1. Introduction
Bananas (
Musa ssp.), including plantains, are the world’s most popular fruit crop grown in about 130 tropical and subtropical countries on an estimated 11 million hectares [
1]. Though grown in the tropics and subtropics, their origin is traced back to Southeast Asian and Western Pacific regions, where their wild relatives exist in natural forests [
2]. Bananas are also the only fruit crop that is a staple food for many farming communities in the tropics. Overall banana production (excluding plantains and others) was estimated at 129 million tons in 2019 [
1], with only 16% exported [
3], and the rest was locally consumed. Latin America and the Caribbean accounted for 72% of all exported bananas, Africa accounted for 4%, and the rest came from Asia [
3]. The East African region produced close to 17 million tons of bananas (including plantains and others) in 2019 [
1] with negligible export.
The dominant banana type in the Great Lakes region of East Africa is the East African Highland bananas commonly known as Matooke (AAA) [
4]. They are grown in Kenya, Uganda, Tanzania, Rwanda, Burundi, and the eastern part of the Democratic Republic of Congo [
4]. There are about 120 Matooke triploids, which are further classified into clone sets, including Mbidde (beer type), Musakala, Nakabululu, Nakitembe, and Nfuuka [
4,
5]. The highly starchy Matooke fruits are mainly consumed in steamed and mashed form, or they are ripened and brewed into banana beer for the beer type [
4,
6]. The Mchare subgroup (AA), which is genetically distinct from Matooke, is common in Tanzania [
7]. They share the same AA genome with “Gros Michel” and Cavendish triploid bananas [
8]. Mchare are genetically homogenous but adapted to wider ecological environments ranging from sea-level to elevations above 1500 m.a.s.l. [
9]. They are grown from islands of Zanzibar, Pemba, off the East African coast to the main land of Tanzania, Kenya, and Central Uganda [
4]. Mchare bananas are classified by FAO as dessert bananas, but they are mainly consumed as cooked [
4] or roasted in the Kilimanjaro region [
9]. Matooke and Mchare can therefore be collectively referred to as East African Highland Cooking bananas (EAHBs). They belong to the same genetic complex transported from South-East Asia [
10].
From the 1970s, banana production in East Africa experienced a drop attributed largely to a complex of diseases (fungal, bacterial, and viral), nematodes, and insect pests [
11]. Low production and productivity is also attributed to poor agronomic practices; abiotic stresses, including poor edaphic factors; and an imminent threat of climate change [
12]. Most banana-breeding programs therefore focus on these production constraints when defining their breeding goals. The major stumbling block to crossbreeding in bananas is low seed set [
6]. Reference [
13] reported an average of 303 ovules per fruit in Matooke, yet the seed set range was only 0–25 per bunch. This implies that an average sized Matooke bunch with 100 fruits could potentially yield over 30,000 seeds. A total of 78 Matooke landraces were screened in Uganda, of which 37 were considered to be seed fertile [
13]. On the other hand, Mchare have not been well characterized, especially for female fertility. The reasons for low seed set are a complex array of factors that have hindered efficient banana breeding. For the triploid bananas, including Matooke, female sterility as a result of meiotic failures has been a major obstacle [
6]. Some cultivars, including those of the Matooke, are rendered “infertile” [
14]; thus, improvement of such cultivars by conventional means is difficult.
Nevertheless, female sterility has also been observed among diploid bananas [
15], suggesting factors beyond meiotic failures contribute to sterility. For example, successful pollination can be achieved when flowers of “Gros Michel” bananas are pollinated between 7:00 a.m. and 10:30 a.m. [
16]. To unveil the mystery of overcoming sterility in
Musa, responsible factors have to be dealt with comprehensively to have a better perspective. In most analyses in
Musa, seed set is considered on a bunch basis. However, bunch size for the same genotype varies depending on environmental and soil fertility factors [
17]. Intriguingly, analyses by Reference [
18] strongly suggested a relationship between bunch size and seed set, with larger bunches setting more seed on a seed set per 100 fruits basis. Furthermore, significant differences in seed set for hand position have been observed in Matooke, and this was linked to stigma receptivity [
19]. Distal hands were found to have more receptive stigmas than proximal hands thus the higher fertility in distal hands. In plantains, seed set also depends on hand position [
20], and there is a general tendency of seed set in the middle hands for both plantain and Matooke. In addition, seed set in “Gros Michel” was found to be predominant in the distal end of the fruit rather than the proximal end [
18].
These observations have not been made on an individual bunch-size basis, especially in EAHBs, and there is a need to look into the behavior of seed set position in hands of different bunch sizes. Correlation coefficients between seed set and weather attributes are mostly less than 0.5 [
21]. This suggests that there are more factors that are yet to be identified and considered. Reference [
22] also found no month effects for pollination success of Matooke after 21 years of crossbreeding. This raises questions about the underlying causes of observations made and how understanding these factors can ultimately lead to overcoming the fertility crisis in
Musa. The aim of this study was to investigate seed set patterns in hands of the bunch, number of fruits per hand in different bunch sizes, seed set position in the fruits, and pollination success in relation to bunch size of EAHBs. Seed set patterns were also compared for different pollination techniques used. The data generated will inform future decisions, on a more targeted approach, of increasing seed set in edible bananas and facilitating banana breeding.
4. Discussion
Getting an in depth understanding of seed set patterns in EAHBs is certainly one of the measures that will help in designing experiments for increasing seed set in
Musa spp. The end goal is to have a fast and an efficient banana breeding pipeline with better hybrids for many farmers in the tropics and subtropical regions globally. Here, there is a deliberate effort to understand the cause of sterility by looking at seed set patterns in relation to position of the hands, bunch size, and position in the fruit in EAHBs. In
Musa spp. sterility has been understood to arise from developmental errors in the sporophyte, the gametophyte as well as pistil–pollen interactions. However, more emphasis has been placed on understanding the gametophyte which is said to account most towards sterility [
17]. Meiotic errors as a result of chromosome mismatch were reported by Reference [
26] to cause embryo sac failure in sterile
Musa spp. Reference [
27], however, found that about 10% of embryo sacs in ovules of triploid bananas were correctly positioned compared to 75% in
Musa acuminata ssp. They also discovered that the presence of a B-genome increased the presence of an embryo sac to 96–100%. However, these observations do not explain the fact that seed set in EAHBs is biased toward distal hands and distal end of the fruit. There is also no documentation of a link between the presence and correct positioning of an embryo sac to seasonal influence of seed set in
Musa spp. with residual fertility.
Because of the nature of banana, an equal number of bunches could not be obtained for all bunch size categories. For a given genotype, bunch size is dependent on soil and environment [
17]. Consequently, the smallest and largest bunch size categories were not well represented thus reliable conclusions cannot be drawn these categories. However, most bunch size categories had a representative number of bunches from which our discussion is based. The observed bias of seed set in distal hands especially in Matooke has been attributed to the high stigma receptivity in distal hands [
19]. However, if this were entirely true, then the use of PGM on stigmas would have increased seed set in the proximal hands as well since all hands are pollinated [
24]. Additionally, since “Enzirabahima” was reported to have fairly high stigma receptivity in all hands, seed set should have been distributed in all hands. Instead, seed set increase was observed in the same hand positions as of bunches pollinated without PGM. Use of PGM should have also resulted in seed set in “Nakitembe” and “Mlelembo” if stigma receptivity was a prime contributor of sterility in
Musa. This implies that there are other factors which come into play after pollen has germinated on the stigmas in different hand positions. The necrosis formed in the prolongation zone of fruits after anthesis especially in triploids [
28] could partly account for absence of seed set in proximal hands. This necrosis acts as a barrier to pollen tube growth. Reference [
16] observed that pollen tube growth through the stigma in “Gros Michel” looked natural, but was slowed or arrested and the tips developed a swelling resulting in pollen tubes not reaching the ovules. It may be worthwhile to investigate rates of necrosis formation in fruits of different hand positions to find a link to seed set if any.
In an attempt to avoid the necrosis formed soon after anthesis in the fruit prolongation zone, early pollinations were made (technique three), but the technique did not increase seed set. Reference [
29] speculated that physiologically immature stigmas may delay or prevent penetration by pollen tubes and this could have been the case with early pollination. Results in the present study suggest that application of PGM on stigmas enhanced pollen germination [
24]. However, this was most effective on stigmas presumed to be physiologically mature after natural flower opening as suggested by Reference [
29]. This observation may also suggest that arrest of pollen tube growth may be a biochemical rather than a physical process. It is supported by the fact that a necrosis is not formed in the prolongation fruit zone of diploids [
28]. Pollination technique three was therefore discontinued in December 2016 since seed set was lower compared with the customary pollination technique described by Reference [
23]. Evening pollinations were also discontinued in 2016 since they did not increase seed set. This resulted from reduced pollen viability with time of the day coupled with low humidity at the time of pollination between 5:00 and 6:30 p.m. The rationale of evening pollination was to have flowers pollinated soon after opening as
Musa flowers start opening from evening through the night [
30]. However, not all female flowers had fully opened at the time of evening pollination and fresh pollen could not be obtained. Fairly reliable comparisons were made between the control and technique two, since a considerable number of bunches were fairly distributed in different months of year during the study period (
Appendix C Table A5,
Table A6 and
Table A7).
Reference [
19] found that bracts of distal female hands opened to a bigger angle than that of proximal hands. This phenomenon was linked to a response of stigma receptivity which increased from proximal to distal hands. However, it might be that ovules in proximal hands and the proximal fruit end have higher abortion rates if pollen tubes reach them. Abortive ovules have been reported in other crops, like hazelnut, whereby some unigenes are upregulated and others down regulated in abortive ovules compared to developing ovules [
31]. The gradual increase of seed set from proximal to distal hands could suggest a mechanism of ovule abortion rate in the same order. The most likely cause of this observation could be auxins in large amounts from the root tips that reach proximal hands first. Auxins induce formation of edible pulp in both seeded and non-seed bananas as well as partially parthenocarpic types [
32,
33]. Moreover, in seeded bananas, synthetic auxins 4-CPA and 2,4,5-T have been reported to hinder seed development [
33]. Auxins therefore play a critical role in parthenocarpy, as well as sterility.
The effect of weather on seed set in
Musa spp. could be as a result of a drop in auxin levels with heat and/or moisture stress. This comes with high temperature, high solar radiation, and low rainfall. These weather conditions correlate with increase in seed set in Matooke [
21]. This is likely to slow down evapo-transpiration pull as the plant tries to conserve moisture. Consequently, materials moving from roots including auxins do not reach the distal hands and fruit tips in adequate amounts. A reduced fruit circumference of “Gros Michel” correlates with increased seed set [
18], which can reflect reduced parthenocarpy. This theory also tends to fit the observed increase of seed set per 100 fruits with increase in bunch size; small bunches are saturated easily. A reduction in auxin levels during moisture stress could be caused by salicylic acid which is involved in response to both abiotic and biotic stress [
34,
35]. Salicylic acid and auxin signaling are mutually antagonist [
35] and production of salicylic acid in response to moisture stress or heat stress could be responsible for reduced auxin levels thus reduced parthenocarpy. This may be linked to the sudden seed set increase observed in bunches from mats on pockets of soils which drain easily. This results in high salicylic acid production in response to moisture stress and consequently high seed set.
There were inconsistences in patterns of seed set in “Nshonowa” as fewer bunches were available compared with the other two cultivars. Using few bunches with seed to calculate averages for “Nshonowa” implied that a single bunch with high seed set affected the overall shape of the curve. It was also noticed that bunches with high seed set had uneven seed distribution among hands. Sudden seed increase was observed in hand position seven of the eight-hand-bunch category. It strongly suggests that there are specific weather conditions for maximum seed set, as hands were pollinated on different days with unique weather conditions. High morning temperatures are likely to overcome the issue of pollen tube growth arrest. In citrus [
36] and in apples [
37], high temperature was reported to overcome self-incompatibility; it could be a similar issue in banana, as high temperature is associated with high seed set [
21]. This could have applied to all the three EAHBs, but use of many bunches evened out this effect in “Enzirabahima” and “Mshale”, since more bunches had seed.
Results in the current study revealed that the hands with the highest number of fruits do not necessary produce the highest number of seeds. This clearly suggests that there are other factors that are more important for seed set. Ideally, it is expected that different sized bunches have same pollination success and same seed set per 100 fruits. However, our observations suggest that bunch size has an influence on fertility based on differences of seed set per 100 fruits for different bunch sizes. Reference [
18] also observed an increase of seed set on 100 fruit basis from small to larger bunches of “Gros Michel” as observed in EAHBs. The current study also revealed that big bunches are more fertile than smaller bunches in terms pollination success. The theory of auxins influencing fertility in different bunch sizes seems to fit this observation. Smaller bunches would be easily saturated by auxins compared with larger bunches. This leads to the low seed set (per 100 fruit basis) and low pollination success in smaller compared to larger bunches. However, there is higher seed set per 100 fruit per hand in smaller bunches if only bunches with seed are considered. However, large bunches generally set more seed per hand as a result of higher pollination success compared to small bunches.
All of these observations call for a slightly different approach to better understand sterility and use it profitably as it is a prerequisite in the final hybrids. It may be the right time for banana researchers to start looking in the direction of hormonal manipulations for increased seed set. However, since segregation data suggest that parthenocarpy and sterility are independent [
26], this would rule out auxins as the sole cause of sterility in
Musa spp. A recent genome wide association study in
Musa found parthenocarpy genes to be potentially linked to seedlessness. However, the prime candidate gene was the gene orthologous to Histadine Kinase CKI1 [
38]. Cytokinins have been reported to determine the fate of seed development [
39] and they could be responsible for sterility in
Musa even if there is successful fertilization. Non-parthenocarpic progeny that has sterile plants could therefore point to ovule abortion. This stems from a mutation in the gene orthologous to Histadine Kinase CKI1 that was linked to seedlessness by Reference [
38].
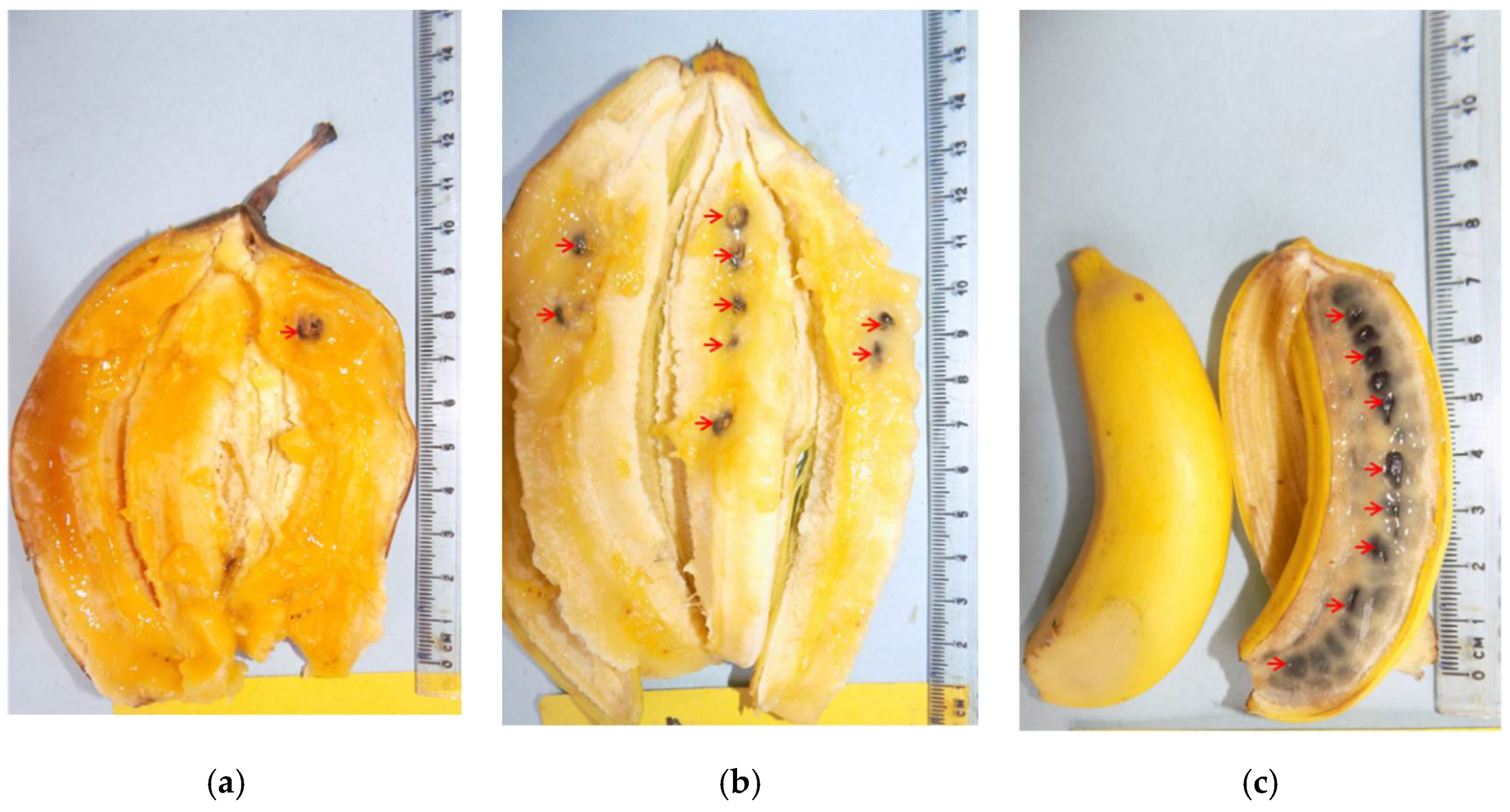
It could be possible that the same gene orthologous to Histadine Kinase CKI1 is responsible for production of poor seed and low embryo rescue rates in some parental combinations. Some edible banana genotypes have been rendered “infertile” and this could be as a result of hyper production auxin individually or in combination with the gene orthologous to Histadine Kinase CKI1 which has been linked to seedlessness. It could be that relative contribution these two factors along with other factors that result in high sterility as observed in “Nakitembe” and “Mlelembo”. Other factors such as fruit length are involved in banana fertility as demonstrated in the current study. In “Calcutta 4”, seeds set covers the entire length of the fruit implying pollen tube growth covered an estimated distance of 10 cm. In “Nshonowa”, pollen tube growth covered about 10 cm and about 7 cm of the fruit pulp had no seed. Moreover, in “Enzirabahima”, pollen tubes cover a distance of about 5 cm with about 8 cm of pulp without seed. This suggests that pollen tube growth within the fruit is not the reason for a biased seed set towards the fruit tip as the distance covered in “Enzirabahima” is shorter. With the same approach of auxin involvement in
Musa fertility, it likely that auxins can cover a limited distance during moisture stress thus longer fingers set more seed. This explains the higher seed set observed in Mchare compared with Matooke in the current study. The drop in seed set from the last hand could also be as a result of reduced fruit length in the last hand. Fruits in the proximal hands are longer with bigger circumference which reduces towards the distal end of the bunch. This was reported in plantain and cooking-banana types: Distal hands have fruits with the least fruit length [
40].