Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review
Abstract
:1. Introduction
Methods
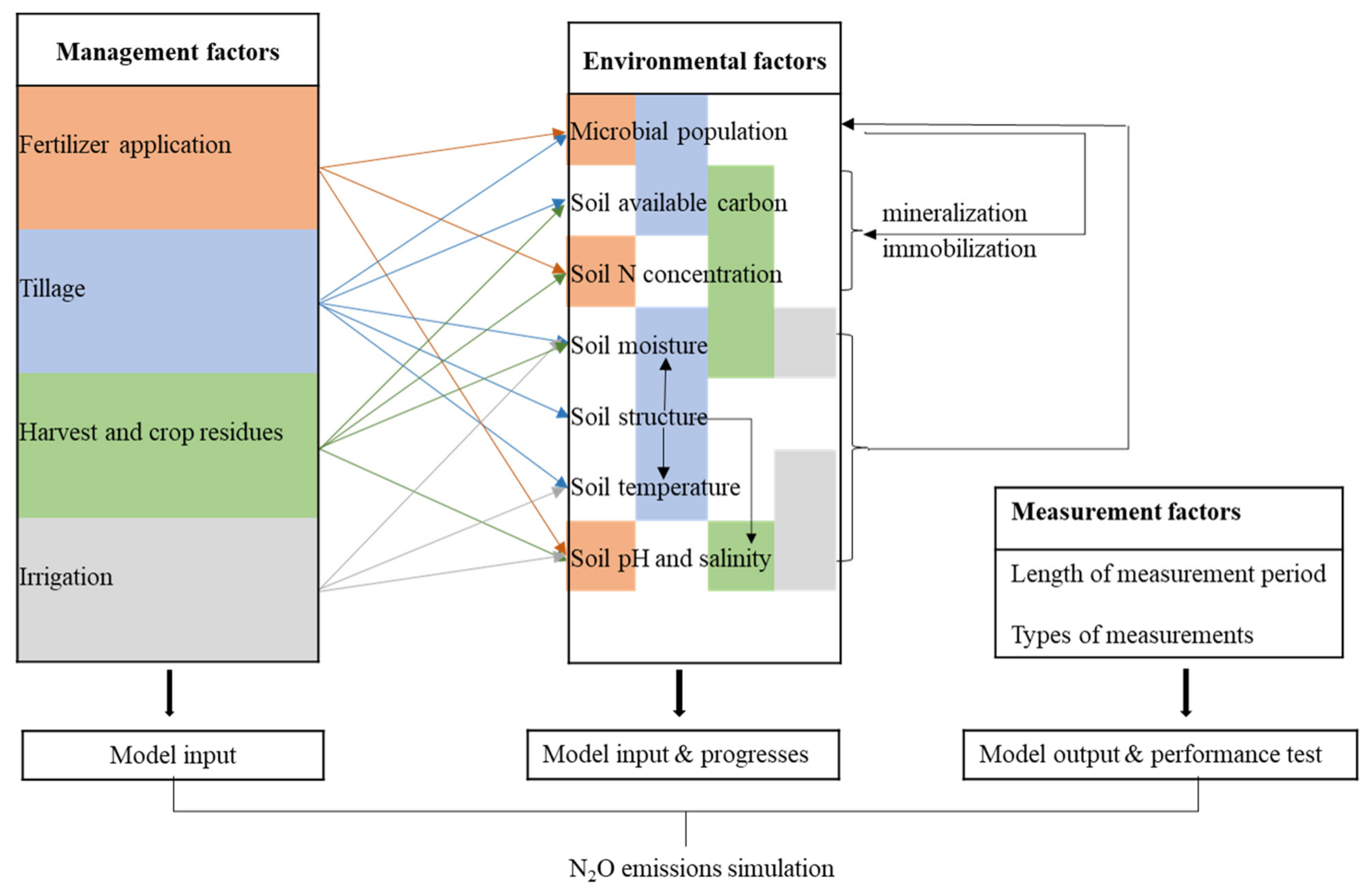
2. Factors That Influence Nitrous Oxide Emissions
2.1. Environmental Factors
2.1.1. Microbial Populations
2.1.2. Soil Available Carbon
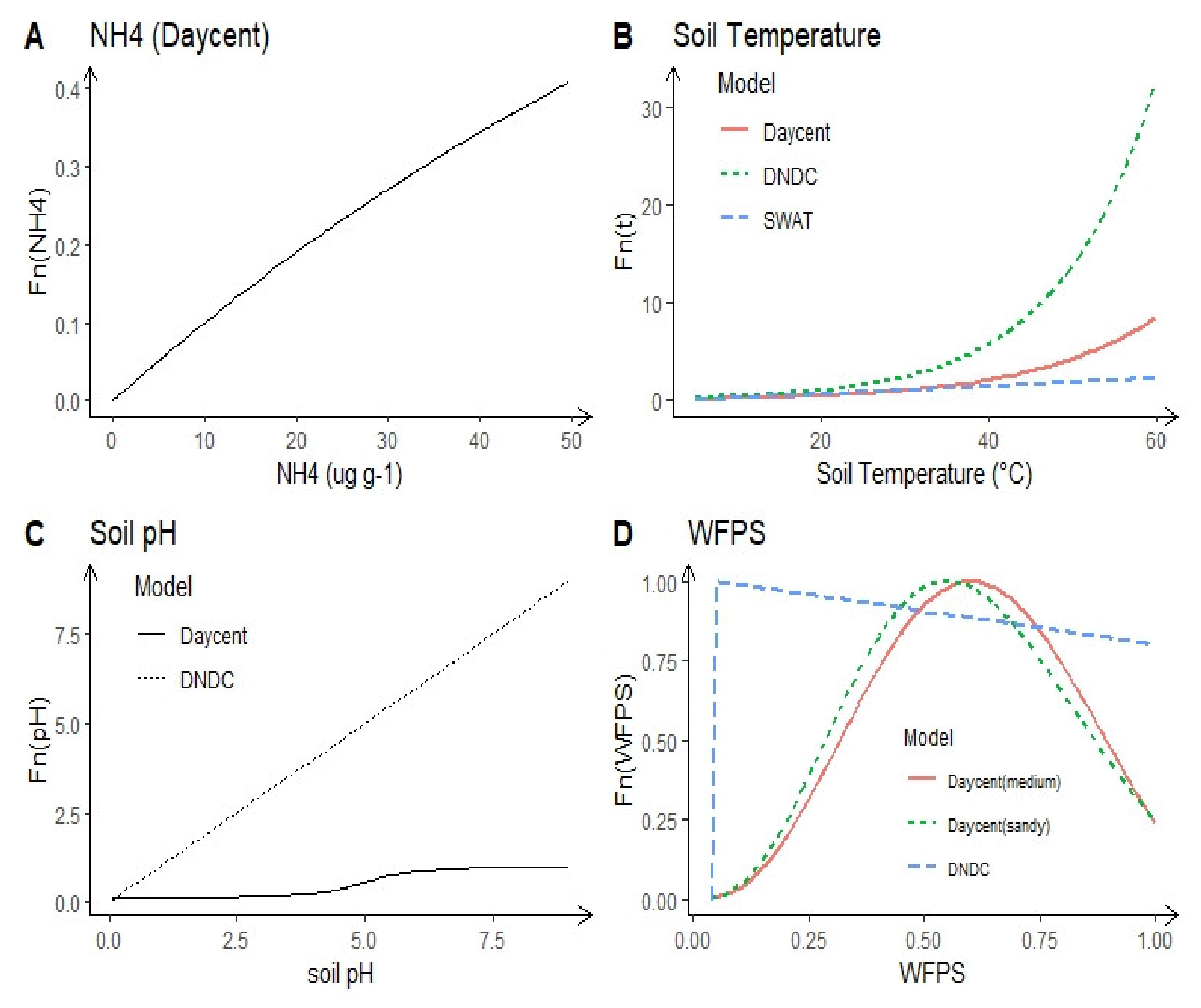
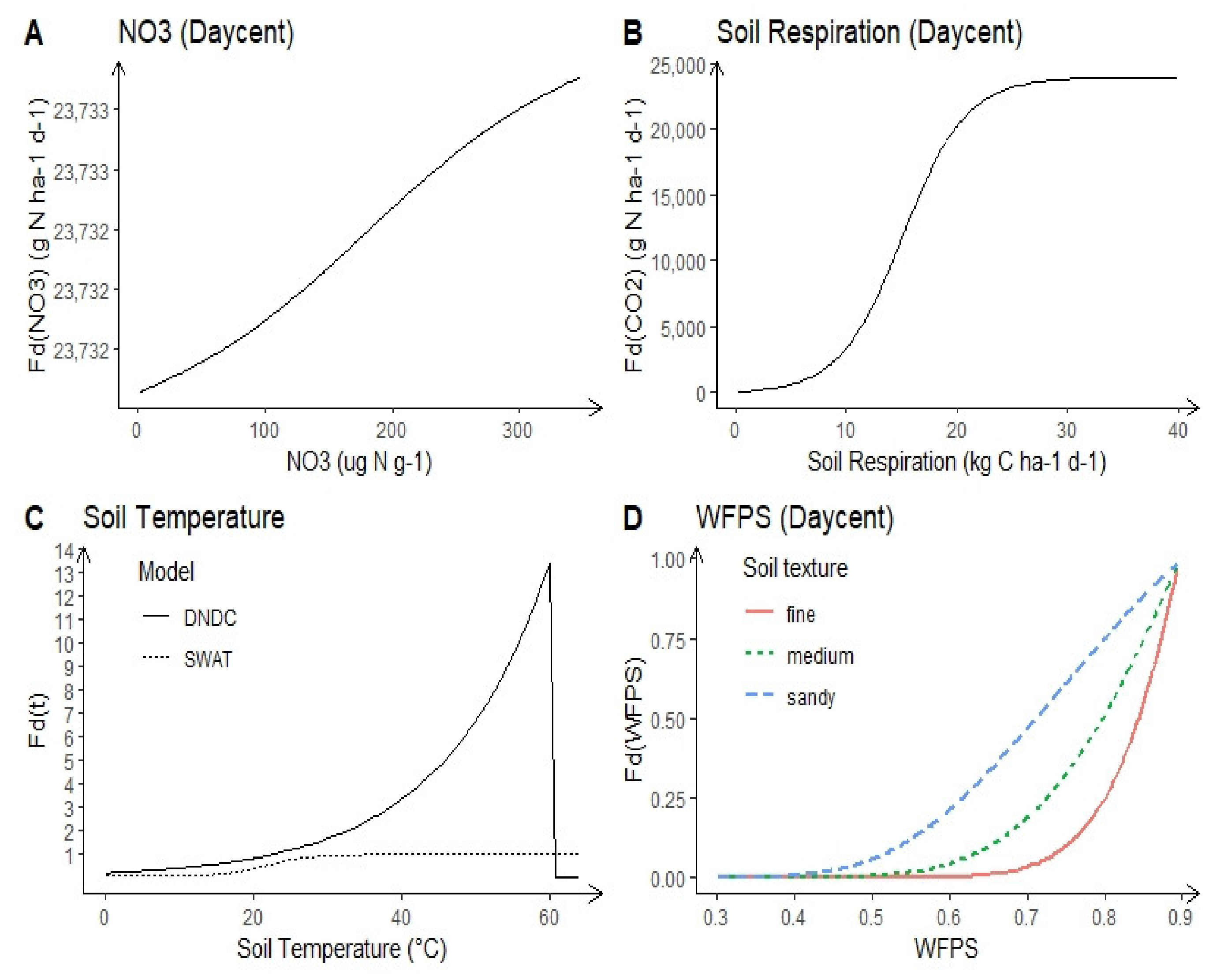
2.1.3. Soil N Concentration
2.1.4. Soil Moisture
2.1.5. Soil Texture
2.1.6. Soil Temperature
2.1.7. Soil pH and Salinity
2.2. Management Factors
2.2.1. Fertilizer Application
2.2.2. Tillage Systems
2.2.3. Harvest and Crop Residues
2.2.4. Irrigation
2.3. Measurement Factors
2.3.1. Length of Measurement Period
2.3.2. Types of Measurement
2.4. Summary of Factors
3. Current Process-Based Simulation Models
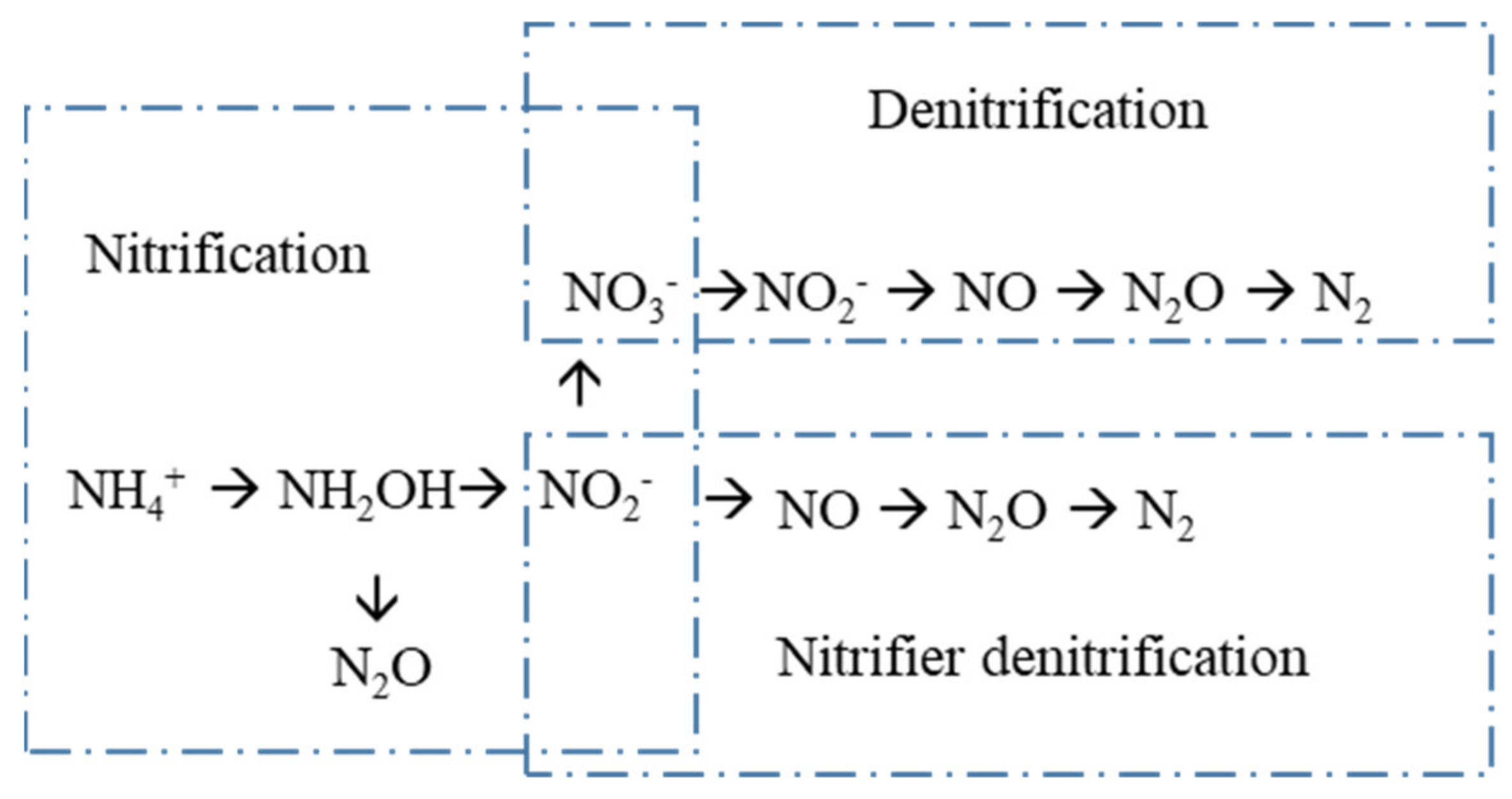
3.1. Nitrification Processes
3.2. Denitrification Processes
3.3. Partitioning N2O from N2
4. Summary & Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowler, D.; Steadman, C.E.; Stevenson, D.; Coyle, M.; Rees, R.M.; Skiba, U.M.; Sutton, M.A.; Cape, J.N.; Dore, A.J.; Vieno, M.; et al. Effects of global change during the 21st century on the nitrogen cycle. Atmos. Chem. Phys. 2015, 15, 13849–13893. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.J.; Tank, J.L.; Hamilton, S.K.; Wollheim, W.M.; Hall, R.O.; Mulholland, P.J.; Peterson, B.J.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Nitrous oxide emission from denitrification in stream and river networks. Proc. Natl. Acad. Sci. USA 2010, 108, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flückiger, J.; Dällenbach, A.; Blunier, T.; Stauffer, B.; Stocker, T.F.; Raynaud, D.; Barnola, J.-M. Variations in Atmospheric N2O Concentration during Abrupt Climatic Changes. Science 1999, 285, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Seitzinger, S.P.; Phillips, L. Nitrogen stewardship in the Anthropocene. Science 2017, 357, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; Masera, O.; Mbow, C.; et al. Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O.R., Pichs-Madruga, Y., Sokona, E., Farahani, S., Kadner, K., Seyboth, A., Adler, I., Baum, S., Brunner, P., Eickemeier, B., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA; Available online: https://www.ipcc.ch/pdf/assessment-report/ar5/wg3/ipcc_wg3_ar5_chapter11.pdf (accessed on 20 August 2018).
- Kravchenko, A.N.; Toosi, E.R.; Guber, A.K.; Ostrom, N.E.; Yu, J.; Azeem, K.; Rivers, M.L.; Robertson, G.P. Hotspots of soil N2O emission enhanced through water absorption by plant residue. Nat. Geosci. 2017, 10, 496–500. [Google Scholar] [CrossRef]
- Mosier, A.R. Nitrous oxide emissions from agricultural soils. Fertil. Res. 1994, 37, 191–200. [Google Scholar] [CrossRef]
- Myhre, G.D.; Shindell, F.-M.; Bréon, W.; Collins, J.; Fuglestvedt, J.; Huang, D.; Koch, J.-F.; Lamarque, D.; Lee, B.; Mendoza, T.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter08_FINAL.pdf (accessed on 20 August 2018).
- Tubiello, F.N.; Salvatore, M.; Cóndor Golec, R.D.; Ferrara, A.; Rossi, S.; Biancalani, R.; Federici, S.; Jacobs, H.; Flammini, A. Agriculture, Forestry and Other Land Use Emissions by Sources and Removals by Sinks, ESS Working Paper No.2; FAO: Rome, Italy, 2014. [Google Scholar] [CrossRef]
- Petrescu, A.M.R.; Peters, G.P.; Janssens-Maenhout, G.; Ciais, P.; Tubiello, F.N.; Grassi, G.; Nabuurs, G.-J.; Leip, A.; Carmona-Garcia, G.; Winiwarter, W.; et al. European anthropogenic AFOLU emissions and their uncertainties: A review and benchmark data. Earth Syst. Sci. Data 2019, 12, 961–1001. [Google Scholar] [CrossRef]
- Ciais, P.C.; Sabine, G.; Bala, L.; Bopp, V.; Brovkin, J.; Canadell, A.; Chhabra, R.; DeFries, J.; Galloway, M.; Heimann, C.; et al. Carbon and Other Biogeochemical Cycles. In In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter06_FINAL.pdf (accessed on 21 August 2018).
- De Klein, C.A.M.; Sherlock, R.R.; Cameron, K.C.; Van Der Weerden, T.J. Nitrous oxide emissions from agricultural soils in New Zealand—A review of current knowledge and directions for future research. J. R. Soc. N. Z. 2001, 31, 543–574. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Akiyama, H.; Bernoux, M.; Chirinda, N.; del Prado, A.; Kasimir, Å.; MacDonald, J.D.; Ogle, S.M.; Regina, K.; van der Weerden, T.J. IPCC: N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application, Chap.11, In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/public/2019rf/pdf/4_Volume4/19R_V4_Ch11_Soils_N2O_CO2.pdf (accessed on 23 September 2019).
- Winiwarter, W.; Klimont, Z. The role of N-gases (N2O, NOx, NH3) in cost-effective strategies to reduce greenhouse gas emissions and air pollution in Europe. Curr. Opin. Environ. Sustain. 2011, 3, 438–445. [Google Scholar] [CrossRef]
- De Klein, C.; Novoa, R.S.A.; Ogle, S.; Smith, K.A.; Rochette, P.; Wirth, C.T.; McConkey, B.G.; Mosier, A.; Williams, S.A. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Volume 4: Agriculture, Forestry and Other Land Use. Chapter 11: N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application. Intergovernmental Panel on Climate Change (IPCC), Technical Report 4-88788-032-4, Geneva, Switzerland. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf (accessed on 19 August 2018).
- Abdalla, M.; Smith, P.; Williams, M. Emissions of nitrous oxide from agriculture: Responses to management and climate change. In Understanding Greenhouse Gas Emissions from Agricultural Management; American Chemical Society (ACS): Washington, DC, USA, 2011; pp. 343–370. [Google Scholar]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecuária Trop. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosystems 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Modeling global annual N2O and NO emissions from fertilized fields. Glob. Biogeochem. Cycles 2002, 16, 28. [Google Scholar] [CrossRef]
- Charles, A.; Rochette, P.; Whalen, J.K.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agric. Ecosyst. Environ. 2017, 236, 88–98. [Google Scholar] [CrossRef]
- Dalal, R.C.; Wang, W.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Uchida, Y.; Akiyama, H. Mitigation of postharvest nitrous oxide emissions from soybean ecosystems: A review. Soil Sci. Plant Nutr. 2013, 59, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the Dinitrogen/Nitrous Oxide Ratio as Affected by Soil Water, Available Carbon, and Nitrate. Soil Sci. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Valentine, D.W.; Schimel, D.S.; Weier, K.; Kulmala, A.E. Generalized model for N2and N2O production from nitrification and denitrification. Glob. Biogeochem. Cycles 1996, 10, 401–412. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, U.; Shrestha, N.K.; Biswas, A.; Wagner-Riddle, C.; Yang, W.; Prasher, S.; Rudra, R.; Daggupati, P. A Review of Ongoing Advancements in Soil and Water Assessment Tool (SWAT) for Nitrous Oxide (N2O) Modeling. Atmosphere 2020, 11, 450. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.-G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total. Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Parton, W.J.; Holland, E.A.; Del Grosso, S.J.; Hartman, M.D.; Martin, R.E.; Mosier, A.R.; Ojima, D.S.; Schimel, D.S. Generalized model for NOxand N2O emissions from soils. J. Geophys. Res. Space Phys. 2001, 106, 17403–17419. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Zhang, Y.; Edis, R.; Ding, H. Comparison of three modeling approaches for simulating denitrification and nitrous oxide emissions from loam-textured arable soils. Glob. Biogeochem. Cycles 2005, 19, 1–15. [Google Scholar] [CrossRef]
- Arnold, J.G.; Srinivasan, R.; Muttiah, R.S.; Williams, J.R. Large area hydrologic modeling and assessment part I: Model development. JAWRA J. Am. Water Resour. Assoc. 1998, 34, 73–89. [Google Scholar] [CrossRef]
- Wu, L.; Rees, R.; Tarsitano, D.; Zhang, X.; Jones, S.; Whitmore, A. Simulation of nitrous oxide emissions at field scale using the SPACSYS model. Sci. Total. Environ. 2015, 530–531, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackmer, A.M.; Bremner, J.M. Inhibitory effect of nitrate on reduction of N2O to N2 by soil microorganisms. Soil Biol. Biochem. 1978, 10, 187–191. [Google Scholar] [CrossRef]
- Williams, E.J.; Hutchinson, G.L.; Fehsenfeld, F.C. NOx and N2O Emissions from Soil. Glob. Biogeochem. Cycles 1992, 6, 351–388. [Google Scholar] [CrossRef]
- Bremner, J.M. Sources of nitrous oxide in soils. Nutr. Cycl. Agroecosystems 1997, 49, 7–16. [Google Scholar] [CrossRef]
- Chen, G.; Kolb, L.; Cavigelli, M.A.; Weil, R.R.; Hooks, C.R. Can conservation tillage reduce N2O emissions on cropland transitioning to organic vegetable production? Sci. Total. Environ. 2018, 618, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Mehnaz, K.R.; Keitel, C.; Dijkstra, F.A. Effects of carbon and phosphorus addition on microbial respiration, N2O emission, and gross nitrogen mineralization in a phosphorus-limited grassland soil. Biol. Fertil. Soils 2018, 54, 481–493. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Steinbach, H.S.; Alvarez, R. Changes in Soil Organic Carbon Contents and Nitrous Oxide Emissions after Introduction of No-Till in Pampean Agroecosystems. J. Environ. Qual. 2006, 35, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liao, T.-T.; Wang, R.; Zheng, X.-H.; Hu, R.-G.; Butterbach-Bahl, K. Effect of carbon substrate concentration on N2, N2O, NO, CO2, and CH4 emissions from a paddy soil in anaerobic condition. Huan Jing Ke Xue 2014, 35, 3595–3604. [Google Scholar]
- Stein, L.Y.; Yung, Y.L. Production, isotopic composition, and atmospheric fate of biologically produced nitrous oxide. Annu. Rev. Earth Planet. Sci. 2003, 31, 329–356. [Google Scholar] [CrossRef]
- Benckiser, G.; Eilts, R.; Linn, A.; Lorch, H.J.; Sümer, E.; Weiske, A.; Wenzhöfer, F. N2O emissions from different cropping systems and from aerated, nitrifying and denitrifying tanks of a municipal waste water treatment plant. Biol. Fertil. Soils 1996, 23, 257–265. [Google Scholar] [CrossRef]
- Miller, M.N.; Zebarth, B.; Dandie, C.E.; Burton, D.L.; Goyer, C.; Trevors, J.T. Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol. Biochem. 2008, 40, 2553–2562. [Google Scholar] [CrossRef]
- Köster, J.R.; Cárdenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Senbayram, M.; Well, R.; Giesemann, A.; Dittert, K. Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification–An N2O isotopomer case study. Soil Biol. Biochem. 2015, 84, 65–74. [Google Scholar] [CrossRef]
- Ribas, A.; Mattana, S.; Llurba, R.; Debouk, H.; Sebastià, M.; Domene, X. Biochar application and summer temperatures reduce N2O and enhance CH4 emissions in a Mediterranean agroecosystem: Role of biologically-induced anoxic microsites. Sci. Total. Environ. 2019, 685, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhu, L. Impact of biochar on soil N2O emissions under different biochar-carbon/fertilizer-nitrogen ratios at a constant moisture condition on a silt loam soil. Sci. Total. Environ. 2017, 584–585, 776–782. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr. Opin. Environ. Sustain. 2011, 3, 389–395. [Google Scholar] [CrossRef]
- Smith, K.A. Changing views of nitrous oxide emissions from agricultural soil: Key controlling processes and assessment at different spatial scales. Eur. J. Soil Sci. 2017, 68, 137–155. [Google Scholar] [CrossRef]
- Farquharson, R. Nitrification rates and associated nitrous oxide emissions from agricultural soils—A synopsis. Soil Res. 2016, 54, 469–480. [Google Scholar] [CrossRef]
- Bol, R.; Toyoda, S.; Yamulki, S.; Hawkins, J.M.B.; Cardenas, L.M.; Yoshida, N. Dual isotope and isotopomer ratios of N2O emitted from a temperate grassland soil after fertiliser application. Rapid Commun. Mass Spectrom. 2003, 17, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, D.; Hawkins, J.M.B.; Jackson, S.M. Use of a flowing helium atmosphere incubation technique to measure the effects of denitrification controls applied to intact cores of a clay soil. Soil Biol. Biochem. 1997, 29, 1337–1344. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Ciarlo, E.; Conti, M.; Bartoloni, N.; Rubio, G. The effect of moisture on nitrous oxide emissions from soil and the N2O/(N2O+N2) ratio under laboratory conditions. Biol. Fertil. Soils 2006, 43, 675–681. [Google Scholar] [CrossRef]
- Friedl, J.; Scheer, C.; Rowlings, D.W.; McIntosh, H.V.; Strazzabosco, A.; Warner, D.I.; Grace, P.R. Denitrification losses from an intensively managed sub-tropical pasture–Impact of soil moisture on the partitioning of N2 and N2O emissions. Soil Biol. Biochem. 2016, 92, 58–66. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Wu, D.; Cárdenas, L.M.; Calvet, S.; Brüggemann, N.; Loick, N.; Liu, S.; Bol, R. The effect of nitrification inhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol. Biochem. 2017, 113, 153–160. [Google Scholar] [CrossRef]
- Choudhary, M.A.; Akramkhanov, A.; Saggar, S. Nitrous oxide emissions in soils cropped with maize under long-term tillage and under permanent pasture in New Zealand. Soil Tillage Res. 2001, 62, 61–71. [Google Scholar] [CrossRef]
- Schwenke, G.D.; Haigh, B.M. The interaction of seasonal rainfall and nitrogen fertiliser rate on soil N2O emission, total N loss and crop yield of dryland sorghum and sunflower grown on sub-tropical Vertosols. Soil Res. 2016, 54, 604–618. [Google Scholar] [CrossRef] [Green Version]
- Lesschen, J.P.; Velthof, G.L.; De Vries, W.; Kros, J. Differentiation of nitrous oxide emission factors for agricultural soils. Environ. Pollut. 2011, 159, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Meurer, K.H.; Franko, U.; Stange, C.F.; Dalla Rosa, J.; Madari, B.E.; Jungkunst, H.F. Direct nitrous oxide (N2O) fluxes from soils under different land use in Brazil—A critical review. Environ. Res. Lett. 2016, 11, 23001. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Z.; Cai, Z.; Reverchon, F. Review of denitrification in tropical and subtropical soils of terrestrial ecosystems. J. Soils Sediments 2013, 13, 699–710. [Google Scholar] [CrossRef]
- Hefting, M.M.; Bobbink, R.; De Caluwe, H. Nitrous Oxide Emission and Denitrification in Chronically Nitrate-Loaded Riparian Buffer Zones. J. Environ. Qual. 2003, 32, 1194–1203. [Google Scholar] [CrossRef]
- Hénault, C.; Grossel, A.; Mary, B.; Roussel, M.; Léonard, J. Nitrous Oxide Emission by Agricultural Soils: A Review of Spatial and Temporal Variability for Mitigation. Pedosphere 2012, 22, 426–433. [Google Scholar] [CrossRef]
- Lai, T.V.; Farquharson, R.; Denton, M.D. High soil temperatures alter the rates of nitrification, denitrification and associated N2O emissions. J. Soils Sediments 2019, 19, 2176–2189. [Google Scholar] [CrossRef]
- Prentice, I.C. Terrestrial nitrogen cycle simulation with a dynamic global vegetation model. Glob. Chang. Biol. 2008, 14, 1745–1764. [Google Scholar] [CrossRef]
- Lai, T.V.; Denton, M.D. N2O and N2 emissions from denitrification respond differently to temperature and nitrogen supply. J. Soils Sediments 2018, 18, 1548–1557. [Google Scholar] [CrossRef]
- Sun, X.; Han, X.; Ping, F.; Zhang, L.; Zhang, K.; Chen, M.; Wu, W. Effect of rice-straw biochar on nitrous oxide emissions from paddy soils under elevated CO2 and temperature. Sci. Total. Environ. 2018, 628, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Vargas, V.P.; Soares, J.R.; Oliveira, B.G.; Lourenço, K.S.; Martins, A.A.; Del Grosso, S.J.; Carmo, J.B.D.; Cantarella, H. Sugarcane Straw, Soil Temperature, and Nitrification Inhibitor Impact N2O Emissions from N Fertilizer. BioEnergy Res. 2019, 12, 801–812. [Google Scholar] [CrossRef]
- Maag, M.; Vinther, F. Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl. Soil Ecol. 1996, 4, 5–14. [Google Scholar] [CrossRef]
- Lassey, K.; Harvey, M. Nitrous oxide: The serious side of laughing gas. Water Atmos. 2007, 15, 10–11. Available online: https://niwa.co.nz/sites/niwa.co.nz/files/import/attachments/oxide.pdf (accessed on 26 February 2019).
- Clough, T.J.; Sherlock, R.R.; Kelliher, F.M. Can liming mitigate N2O fluxes from a urine-amended soil? Soil Res. 2003, 41, 439–457. [Google Scholar] [CrossRef]
- Šimek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Rochester, I.J. Estimating nitrous oxide emissions from flood-irrigated alkaline grey clays. Soil Res. 2003, 41, 197–206. [Google Scholar] [CrossRef]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Tsuneda, S.; Mikami, M.; Kimochi, Y.; Hirata, A. Effect of salinity on nitrous oxide emission in the biological nitrogen removal process for industrial wastewater. J. Hazard. Mater. 2005, 119, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, J.; Liao, L.; Li, Y.; Wang, H.; Rahim, S.F. Water Salinity Should Be Reduced for Irrigation to Minimize Its Risk of Increased Soil N2O Emissions. Int. J. Environ. Res. Public Health 2018, 15, 2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, M.J.; Cloy, J.M.; Topp, C.F.E.; Ball, B.C.; Bagnall, A.; Rees, R.M.; Chadwick, D.R. Quantifying N2O emissions from intensive grassland production: The role of synthetic fertilizer type, application rate, timing and nitrification inhibitors. J. Agric. Sci. 2016, 154, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Snyder, C.; Bruulsema, T.; Jensen, T.; Fixen, P. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Zimmermann, J.; Carolan, R.; Forrestal, P.; Harty, M.; Lanigan, G.; Richards, K.; Roche, L.; Whitfield, M.; Jones, M. Assessing the performance of three frequently used biogeochemical models when simulating N2O emissions from a range of soil types and fertiliser treatments. Geochemistry 2018, 331, 53–69. [Google Scholar] [CrossRef]
- Leip, A.; Busto, M.; Winiwarter, W. Developing spatially stratified N2O emission factors for Europe. Environ. Pollut. 2011, 159, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Dechow, R.; Freibauer, A. Assessment of German nitrous oxide emissions using empirical modelling approaches. Nutr. Cycl. Agroecosystems 2011, 91, 235–254. [Google Scholar] [CrossRef]
- Hoben, J.P.; Gehl, R.J.; Millar, N.; Grace, P.R.; Robertson, G.P. Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Glob. Chang. Biol. 2010, 17, 1140–1152. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Zhou, Z.; Wang, B.; Xing, Y.; Cheng, H. Nitrous oxide emissions from different land use patterns in a typical karst region, Southwest China. Chin. J. Geochem. 2013, 32, 137–145. [Google Scholar] [CrossRef]
- de Morais, R.F.; Boddey, R.M.; Urquiaga, S.; Jantalia, C.P.; Alves, B.J. Ammonia volatilization and nitrous oxide emissions during soil preparation and N fertilization of elephant grass (Pennisetum purpureum Schum.). Soil Biol. Biochem. 2013, 64, 80–88. [Google Scholar] [CrossRef]
- Pal, P.; Clough, T.J.; Kelliher, F.M.; Sherlock, R.R. Nitrous Oxide Emissions from In Situ Deposition of 15 N-Labeled Ryegrass Litter in a Pasture Soil. J. Environ. Qual. 2013, 42, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Ju, X.; Su, F.; Meng, Q.; Oenema, O.; Christie, P.; Chen, X.; Zhang, F. Nitrous oxide and methane emissions from optimized and alternative cereal cropping systems on the North China Plain: A two-year field study. Sci. Total. Environ. 2014, 472, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Lebender, U.; Senbayram, M.; Lammel, J.; Kuhlmann, H. Impact of mineral N fertilizer application rates on N2O emissions from arable soils under winter wheat. Nutr. Cycl. Agroecosyst. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Shi, Y.-F.; Wu, W.-L.; Meng, F.-Q.; Zheng, L.; Wang, D.-P.; Ye, H.; Ding, G.-W. Nitrous Oxide and Methane Fluxes during the Maize Season Under Optimized Management in Intensive Farming Systems of the North China Plain. Pedosphere 2014, 24, 487–497. [Google Scholar] [CrossRef]
- Sordi, A.; Dieckow, J.; Bayer, C.; Alburquerque, M.A.; Piva, J.T.; Zanatta, J.A.; Tomazi, M.; da Rosa, C.M.; de Moraes, A. Nitrous oxide emission factors for urine and dung patches in a subtropical Brazilian pastureland. Agric. Ecosyst. Environ. 2014, 190, 94–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Y.; Zhou, Y.; Liu, J.; Zhang, C. Nitrous oxide emissions from maize–wheat field during 4 successive years in the North China Plain. Biogeosciences 2014, 11, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Aita, C.; Schirmann, J.; Pujol, S.B.; Giacomini, S.J.; Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gonzatto, R.; Giacomini, D.A.; Doneda, A.A. Reducing nitrous oxide emissions from a maize-wheat sequence by decreasing soil nitrate concentration: Effects of split application of pig slurry and dicyandiamide. Eur. J. Soil Sci. 2015, 66, 359–368. [Google Scholar] [CrossRef]
- Hinton, N.; Cloy, J.; Bell, M.; Chadwick, D.; Topp, C.; Rees, R. Managing fertiliser nitrogen to reduce nitrous oxide emissions and emission intensities from a cultivated Cambisol in Scotland. Geoderma Reg. 2015, 4, 55–65. [Google Scholar] [CrossRef]
- Huérfano, X.; Fuertes-Mendizábal, T.; Duñabeitia, M.K.; González-Murua, C.; Estavillo, J.M.; Menéndez, S. Splitting the application of 3,4-dimethylpyrazole phosphate (DMPP): Influence on greenhouse gases emissions and wheat yield and quality under humid Mediterranean conditions. Eur. J. Agron. 2015, 64, 47–57. [Google Scholar] [CrossRef]
- Martins, M.R.; Jantalia, C.P.; Polidoro, J.C.; Batista, J.N.; Alves, B.J.; Boddey, R.M.; Urquiaga, S. Nitrous oxide and ammonia emissions from N fertilization of maize crop under no-till in a Cerrado soil. Soil Tillage Res. 2015, 151, 75–81. [Google Scholar] [CrossRef]
- Shepherd, A.; Yan, X.; Nayak, D.; Newbold, J.; Moran, D.; Dhanoa, M.S.; Goulding, K.; Smith, P.; Cárdenas, L.M. Disaggregated N2O emission factors in China based on cropping parameters create a robust approach to the IPCC Tier 2 methodology. Atmos. Environ. 2015, 122, 272–281. [Google Scholar] [CrossRef] [Green Version]
- van der Weerden, T.; Cox, N.; Luo, J.; Di, H.; Podolyan, A.; Phillips, R.; Saggar, S.; de Klein, C.; Ettema, P.; Rys, G. Refining the New Zealand nitrous oxide emission factor for urea fertiliser and farm dairy effluent. Agric. Ecosyst. Environ. 2016, 222, 133–137. [Google Scholar] [CrossRef]
- Harty, M.; Forrestal, P.; Watson, C.; McGeough, K.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.; Richards, K.; Lanigan, G. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total. Environ. 2016, 563–564, 576–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krol, D.; Carolan, R.; Minet, E.; McGeough, K.; Watson, C.; Forrestal, P.; Lanigan, G.; Richards, K. Improving and disaggregating N2O emission factors for ruminant excreta on temperate pasture soils. Sci. Total. Environ. 2016, 568, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, B.C.T.; Denmead, O.T.; White, I.; Naylor, T.; Salter, B.; Wilson, S.R.; Griffith, D.W. Emissions of nitrogen gases from sugarcane soils. In Proceedings of the 2009 Conference of the Australian Society of Sugar Cane Technologists held at Ballina, Ballina, NSW, Australia, 5–8 May 2009; Australian Society of Sugar Cane Technologists. pp. 85–92. Available online: http://hdl.handle.net/11079/14180 (accessed on 4 March 2019).
- Roche, L.; Forrestal, P.; Lanigan, G.; Richards, K.; Shaw, L.; Wall, D. Impact of fertiliser nitrogen formulation, and N stabilisers on nitrous oxide emissions in spring barley. Agric. Ecosyst. Environ. 2016, 233, 229–237. [Google Scholar] [CrossRef]
- Faubert, P.; Lemay-Bélisle, C.; Bertrand, N.; Bouchard, S.; Chantigny, M.H.; Durocher, S.; Paré, M.C.; Rochette, P.; Tremblay, P.; Ziadi, N.; et al. Greenhouse gas emissions following land application of pulp and paper mill sludge on a clay loam soil. Agric. Ecosyst. Environ. 2017, 250, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Forte, A.; Fagnano, M.; Fierro, A. Potential role of compost and green manure amendment to mitigate soil GHGs emissions in Mediterranean drip irrigated maize production systems. J. Environ. Manag. 2017, 192, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Gillette, K.; Ma, L.; Malone, R.W.; Fang, Q.; Halvorson, A.D.; Hatfield, J.L.; Ahuja, L. Simulating N 2 O emissions under different tillage systems of irrigated corn using RZ-SHAW model. Soil Tillage Res. 2017, 165, 268–278. [Google Scholar] [CrossRef]
- Htun, Y.M.; Tong, Y.; Gao, P.; Xiaotang, J. Coupled effects of straw and nitrogen management on N 2 O and CH 4 emissions of rainfed agriculture in Northwest China. Atmos. Environ. 2017, 157, 156–166. [Google Scholar] [CrossRef]
- Laville, P.; Bosco, S.; Volpi, I.; Virgili, G.; Neri, S.; Continanza, D.; Bonari, E. Temporal integration of soil N2O fluxes: Validation of IPNOA station automatic chamber prototype. Environ. Monit. Assess. 2017, 189, 485. [Google Scholar] [CrossRef] [PubMed]
- Krauss, M.; Ruser, R.; Müller, T.; Hansen, S.; Mäder, P.; Gattinger, A. Impact of reduced tillage on greenhouse gas emissions and soil carbon stocks in an organic grass-clover ley-winter wheat cropping sequence. Agric. Ecosyst. Environ. 2017, 239, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Pugesgaard, S.; Petersen, S.O.; Chirinda, N.; Olesen, J.E. Crop residues as driver for N2O emissions from a sandy loam soil. Agric. For. Meteorol. 2017, 233, 45–54. [Google Scholar] [CrossRef]
- Xie, B.; Gu, J.; Yu, J.; Han, G.; Zheng, X.; Xu, Y.; Lin, H. Effects of N Fertilizer Application on Soil N2O Emissions and CH4 Uptake: A Two-Year Study in an Apple Orchard in Eastern China. Atmosphere 2017, 8, 181. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhu, B.; Wang, X.; Wang, Y. Long-term field measurements of annual methane and nitrous oxide emissions from a Chinese subtropical wheat-rice rotation system. Soil Biol. Biochem. 2017, 115, 21–34. [Google Scholar] [CrossRef]
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term effects of contrasting tillage on soil organic carbon, nitrous oxide and ammonia emissions in a Mediterranean Vertisol under different crop sequences. Sci. Total. Environ. 2018, 619–620, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Kou, Y.; Yang, W.; Chen, G.; Xu, H. Effects of urease and nitrification inhibitors on nitrous oxide emissions and nitrifying/denitrifying microbial communities in a rainfed maize soil: A 6-year field observation. Soil Tillage Res. 2018, 180, 82–90. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Bareche, J.; Pareja-Sánchez, E.; Justes, É.; Cantero-Martínez, C. No-tillage reduces long-term yield-scaled soil nitrous oxide emissions in rainfed Mediterranean agroecosystems: A field and modelling approach. Agric. Ecosyst. Environ. 2018, 262, 36–47. [Google Scholar] [CrossRef]
- Reinsch, T.; Loges, R.; Kluß, C.; Taube, F. Renovation and conversion of permanent grass-clover swards to pasture or crops: Effects on annual N 2 O emissions in the year after ploughing. Soil Tillage Res. 2018, 175, 119–129. [Google Scholar] [CrossRef]
- Simon, P.L.; Dieckow, J.; de Klein, C.A.; Zanatta, J.A.; van der Weerden, T.J.; Ramalho, B.; Bayer, C. Nitrous oxide emission factors from cattle urine and dung, and dicyandiamide (DCD) as a mitigation strategy in subtropical pastures. Agric. Ecosyst. Environ. 2018, 267, 74–82. [Google Scholar] [CrossRef]
- Campanha, M.M.; de Oliveira, A.D.; Marriel, I.E.; Neto, M.M.G.; Malaquias, J.V.; Landau, E.C.; Filho, M.R.D.A.; Ribeiro, F.P.; de Carvalho, A.M. Effect of soil tillage and N fertilization on N2O mitigation in maize in the Brazilian Cerrado. Sci. Total. Environ. 2019, 692, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Foldal, C.; Kitzler, B.; Haas, E.; Strauss, P.; Eder, A.; Zechmeister-Boltenstern, S.; Amon, B. N2O emissions and NO3− leaching from two contrasting regions in Austria and influence of soil, crops and climate: A modelling approach. Nutr. Cycl. Agroecosystems 2019, 113, 95–111. [Google Scholar] [CrossRef] [Green Version]
- Mumford, M.; Rowlings, D.; Scheer, C.; De Rosa, D.; Grace, P. Effect of irrigation scheduling on nitrous oxide emissions in intensively managed pastures. Agric. Ecosyst. Environ. 2019, 272, 126–134. [Google Scholar] [CrossRef]
- Myrgiotis, V.; Williams, M.; Rees, R.M.; Topp, C.F. Estimating the soil N2O emission intensity of croplands in northwest Europe. Biogeosciences 2019, 16, 1641–1655. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Treu, R.; Wang, J.; Hao, X.; Thomas, B.W. Modeling growing season and annual cumulative nitrous oxide emissions and emission factors from organically fertilized soils planted with barley in Lethbridge, Alberta, Canada. Agric. Syst. 2019, 176, 102654. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, F.; Li, H.; Wang, L.; Wu, S.; Xiao, G.; Wu, W. Optimized fertigation maintains high yield and mitigates N2O and NO emissions in an intensified wheat–maize cropping system. Agric. Water Manag. 2019, 211, 26–36. [Google Scholar] [CrossRef]
- Baral, K.R.; Lærke, P.E.; Petersen, S.O. Nitrous oxide emissions after renovation of festulolium, and mitigation potential of 3,4-dimethyl pyrazole phosphate (DMPP). Geoderma 2020, 376, 114551. [Google Scholar] [CrossRef]
- Cowan, N.; Levy, P.; Maire, J.; Coyle, M.; Leeson, S.; Famulari, D.; Carozzi, M.; Nemitz, E.; Skiba, U. An evaluation of four years of nitrous oxide fluxes after application of ammonium nitrate and urea fertilisers measured using the eddy covariance method. Agric. For. Meteorol. 2020, 280, 107812. [Google Scholar] [CrossRef]
- Krol, D.; Forrestal, P.; Wall, D.; Lanigan, G.; Sanz-Gomez, J.; Richards, K. Nitrogen fertilisers with urease inhibitors reduce nitrous oxide and ammonia losses, while retaining yield in temperate grassland. Sci. Total. Environ. 2020, 725, 138329. [Google Scholar] [CrossRef] [PubMed]
- Kudeyarov, V.N. Nitrous Oxide Emission from Fertilized Soils: An Analytical Review. Eurasian Soil Sci. 2020, 53, 1396–1407. [Google Scholar] [CrossRef]
- Wang, R.; Pan, Z.; Zheng, X.; Ju, X.; Yao, Z.; Butterbach-Bahl, K.; Zhang, C.; Wei, H.; Huang, B. Using field-measured soil N2O fluxes and laboratory scale parameterization of N2O/(N2O+N2) ratios to quantify field-scale soil N2 emissions. Soil Biol. Biochem. 2020, 148, 107904. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; Cantero-Martínez, C.; Álvaro-Fuentes, J.; Plaza-Bonilla, D. Impact of tillage and N fertilization rate on soil N2O emissions in irrigated maize in a Mediterranean agroecosystem. Agric. Ecosyst. Environ. 2020, 287, 106687. [Google Scholar] [CrossRef]
- Yang, Y.; Tong, Y.; Gao, P.; Htun, Y.M.; Feng, T. Evaluation of N2O emission from rainfed wheat field in northwest agricultural land in China. Environ. Sci. Pollut. Res. 2020, 27, 43466–43479. [Google Scholar] [CrossRef] [PubMed]
- Grave, R.A.; Nicoloso, R.D.S.; Cassol, P.C.; da Silva, M.L.B.; Mezzari, M.P.; Aita, C.; Wuaden, C.R. Determining the effects of tillage and nitrogen sources on soil N2O emission. Soil Tillage Res. 2018, 175, 1–12. [Google Scholar] [CrossRef]
- Chen, S.; Hao, T.; Goulding, K.; Misselbrook, T.; Liu, X. Impact of 13-years of nitrogen addition on nitrous oxide and methane fluxes and ecosystem respiration in a temperate grassland. Environ. Pollut. 2019, 252, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, N.; Baruah, K.K.; Bhattacharyya, P. Emission estimation of nitrous oxide (N2O) from a wheat cropping system under varying tillage practices and different levels of nitrogen fertiliser. Soil Res. 2016, 54, 767–776. [Google Scholar] [CrossRef]
- Schwenke, G.D.; Haigh, B.M. Can split or delayed application of N fertiliser to grain sorghum reduce soil N2O emissions from sub-tropical Vertosols and maintain grain yields? Soil Res. 2019, 57, 859–874. [Google Scholar] [CrossRef]
- Yang, X.M.; Drury, C.F.; Reeb, M.R. No-Tillage had Warmer Over-Winter Soil Temperatures than Conventional Tillage in a Brookston Clay Loam Soils in Southwestern Ontario. Soil Sci. Soc. Am. J. 2018, 82, 307–314. [Google Scholar] [CrossRef]
- Žurovec, O.; Sitaula, B.K.; Čustović, H.; Žurovec, J.; Dörsch, P. Effects of tillage practice on soil structure, N2O emissions and economics in cereal production under current socio-economic conditions in central Bosnia and Herzegovina. PLoS ONE 2017, 12, e0187681. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chadwick, D.R.; Cheng, Y.; Yan, X. Global analysis of agricultural soil denitrification in response to fertilizer nitrogen. Sci. Total. Environ. 2018, 616–617, 908–917. [Google Scholar] [CrossRef]
- Duan, Y.-F.; Hallin, S.; Jones, C.M.; Priemé, A.; Labouriau, R.; Petersen, S.O. Catch Crop Residues Stimulate N2O Emissions during Spring, Without Affecting the Genetic Potential for Nitrite and N2O Reduction. Front. Microbiol. 2018, 9, 2629. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Greer, K.D.; Nafziger, E.D.; Villamil, M.B.; Pittelkow, C.M. Soil N2 O emissions as affected by long-term residue removal and no-till practices in continuous corn. GCB Bioenergy 2018, 10, 972–985. [Google Scholar] [CrossRef]
- Badagliacca, G.; Ruisi, P.; Rees, R.M.; Saia, S. An assessment of factors controlling N2O and CO2 emissions from crop residues using different measurement approaches. Biol. Fertil. Soils 2017, 53, 547–561. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, Y.; Liang, H.; Gao, D. Long-term harvesting of reeds affects greenhouse gas emissions and microbial functional genes in alkaline wetlands. Water Res. 2019, 164, 114936. [Google Scholar] [CrossRef] [PubMed]
- da Silva Cardoso, A.; de Figueiredo Brito, L.; Janusckiewicz, E.R.; da Silva Morgado, E.; Barbero, R.P.; Koscheck, J.F.W.; Reis, R.A.; Ruggieri, A.C. Impact of Grazing Intensity and Seasons on Greenhouse Gas Emissions in Tropical Grassland. Ecosystems 2017, 20, 845–859. [Google Scholar] [CrossRef]
- Deng, J.; Li, C.; Burger, M.; Horwath, W.R.; Smart, D.; Six, J.; Guo, L.; Salas, W.; Frolking, S. Assessing Short-Term Impacts of Management Practices on N2O Emissions from Diverse Mediterranean Agricultural Ecosystems Using a Biogeochemical Model. J. Geophys. Res. Biogeosci. 2018, 123, 1557–1571. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.A.; McTaggart, I.P.; Tsuruta, H. Emissions of N2O and NO associated with nitrogen fertilization in intensive agriculture, and the potential for mitigation. Soil Use Manag. 1997, 13, 296–304. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Drastig, K.; Meyer-Aurich, A.; Ellmer, F.; Baumecker, M. Irrigation, soil organic carbon and N2O emissions. A review. Agron. Sustain. Dev. 2013, 33, 733–749. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Martín, L.; Arce, A.; Benito, A.; Garcia-Torres, L.; Vallejo, A. Influence of drip and furrow irrigation systems on nitrogen oxide emissions from a horticultural crop. Soil Biol. Biochem. 2008, 40, 1698–1706. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Li, Z.; Wang, S.; Qu, Y. Effects of Irrigation Regime and Nitrogen Fertilizer Management on CH4, N2O and CO2 Emissions from Saline–Alkaline Paddy Fields in Northeast China. Sustainability 2018, 10, 475. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Liu, H.; Zhang, X.; Ma, J.; Han, B.; Li, W.; Zou, H.; Zhang, Y.; Lin, X. Impacts of irrigation methods on greenhouse gas emissions/absorptions from vegetable soils. J. Soils Sediments 2019, 20, 723–733. [Google Scholar] [CrossRef]
- Shang, Z.; Abdalla, M.; Kuhnert, M.; Albanito, F.; Zhou, F.; Xia, L.; Smith, P. Measurement of N2O emissions over the whole year is necessary for estimating reliable emission factors. Environ. Pollut. 2020, 259, 113864. [Google Scholar] [CrossRef]
- Groffman, P.M.; Altabet, M.A.; Böhlke, J.K.; Butterbach-Bahl, K.; David, M.B.; Firestone, M.K.; Giblin, A.E.; Kana, T.M.; Nielsen, L.P.; Voytek, M.A. Methods for Measuring Denitrification: Diverse Approaches to a Difficult Problem. Ecol. Appl. 2006, 16, 2091–2122. [Google Scholar] [CrossRef]
- Beauchamp, E.G. Nitrous oxide emission from agricultural soils. Can. J. Soil Sci. 1997, 77, 113–123. [Google Scholar] [CrossRef]
- Skiba, U.; Hargreaves, K.; Fowler, D.; Smith, K. Fluxes of nitric and nitrous oxides from agricultural soils in a cool temperate climate. Atmos. Environ. Part A. Gen. Top. 1992, 26, 2477–2488. [Google Scholar] [CrossRef]
- Schäfer, K.G.; Böttcher, J.; Weymann, D.; Von Der Heide, C.; Duijnisveld, W.H.M. Evaluation of a Closed Tunnel for Field-Scale Measurements of Nitrous Oxide Fluxes from an Unfertilized Grassland Soil. J. Environ. Qual. 2012, 41, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Venterea, R.T. Simplified Method for Quantifying Theoretical Underestimation of Chamber-Based Trace Gas Fluxes. J. Environ. Qual. 2010, 39, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensen, A.; Skiba, U.; Famulari, D. Low cost and state of the art methods to measure nitrous oxide emissions. Environ. Res. Lett. 2013, 8, 025022. [Google Scholar] [CrossRef]
- Adu, J.; Kumarasamy, M.V. Assessing Non-Point Source Pollution Models: A Review. Pol. J. Environ. Stud. 2018, 27, 1913–1922. [Google Scholar] [CrossRef]
- Frolking, S.; Mosier, A.; Ojima, D.; Li, C.; Parton, W.; Potter, C.; Priesack, E.; Stenger, R.; Haberbosch, C.; Dörsch, P.; et al. Comparison of N2O emissions from soils at three temperate agricultural sites: Simulations of year-round measurements by four models. Nutr. Cycl. Agroecosyst. 1998, 52, 77–105. [Google Scholar] [CrossRef]
- Heinen, M. Simplified denitrification models: Overview and properties. Geoderma 2006, 133, 444–463. [Google Scholar] [CrossRef]
- Li, X.; Yeluripati, J.; Jones, E.O.; Uchida, Y.; Hatano, R. Hierarchical Bayesian calibration of nitrous oxide (N2O) and nitrogen monoxide (NO) flux module of an agro-ecosystem model: ECOSSE. Ecol. Model. 2015, 316, 14–27. [Google Scholar] [CrossRef]
- Snow, V.; Rotz, C.; Moore, A.; Martin-Clouaire, R.; Johnson, I.; Hutchings, N.; Eckard, R. The challenges–and some solutions–to process-based modelling of grazed agricultural systems. Environ. Model. Softw. 2014, 62, 420–436. [Google Scholar] [CrossRef]
- Williams, J.R.; Izaurralde, R.C.; Steglich, E.M. Agricultural policy/environmental extender model. Theor. Doc. 2008, 604, 2008–2017. Available online: https://agrilifecdn.tamu.edu/epicapex/files/2017/03/THE-APEX0806-theoretical-documentation-Oct-2015.pdf (accessed on 21 December 2018).
- Lehuger, S.; Gabrielle, B.; Van Oijen, M.; Makowski, D.; Germon, J.-C.; Morvan, T.; Hénault, C. Bayesian calibration of the nitrous oxide emission module of an agro-ecosystem model. Agric. Ecosyst. Environ. 2009, 133, 208–222. [Google Scholar] [CrossRef] [Green Version]
- Youssef, M.A.; Skaggs, R.W.; Chescheir, G.M.; Gilliam, J.W. The nitrogen simulation model, DRAINMOD-N II. Trans. ASAE 2005, 48, 611–626. [Google Scholar] [CrossRef]
- Gassman, P.W.; Williams, J.R.; Benson, V.W.; Izaurralde, R.C.; Hauck, L.M.; Jones, C.A.; Atwood, J.D.; Kiniry, J.R.; Flowers, J.D. Historical development and applications of the EPIC and APEX models. In Proceedings of the 2005 ASAE Annual Meeting (p. 1). American Society of Agricultural and Biological Engineers, Ottawa, ON, Canada, 1–4 August 2004; p. 1. [Google Scholar] [CrossRef] [Green Version]
- Chatskikh, D.; Olesen, J.E.; Berntsen, J.; Regina, K.; Yamulki, S. Simulation of Effects of Soils, Climate and Management on N2O Emission from Grasslands. Biogeochemistry 2005, 76, 395–419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Peng, C.; Wang, M.; Zhou, X.; Li, M.; Wang, K.; Ding, J.; Zhu, Q. Process-based TRIPLEX-GHG model for simulating N2O emissions from global forests and grasslands: Model development and evaluation. J. Adv. Model. Earth Syst. 2017, 9, 2079–2102. [Google Scholar] [CrossRef]
- Adams, H.D.; Williams, A.P.; Xu, C.; Rauscher, S.A.; Jiang, X.; McDowell, N.G. Empirical and process-based approaches to climate-induced forest mortality models. Front. Plant Sci. 2013, 4, 438. [Google Scholar] [CrossRef] [Green Version]
- Izaurralde, R.C.; McGill, W.B.; Williams, J.R.; Jones, C.D.; Link, R.P.; Manowitz, D.H.; Schwab, D.E.; Zhang, X.; Robertson, G.P.; Millar, N. Simulating microbial denitrification with EPIC: Model description and evaluation. Ecol. Model. 2017, 359, 349–362. [Google Scholar] [CrossRef]
- Foltz, M.E.; Zilles, J.L.; Koloutsou-Vakakis, S. Prediction of N2O emissions under different field management practices and climate conditions. Sci. Total. Environ. 2019, 646, 872–879. [Google Scholar] [CrossRef]
- Bouraoui, F.; Grizzetti, B. Modelling mitigation options to reduce diffuse nitrogen water pollution from agriculture. Sci. Total. Environ. 2014, 468–469, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Thomas, B.W.; Du, X.; Hao, X.; Wang, J. Modeling nitrous oxide emissions from rough fescue grassland soils subjected to long-term grazing of different intensities using the Soil and Water Assessment Tool (SWAT). Environ. Sci. Pollut. Res. 2018, 25, 27362–27377. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Wang, J. Current and future hot-spots and hot-moments of nitrous oxide emission in a cold climate river basin. Environ. Pollut. 2018, 239, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, J.; Lu, C.; Xu, R.; Canadell, J.G.; Jackson, R.B.; Arneth, A.; Chang, J.; Chen, G.; Ciais, P.; et al. The global N2O Model Intercomparison Project. Bull. Am. Meteorol. Soc. 2018, 99, 1231–1251. [Google Scholar] [CrossRef] [Green Version]
- Li, C. User’s Guide for the DNDC Model (Version 9.5) 2012. University of New Hampshire. Available online: http://www.dndc.sr.unh.edu/model/GuideDNDC95.pdf (accessed on 25 January 2020).
- Neitsch, S.L.; Arnold, J.G.; Kiniry, J.R.; Williams, J.R. Soil and water assessment tool theoretical documentation version 2009. Texas Wat. Resour. Inst. 2011. Available online: https://swat.tamu.edu/media/99192/swat2009-theory.pdf (accessed on 9 March 2018).
- Yang, Q.; Zhang, X.; Abraha, M.; Del Grosso, S.; Robertson, G.P.; Chen, J. Enhancing the soil and water assessment tool model for simulating N2O emissions of three agricultural systems. Ecosyst. Health Sustain. 2017, 3, e01259. [Google Scholar] [CrossRef] [Green Version]
- Wagena, M.B.; Bock, E.M.; Sommerlot, A.R.; Fuka, D.R.; Easton, Z.M. Development of a nitrous oxide routine for the SWAT model to assess greenhouse gas emissions from agroecosystems. Environ. Model. Softw. 2017, 89, 131–143. [Google Scholar] [CrossRef] [Green Version]
- David, M.B.; Del Grosso, S.J.; Hu, X.; Marshall, E.P.; McIsaac, G.F.; Parton, W.J.; Tonitto, C.; Youssef, M.A. Modeling denitrification in a tile-drained, corn and soybean agroecosystem of Illinois, USA. Biogeochemistry 2009, 93, 7–30. [Google Scholar] [CrossRef]
- Del Grosso, S.J.; Parton, W.J.; Adler, P.R.; Davis, S.C.; Keough, C.; Marx, E. DayCent model simulations for estimating soil carbon dynamics and greenhouse gas fluxes from agricultural production systems. In Managing Agricultural Greenhouse Gases: Coordinated Agricultural Research through GRACEnet to Address our Changing Climate; Academic Press: San Diego, CA, USA, 2012; pp. 241–250. [Google Scholar]
- Bosatta, E. A qualitative analysis of the stability of the root-microorganism soil system I. carbon-nitrogen status and nitrogen mineralization. Ecol. Model. 1981, 13, 223–236. [Google Scholar] [CrossRef]
- Cui, F.; Zheng, X.; Liu, C.; Wang, K.; Zhou, Z.; Deng, J. Assessing biogeochemical effects and best management practice for a wheat–maize cropping system using the DNDC model. Biogeosciences 2014, 11, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R.K.; Jones, C.D.; Ingraham, P.; Collier, S.; Izaurralde, R.C.; Jokela, W.; Osterholz, W.; Salas, W.; Vadas, P.; Ruark, M.D. Underestimation of N2 O emissions in a comparison of the DayCent, DNDC, and EPIC models. Ecol. Appl. 2018, 28, 694–708. [Google Scholar] [CrossRef]
- Fitton, N.; Datta, A.; Smith, K.; Williams, J.R.; Hastings, A.; Kuhnert, M.; Topp, C.F.E.; Smith, P. Assessing the sensitivity of modelled estimates of N2O emissions and yield to input uncertainty at a UK cropland experimental site using the DailyDayCent model. Nutr. Cycl. Agroecosyst. 2014, 99, 119–133. [Google Scholar] [CrossRef]
- Necpálová, M.; Anex, R.P.; Fienen, M.N.; Del Grosso, S.J.; Castellano, M.J.; Sawyer, J.E.; Iqbal, J.; Pantoja, J.L.; Barker, D.W. Understanding the DayCent model: Calibration, sensitivity, and identifiability through inverse modeling. Environ. Model. Softw. 2015, 66, 110–130. [Google Scholar] [CrossRef] [Green Version]
- Myrgiotis, V.; Rees, R.M.; Topp, C.F.; Williams, M. A systematic approach to identifying key parameters and processes in agroecosystem models. Ecol. Model. 2018, 368, 344–356. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Chen, S. A review on parameterization and uncertainty in modeling greenhouse gas emissions from soil. Geoderma 2012, 170, 206–216. [Google Scholar] [CrossRef]
- Myrgiotis, V.; Williams, M.; Rees, R.M.; Smith, K.E.; Thorman, R.E.; Topp, C.F. Model evaluation in relation to soil N2O emissions: An algorithmic method which accounts for variability in measurements and possible time lags. Environ. Model. Softw. 2016, 84, 251–262. [Google Scholar] [CrossRef] [Green Version]

| Environmental Factors | Management Factors | Measurement Factors |
|---|---|---|
| Microbial population | Fertilizer application | Length of measurement period |
| Soil available carbon | Tillage system | Types of measurements |
| Soil N concentration | Harvest and crop residues | |
| Soil moisture | Irrigation | |
| Soil texture | ||
| Soil temperature | ||
| Soil pH and salinity |
| Processes | Soil N | SOC | Soil Moisture (Water-Filled-Pore-Space (WFPS)) | Soil Temperature | Soil pH |
|---|---|---|---|---|---|
| Nitrification | + | + | ~60%: + | + | Need more research |
| Denitrification | + | + | 60–80%: + | + | Need more research |
| N2/N2O ratio | − | + (depends on N) | >90%: + | + | <6.0: more N2O; =6.0:equivalent; >6.0: more N2 |
| Source | Crops | EF (%) | Country | Fertilizer Type | Soil Type | N Fertilizer (kg/ha) |
|---|---|---|---|---|---|---|
| Rochester et al. [74] | Cotton | 1.1 | Australia | Mineral N | Clay | 180 |
| Dechow et al. [82] | Grassland | 0.92 | Germany | Mineral N | 100 | |
| Cropland | 0.9 | Germany | Mineral N | 0–225 | ||
| Hoben et al. [83] | Corn | 0.6–1.5 | USA | Mineral N | Loam | 0–225 |
| Lesschen et al. [60] | Grassland | 1.1 | Europe | Mineral N | 300–400 | |
| Grassland | 0.83 | Netherlands | Organic N | |||
| Cheng et al. [84] | Corn | 0.34 | China | Mineral N | Sand | 266 |
| de Morais et al. [85] | Grassland | 0.51 | Brazil | Mineral N | Clay | 80/100 |
| Pal et al. [86] | Pasture | 1.2 | New Zealand | Organic N | Clay loam | 213 |
| Gao et al. [87] | Winter wheat | 0.17 | China | Mineral N | Silty loam | 300 |
| Corn | 0.53 | China | Mineral N | Silty loam | 250 | |
| Lebender et al. [88] | Winter wheat | 0.46 | Germany | Mineral N | ||
| Shi et al. [89] | Corn | 0.42 | China | Mineral N | Sandy loam | 300 |
| Corn | 0.29 | China | Mineral N | Sandy loam | 186 | |
| Sordi et al. [90] | Pasture | 0.15 | Brazil | Organic N | Clay | |
| Pasture | 0.26 | Brazil | Organic N | Clay | ||
| Zhang et al. [91] | Corn | 2.5 | China | Mineral N | Clay | 173 |
| Winter wheat | 2 | China | Mineral N | Clay | 165 | |
| Aita et al. [92] | Corn | 1.39 | Brazil | Mineral N | Loam | 130 |
| Corn | 1.18 | Brazil | Organic N | Loam | 333 | |
| Winter wheat | 1.14 | Brazil | Mineral N | Loam | 110 | |
| Winter wheat | 1.55 | Brazil | Organic N | Loam | 269 | |
| Hinton et al. [93] | Spring barley | 1.35 | UK | Mineral N | Sandy loam | 120 |
| Huérfano et al. [94] | Winter wheat | 0.21 | Spain | Mineral N | Clay loam | 180 |
| Martins et al. [95] | Corn | 0.2 | Brazil | Mineral N | Sandy loam | 120 |
| Shepherd et al. [96] | Corn | 1.4 | China | Mineral N | Clay | 150 |
| Wheat | 0.71 | China | Mineral N | Silty clay | 150 | |
| Wheat | 1 | China | Mineral N | Clay loam | 150 | |
| Bell et al. [78] | Grassland | 1.06–1.34 | UK | Mineral N | Sandy loam | 80–320 |
| Van der Weerden et al. [97] | Pasture | 0.6 | New Zealand | Mineral N | 50 | |
| Pasture | 0.3 | New Zealand | Organic N | 101 | ||
| Harty et al. [98] | Pasture | 1.49 | Ireland | Mineral N | Clay/sandy loam | 200 |
| Krol et al. [99] | Grassland | 0.31 | Ireland | Organic N | Sandy loam | 280 |
| Grassland | 1.18 | Ireland | Organic N | Sandy loam | 507 | |
| Macdonald et al. [100] | Sugarcane | 3 | Australia | Mineral N | Sandy loam | |
| Roche et al. [101] | Spring barley | 0.35 | Ireland | Mineral N | Loam | 150 |
| Spring barley | 0.27 | Ireland | Mineral N | Loam | ||
| Faubert et al. [102] | Spring barley | 0.8–3.1 | Canada | Organic N | Clay loam | 90–120 |
| Forte et al. [103] | Corn | 0.55 | Italy | Mineral N | Sandy-clay-loam | 130 |
| Gillette et al. [104] | Corn | 0.66 | USA | Mineral N | Clay loam | 224 |
| Corn | 0.75 | USA | Mineral N | Clay loam | 246 | |
| Htun et al. [105] | Winter wheat | 0.43 | China | Mineral N | Silty loam | 220 |
| Laville et al. [106] | Corn | 1.8 | Italy | Mineral N | Sandy loam | 170 |
| Krauss et al. [107] | Winter wheat | 1.64 | Switzerland | Organic N | Clay | |
| Grassland | 0.71 | Switzerland | Organic N | |||
| Pugesgaard et al. [108] | Spring barley | 0.65 | Denmark | Organic N | Sandy loam | 150 |
| Xie et al. [109] | Apple orchard | 1.34 | China | Organic N | Sand | |
| Zhou et al. [110] | Wheat | 1.05 | China | Mineral N | Loam | 0–250 |
| Badagliacca et al. [111] | Winter wheat | ~1.9 | Italy | Mineral N | Clay | 120 |
| Dong et al. [112] | Corn | 0.308 | China | Mineral N | Clay | 180 |
| Plaza-Bonilla et al. [113] | Winter wheat | ~0.57 | Spain | Mineral N | Loam | 0–120 |
| Reinsch et al. [114] | Grassland | 0.27 | Germany | Organic N | Sandy loam | 180 |
| Corn | 0.74 | Germany | Organic N | Sandy loam | 180 | |
| Simon et al. [115] | Pasture | 0.34 | Brazil | Organic N | Clay | 516 |
| Pasture | 0.11 | Brazil | Organic N | Clay | ||
| Campanha et al. [116] | Corn | 0.96 | Brazil | Mineral N | Clay | 0–275 |
| Kasper et al. [117] | Corn | 0.71 | Austria | Mineral N | Clay loam | |
| Mumford et al. [118] | Pasture | 0.49–1.17 | Australia | Mineral N | Clay | 340 |
| Myrgiotis et al. [119] | Winter wheat | 0.25 | UK | Mineral N | ||
| Spring barley | 0.57 | UK | Mineral N | |||
| Shen et al. [120] | Spring barley | 0.085–1.1 | Canada | Organic N | Clay loam | 100–800 |
| Zhang et al. [121] | Winter wheat | 0.19–0.25 | China | Mineral N | Loam | 420/600 |
| Corn | 0.38–0.63 | China | Mineral N | Loam | ||
| Baral et al. [122] | Spring barley | 0.53 | Denmark | Mineral N | Sand | 169 |
| Cowan et al. [123] | Grassland | 0.9 | UK | Mineral N | Clay | 20–220 |
| Krol et a. [124] | Grassland | 0.58 | Ireland | Mineral N | Loam | 200 |
| Kudeyarov et al. [125] | Cereal crops | 0.66–0.7 | Russia | Mineral N | 67 | |
| Wang et al. [126] | Corn | 1.85 | China | Mineral N | Clay loam | 130 |
| Pareja-Sanchez et al. [127] | Corn | 0.2 | Spain | Mineral N | Sandy loam | 0/60/120 |
| Yang et al. [128] | Winter wheat | 0.41 | China | Mineral N | Silty loam | 220 |
| Model | Description | Nitrification | Denitrification | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | SOC | WFPS | T | pH | N | SOC | WFPS | T | pH | |||
| APEX | APEX is a field-scale model and is used to evaluate various land management strategies at a daily time step. | √ | √ | √ | √ | √ | √ | √ | √ | Williams et al. [159] | ||
| CERES_EGC | CERES-EGC is a field-scale and process-based agro-ecosystem model and is used to simulate NO3− leaching, emissions of N2O and nitrogen oxides at a daily time step. | √ | √ | √ | √ | √ | √ | Lehuger et al. [160] | ||||
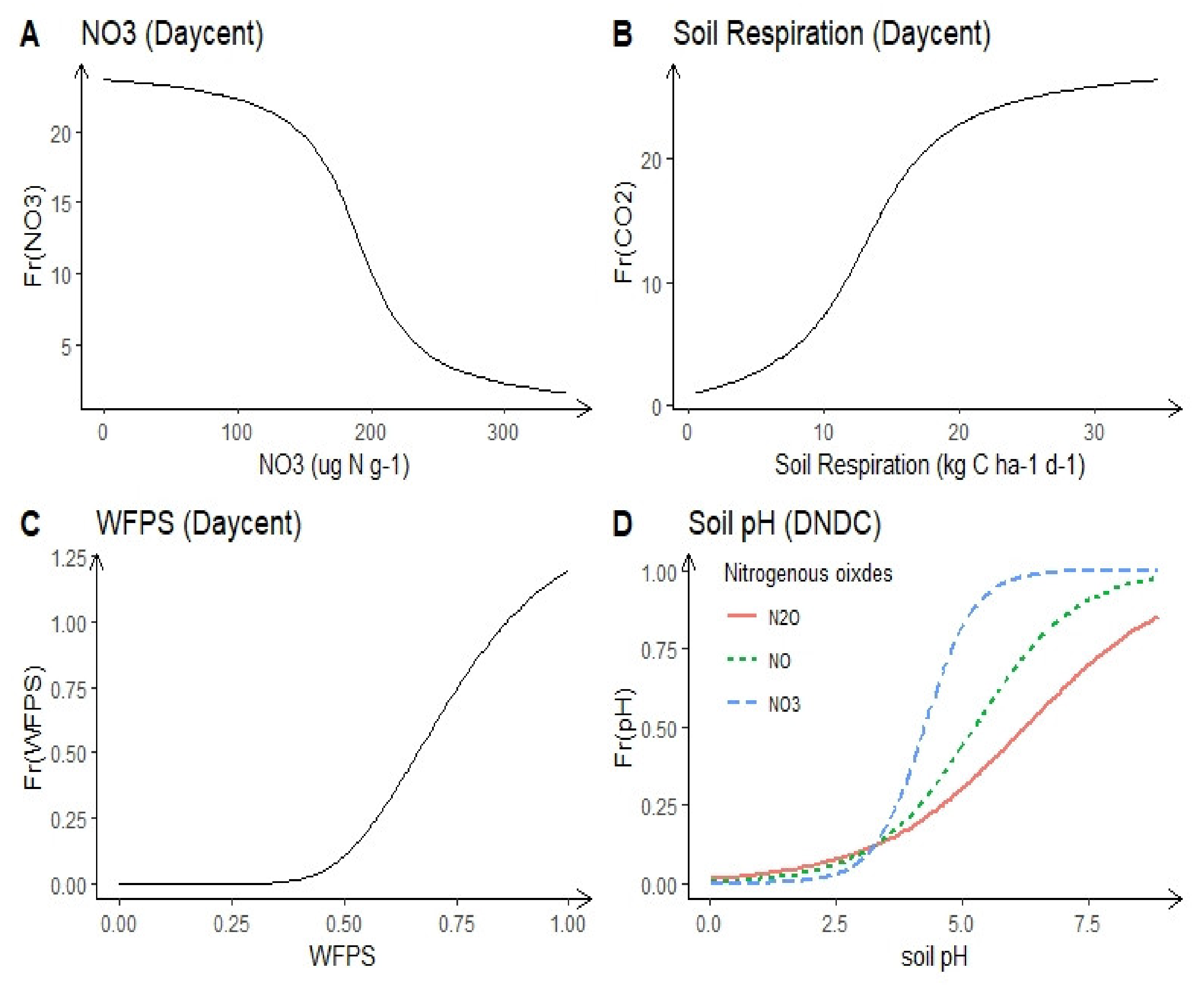
| Daily Century (DAYCENT) | DAYCENT is the daily time step version of the CENTURY, and is used to simulate exchanges of C, nutrients, and trace gases among the atmosphere, soil and plants. | √ | √ | √ | √ | √ | √ | √ | Parton et al. [30] | |||
| DNDC | DNDC is a field-scale and process-based model and is used to study N and C dynamics in agroecosystems at daily time step. | √ | √ | √ | √ | √ | √ | √ | √ | √ | Li et al. [31] | |
| DRAINMOD-N II | DRAINMOD-N II is a field-scale, daily time step and process-based model and is used to simulate C and N dynamics for artificially drained soils. | √ | √ | √ | √ | √ | √ | √ | Youssef et al. [161] | |||
| EPIC | EPIC is a field-scale agroecosystem model that simulates crop production. | √ | √ | √ | √ | √ | √ | √ | √ | Gassman et al. [162] | ||
| FASSET | FASSET is used to simulate crop growth and yield, as well as daily soil N and C fluxes in the plant–soil–atmosphere continuum. | √ | √ | √ | √ | √ | √ | Chatskikh et al. [163] | ||||
| SPACSYS | SPACSYS is a field-scale model and is used to simulate daily N and C emissions from arable land and grassland. | √ | √ | √ | √ | √ | √ | √ | √ | √ | Wu et al. [33] | |
| SWAT | SWAT is a field or catchment scale, process based model and is run at the daily time step for simulating the impacts of agricultural management practices on hydrology and water quality. | √ | √ | √ | √ | √ | √ | √ | √ | Arnold et al. [32] | ||
| TRIPLEX_GHG | TRIPLEX-GHG is developed to simulate N2O emissions from global forests and grassland. | √ | √ | √ | √ | √ | √ | √ | √ | √ | Zhang et al. [164] | |
| Model | Input Data | Physical Processes and Products Partitioning | Considered Environmental Factors |
|---|---|---|---|
| DAYCENT | Daily weather variables, site-specific soil properties, and land use. | Nitrification | Soil N, temperature, WFPS and pH |
| Denitrification | Soil N, SOC and WFPS | ||
| N2/N2O | Soil N, SOC and WFPS | ||
| NOx/N2O | Soil WFPS | ||
| DNDC | Daily weather variables, soil properties, and management practices. | Nitrification | Nitrifiers, soil N, WFPS, temperature, and pH |
| Denitrification | De-nitrifiers, SOC, soil N, temperature, and pH | ||
| NOx, N2 | Soil pH | ||
| SWAT | DEM, soil properties, daily weather variables, and management practices. | Nitrification | Soil N, WFPS, temperature and pH |
| Denitrification | Soil N, SOC, moisture, temperature and pH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy 2021, 11, 770. https://doi.org/10.3390/agronomy11040770
Wang C, Amon B, Schulz K, Mehdi B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy. 2021; 11(4):770. https://doi.org/10.3390/agronomy11040770
Chicago/Turabian StyleWang, Cong, Barbara Amon, Karsten Schulz, and Bano Mehdi. 2021. "Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review" Agronomy 11, no. 4: 770. https://doi.org/10.3390/agronomy11040770
APA StyleWang, C., Amon, B., Schulz, K., & Mehdi, B. (2021). Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy, 11(4), 770. https://doi.org/10.3390/agronomy11040770






