.C., Muralirangan, M., Eds.; CAB International: Wallingford, UK, 1997; pp. 421–442. [Google Scholar]
- Lockwood, J.A.; Bomar, C.R.; Ewen, A.B. The History of Biological Control with Nosema locustae: Lessons for Locust Management. Int. J. Trop. Insect Sci. 1999, 19, 333–350. [Google Scholar] [CrossRef]
- Henry, J.E.; Oma, E.A. Pest Control by Nosema locustae, a Pathogen of Grasshoppers and Crickets. In Microbial Control of Pests and Plant Diseases (1970–1980); Burges, H.D., Ed.; Academic Press: New York, NY, USA, 1981; pp. 573–586. [Google Scholar]
- Henry, J.E. Natural and Applied Control of Insects by Protozoa. Annu. Rev. Entomol. 1981, 26, 49–73. [Google Scholar] [CrossRef]
- Brooks, W.M. Entomogenous Protozoa. In CRC Handbook of Natural Pesticides; Apple Academic Press: Palm Bay, FL, USA, 2019; Volume 5, pp. 1–149. [Google Scholar]
- Lomer, C.J.; Bateman, R.P.; Johnson, D.L.; Langewald, J.; Thomas, M.B. Biological control of Locusts and Grasshoppers. Annu. Rev. Entomol. 2001, 46, 667–702. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.L. Nosematidae and other Protozoa as Agents for Control of Grasshoppers and Locusts: Current Status and Prospects. Memoirs Entomol. Soc. Can. 1997, 129, 375–389. [Google Scholar] [CrossRef]
- Lange, C.E.; Sokolova, Y.Y. The Development of the Microsporidium Paranosema (Nosema) locustae for Grasshopper Control: John Henry’s Innovation with Worldwide Lasting Impacts. Protistology 2017, 11, 170–174. [Google Scholar] [CrossRef]
- Canning, E.U. A New Microsporidian, Nosema locustae n.sp., from the Fat Body of the African Migratory Locust, Locusta migratoria migratorioides R. & F. Parasitol. 1953, 43, 287–290. [Google Scholar] [CrossRef]
- Lange, C.E. The Host and Geographical Range of the Grasshopper Pathogen Paranosema (Nosema) locustae Revisited. J. Orthoptera Res. 2005, 14, 137–141. [Google Scholar] [CrossRef]
- Henry, J.E. Epizootiology of Infections by Nosema locustae Canning (Microsporidia: Nosematidae) in Grasshoppers. Acrida 1972, 1, 111. [Google Scholar]
- Henry, J.E. Extension of the Host Range of Nosema locustae in Orthoptera. Ann. Entomol. Soc. Am. 1969, 62, 452–453. [Google Scholar] [CrossRef]
- Dong, Y. Preliminary Experiments on Application of Nosema locustae to Control Grasshoppers in Inner Mogolia Rangland. Master′s Thesis, Agricultural University, Beijing, China, 1989. [Google Scholar]
- Wang, L.; Yan, Y.; Dong, Y.; Lin, Z. Observations of a Microsporidian Parasite Nosema locustae Canning in the Oriental Migratory Locust (Locusta migratoria manilensis (Meyen)) and other Hosts from Inner Mogolia & Xinjiang. Acta Agric. Univ. Pekin. 1987, 13, 459–462. [Google Scholar]
- Chen, R.; Liu, Q.; Huang, H. Application of Insect Pathogenic Microbials to Control Ceracris kiangsu Tsai. Nat. Enem. Insects 2002, 24, 123–127. [Google Scholar]
- Liu, Q.; Chen, R. A Preliminary Study on Chondracris rosea Control by Inoculating Nosema locustae. Guangdong For. Sci. Technol. 1996, 12, 30–36. [Google Scholar]
- Ma, D.; Bai, S.; Li, Z. Studies on Sustainable Controlling Rangeland Grasshopper with Nosema locustae in Caidamu of Qinghai Province. J. Shandong Agric. Univ. Nat. Sci. 2005, 36, 199–205. [Google Scholar]
- Chen, Y.; Chen, Z.; Luo, R.; Chen, W.; Lu, S.; Li, Y. A Study on Application of Nosema locustae to Control Fruhstorferiola tonkinesis. In Proceedings of the 8th Conference of Chinese Entomology Society, Hebi, China, 11–13 October 2007. [Google Scholar]
- Tounou, A.-K.; Kooyman, C.; Douro-Kpindou, O.-K.; Poehling, H.-M. Interaction between Paranosema locustae and Metarhizium anisopliae var. acridum, two Pathogens of the Desert Locust, Schistocerca gregaria under Laboratory Conditions. J. Invertebr. Pathol. 2008, 97, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.E.; Mariottini, Y.; Plischuk, S.; Cigliano, M.M. Naturalized, Newly-Associated Microsporidium Continues Causing Epizootics and Expanding its Host Range. Protistology 2020, 14, 32–37. [Google Scholar] [CrossRef]
- Menapace, D.M.; Sackett, R.; Wilson, W.T. Adult Honey Bees are not Susceptible to Infection by Nosema locustae. J. Econ. Entomol. 1978, 71, 304–306. [Google Scholar] [CrossRef]
- Krall, S.; Knausenberger, W. Efficiency and Environmental Impact for Biological Control of Nosema locustae on Grasshoppers in Cape Verde, a Synthesis Report; Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ): Eschborn, Germany, 1992; p. 16. [Google Scholar]
- Yuan, S.; Jiang, J.; Zhang, L.; Wu, S.; Liu, X.; An, X.; Li, G. Toxic Effects of Nosema locustae on Environmental Non-Target Bene-ficial Organisms. Mod. Agrochem. 2020, 19, 39–43. [Google Scholar]
- Tounou, A.; Kooyman, C.; Douro-Kpindou, O.; Gumedzoe, Y.; Poehlingn, H. Laboratory Assessment of the Potential of Paranosema locustae to Control Immature Stages of Schistocerca gregaria and Oedaleus senegalensis and Vertical Transmission of the Pathogen in Host Populations. Biocontrol Sci. Technol. 2011, 21, 605–617. [Google Scholar] [CrossRef]
- Zang, J.; Cao, J.; Li, G.; Li, C.; Yan, Y. Biological Control of Oxya chinensis by using Nosema locustae. Chin. J. Boil. Control 2001, 17, 126–128. Available online: http://www.zgswfz.com.cn/EN/Y2001/V17/I3/126 (accessed on 3 March 2021).
- Ding, X.; Zhang, L. Virulence of Metarhizium anisopliae and Nosema locustae Against the Nymphs of Locusta migratoria manilensis. J. Beijing Univ. Agric. 2009, 24, 9–14. [Google Scholar]
- Tokarev, Y.S.; Levchenko, M.V.; Naumov, A.M.; Senderskiy, I.V.; Lednev, G.R. Interactions of two Insect Pathogens, Paranosema locustae (Protista: Microsporidia) and Metarhizium acridum (Fungi: Hypocreales), during a Mixed Infection of Locusta migratoria (Insecta: Orthoptera) Nymphs. J. Invertebr. Pathol. 2011, 106, 336–338. [Google Scholar] [CrossRef]
- Lv, M.; Mohamed, A.A.; Zhang, L.; Zhang, P.; Zhang, L. A Family of CSαβ Defensins and Defensin-Like Peptides from the Migratory Locust, Locusta migratoria, and Their Expression Dynamics during Mycosis and Nosemosis. PLoS ONE 2016, 11, e0161585. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; You, Y.; Zhang, K.; Zhang, L. A Novel Spore Wall Protein from Antonospora locustae (Microsporidia: Nosematidae) Contributes to Sporulation. J. Eukaryot. Microbiol. 2017, 64, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yan, Y.H.; Li, G.B.; Cao, Y.Z. The Effect of Nosema locustae Infection on Flying Ability of Locust (Locusta migratoria manilensis). Acta Agrestia Sin. 1995, 3, 324–327. [Google Scholar]
- Chen, J.; Shen, J.; Song, D.; Zhang, L.; Yan, Y. Effect of Nosema locustae on the Content of Fat in Locusta migratoria manilensis. Acta Entomol. Sin. 2000, 43, 109–113. [Google Scholar]
- Zhang, L. Epizootic and Transmission of Nosema locustae in the Populations of Grasshoppers and Locusts. Ph.D. Thesis, Beijing Agricultural University, Beijing, China, 1994. [Google Scholar]
- Chen, J.; Shen, J.; Song, D.; Zhang, L.; Yan, Y. Effect of Nosema locustae on the Content of Vitellogenin of Locusta migratoria manilensis. Acta Entomol. Sin. 2002, 45, 170–174. [Google Scholar]
- Raina, S.K.; Das, S.; Rai, M.M.; Khurad, A.M. Transovarial Transmission of Nosema locustae (Microsporida: Nosematidae) in the Migratory Locust Locusta migratoria migratorioides. Parasitol. Res. 1995, 81, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, X.; Li, R.; Zhang, L.; Huang, R.; Wang, L.; Song, Y.; Xing, Z.; Liu, T.; Nie, X.; et al. Complete Genome of a Unicellular Parasite (Antonospora locustae) and Transcriptional Interactions with its Host Locust. Microb. Genom. 2020, 6, e000421. [Google Scholar] [CrossRef]
- Polonais, V.; Prensier, G.; Metenier, G.; Vivarès, C.P.; Delbac, F. Microsporidian Polar Tube Proteins: Highly Divergent but Closely Linked Genes Encode PTP1 and PTP2 in Members of the Evolutionarily Distant Antonospora and Encephalitozoon Groups. Fungal Genet. Biol. 2005, 42, 791–803. [Google Scholar] [CrossRef]
- Polonais, V.; Belkorchia, A.; Roussel, M.; Peyretaillade, E.; Peyret, P.; Diogon, M.; Delbac, F. Identification of Two new Polar Tube Proteins Related to Polar Tube Protein 2 in the Microsporidian Antonospora locustae. FEMS Microbiol. Lett. 2013, 346, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Fast, N.M.; Logsdon, J.M.; Doolittle, W.F. Phylogenetic Analysis of the TATA Box Binding Protein (TBP) Gene from Nosema locustae: Evidence for a Microsporidia-Fungi Relationship and Spliceosomal Intron Loss. Mol. Biol. Evol. 1999, 16, 1415–1419. [Google Scholar] [CrossRef] [Green Version]
- Edlund, T.D.; Li, J.; Visvesvara, G.S.; Vodkin, M.H.; McLaughlin, G.L.; Katiyar, S.K. Phylogenetic Analysis of β-Tubulin Sequences from Amitochondrial Protozoa. Mol. Phylogenetics Evol. 1996, 5, 359–367. [Google Scholar] [CrossRef]
- Keeling, P.J.; Doolittle, W.F. Alpha-Tubulin from Early-Diverging Eukaryotic Lineages and the Evolution of the Tubulin Family. Mol. Biol. Evol. 1996, 13, 1297–1305. [Google Scholar] [CrossRef] [Green Version]
- Germot, A.; Philippe, H.; Le Guyader, H. Evidence for Loss of Mitochondria in Microsporidia from a Mitochondrial-Type HSP70 in Nosema locustae. Mol. Biochem. Parasitol. 1997, 87, 159–168. [Google Scholar] [CrossRef]
- Hirt, R.P.; Healy, B.; Vossbrinck, C.R.; Canning, E.U.; Embley, T. A Mitochondrial Hsp70 Orthologue in Vairimorpha necatrix: Molecular Evidence that Microsporidia Once Contained Mitochondria. Curr. Biol. 1997, 7, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Hirt, R.P.; Logsdon, J.M.; Healy, B.; Dorey, M.W.; Doolittle, W.F.; Embley, T.M. Microsporidia are Related to Fungi: Evidence from the Largest Subunit of RNA Polymerase II and other Proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Peyretaillade, E. Microsporidian Encephalitozoon cuniculi, a Unicellular Eukaryote with an Unusual Chromosomal Dispersion of Ribosomal Genes and a LSU rRNA Reduced to the Universal Core. Nucleic Acids Res. 1998, 26, 3513–3520. [Google Scholar] [CrossRef] [Green Version]
- Burri, L.; Williams, B.A.P.; Bursac, D.; Lithgow, T.; Keeling, P.J. Microsporidian Mitosomes Retain Elements of the General Mitochondrial Targeting System. Proc. Natl. Acad. Sci. USA 2006, 103, 15916–15920. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.E. Effect of Grasshoppers Species, Cage Density, Light Intensity, and Method of Inoculation on Mass Production of Nosema locustae (Microsporida: Nosematiodae). J. Econ. Entomol. 1985, 78, 1245–1250. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X. Mass Production and Application of Nosema locustae Against Grasshoppers. Acta Agrestia Sin. 1994, 2, 49–54. [Google Scholar]
- Zhang, J. Research and Application of New Green Control Technology Extension Mode of Locusts in China. China agricultural university. Ph.D. Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- Beijing JiaJing Biotecnology Ltd. Available online: http://www.jiajingbiotech.com (accessed on 26 March 2021).
- Zhang, L.; Shi, W.; Yan, Y.; Lin, B.; Wu, M.; Ye, S.; Li, D. Relationships between Application Rate of Nosema locustae and Infection of Locusta migratoria manilensis in Hainan Province. J. China Agric. Univ. 2001, 6, 90–95. [Google Scholar]
- Henry, J. Experimental Application of Nosema locustae for Control of Grasshoppers. J. Invertebr. Pathol. 1971, 18, 389–394. [Google Scholar] [CrossRef]
- Ewen, A.B.; Mukerji, M. Evaluation of Nosema locustae (Microsporida) as a Control Agent of Grasshopper Populations in Saskatchewan. J. Invertebr. Pathol. 1980, 35, 295–303. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, Y.; Wang, G.; Zhang, Z.; Pan, J.; Yang, Z. A Preliminary Survey on Epizootics of Infection of Nosema locustae Among Grasshoppers in Rangeland. Acta Agrestica Sin. 1995, 3, 223–229. [Google Scholar]
- Zhang, F.; Bai, Y.; Wang, L.; Wang, X.; Zhang, L. A Preliminary Study on Strategies of Management of Grasshoppers in crop-Grassland Ecosystem in Inner Mongolia. Entomol. J. East China 2007, 16, 113–118. [Google Scholar]
- Gong, A.; Liu, X.; Jiang, X.; Zhang, L. Transmission of Nosema locustae Disease in Grasshopper Populations in Qinghai Grassland. Chin. J. Biol. Control 2003, 19, 118–121. [Google Scholar]
- Johnson, D.L.; Dolinski, M. Attempts to Increase the Prevalence and Severity of Infection of Grasshoppers with the Entomopathogen Nosema locustae Canning (Microsporida: Nosematidae) by Repeated Field Application. Mem. Entomol. Soc. Can. 1997, 129, 391–400. [Google Scholar] [CrossRef]
- Phithalsoun, S.; Zhang, L. An Effective Biological Control Method of Yellow-Spined Bamboo Locust (Ceracris kiangsu) has been de-Veloped in Lao PDR and Vietnam. Metaleptea 2018, 38, 15–16. [Google Scholar]
- Tounou, A.K.; Kooyman, C.; Douro-Kpindou, O.K.; Poehling, H.M. Combined Field Efficacy of Paranosema locustae and Metarhizium anisopliae var. acridum for the Control of Sahelian Grasshoppers. BioControl 2007, 53, 813–828. [Google Scholar] [CrossRef]
- Lima, M.L.L.; Brito, J.M.; Henry, J.E. Biological Control of Grasshoppers in the Cape Verde Islands. In Biological Control of Locusts and Grasshoppers; Lomer, C.J., Prior, C., Eds.; CAB International: Wallingford, UK, 1992; pp. 287–295. [Google Scholar]
- Lange, C. Long-term Patterns of Occurrence of Nosema locustae and Perezia dichroplusae (Microsporidia) in Grasshoppers (Orthoptera: Acrididae) of the Pampas, Argentina. Acta Protozool. 2003, 42, 309–315. [Google Scholar]
- Lange, C.E.; Azzaro, F.G. New Case of Long-Term Persistence of Paranosema locustae (Microsporidia) in Melanopline Grasshoppers (Orthoptera: Acrididae: Melanoplinae) of Argentina. J. Invertebr. Pathol. 2008, 99, 357–359. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L. Selection of Antonospora locustae (Protozoa: Microsporidae) with Higher Virulence Against Locusta migratoria manilensis (Orthoptera:Acrididae). Biocontrol Sci. Technol. 2009, 19, 421–427. [Google Scholar] [CrossRef]

| Species | Inoculation Infection (Caging) | Field Trial Infection | References |
|---|---|---|---|
| Asia | |||
| Bryodemella holdereri (Krauss, 1901) | X | [23] | |
| Calliptamus italicus (Linnaeus, 1758) | X | [24] | |
| C. abbreviatus Ikonnikov, 1913 | X | [23] | |
| Ceracris kiangsu Tsai, 1929 | X | X | [25] |
| Chondracris rosea (De Geer, 1773) | X | X | [26] |
| Chorthippus dubius (Zubovski, 1898) | X | [27] | |
| C. brunneus (Thunberg, 1815) | X | [23] | |
| Damalacantha vacca (Fischer von Waldheim, 1846) | X | ||
| Deracanthella aranea (Fischer von Waldheim, 1833) | X | ||
| Dociostaurus kraussi (Ingenitskii, 1897) | X | [24] | |
| Fruhstorferiola tonkinensis (Willemse, 1921) | X | [28] | |
| Gampsocleis sedakovii (Fischer von Waldheim, 1846) | X | [23] | |
| Haplotropis brunneriana Saussure, 1888 | X | [24] | |
| Oedaleus decorus (Germar, 1825) | X | ||
| Arcyptera meridionalis Ikonnikov, 1911 | X | X | |
| Sphingonotus mongolicus Saussure, 1888 | X | [23] | |
| Africa | |||
| Acrotylus blondeli Saussure, 1884 | X | [29] | |
| Acrotylus patruelis (Herrich-Schäffer, 1838) | X | ||
| Aiolopus thalassinus (Fabricius, 1781) | X | ||
| Anacridium melanorhodon (Walker, 1870) | X | ||
| America | |||
| Amblytropidia australis Bruner, 1904 | X | [30] | |
| Dichroplus vittigerus (Blanchard, 1851) | X | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Lecoq, M. Nosema locustae (Protozoa, Microsporidia), a Biological Agent for Locust and Grasshopper Control. Agronomy 2021, 11, 711. https://doi.org/10.3390/agronomy11040711
Zhang L, Lecoq M. Nosema locustae (Protozoa, Microsporidia), a Biological Agent for Locust and Grasshopper Control. Agronomy. 2021; 11(4):711. https://doi.org/10.3390/agronomy11040711
Chicago/Turabian StyleZhang, Long, and Michel Lecoq. 2021. "Nosema locustae (Protozoa, Microsporidia), a Biological Agent for Locust and Grasshopper Control" Agronomy 11, no. 4: 711. https://doi.org/10.3390/agronomy11040711
APA StyleZhang, L., & Lecoq, M. (2021). Nosema locustae (Protozoa, Microsporidia), a Biological Agent for Locust and Grasshopper Control. Agronomy, 11(4), 711. https://doi.org/10.3390/agronomy11040711







