The Applicability of Species- and Trichothecene-Specific Primers in Monitoring the Fusarium graminearum Species Complex and Its Impact on the Surveillance of Fusarium Head Blight in Winter Wheat in Serbia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trials and Conventional Disease Assessment
2.2. Identification of Fusarium Species and Trichothecene Genotyping
2.2.1. Identification of Fusarium spp.
2.2.2. Trichothecene Genotyping
2.2.3. Qualitative Tri-5-Specific PCR Assay and HPLC Analysis
2.3. Statistical Methods
3. Results
3.1. Effectiveness of Species and Trichothecene-Specific Primers for the Characterization of the F. graminearum Population in Serbia
Trichothecene Genotypes of the Population and the Effectiveness of Primer Sets for Multilocus Genotyping
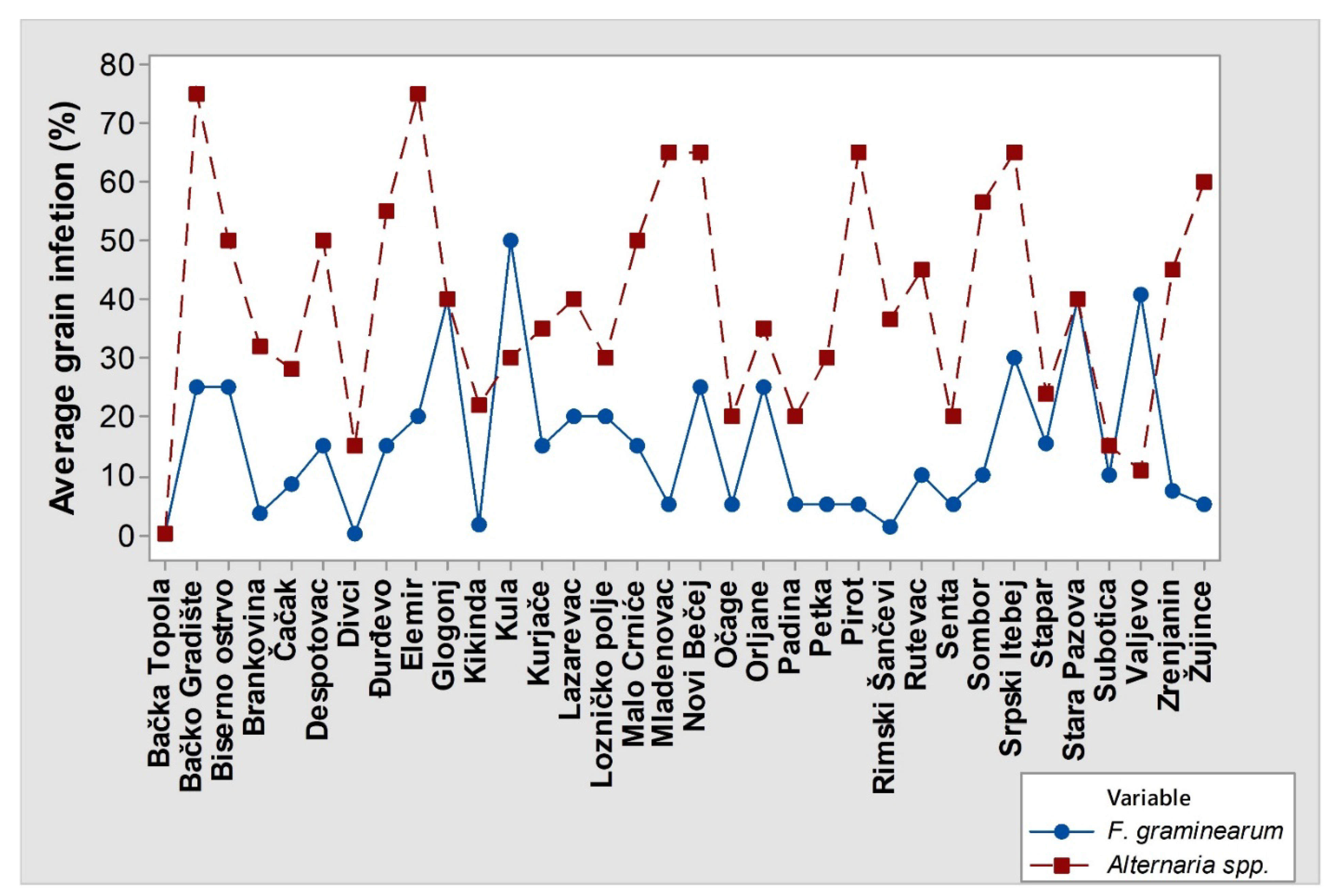
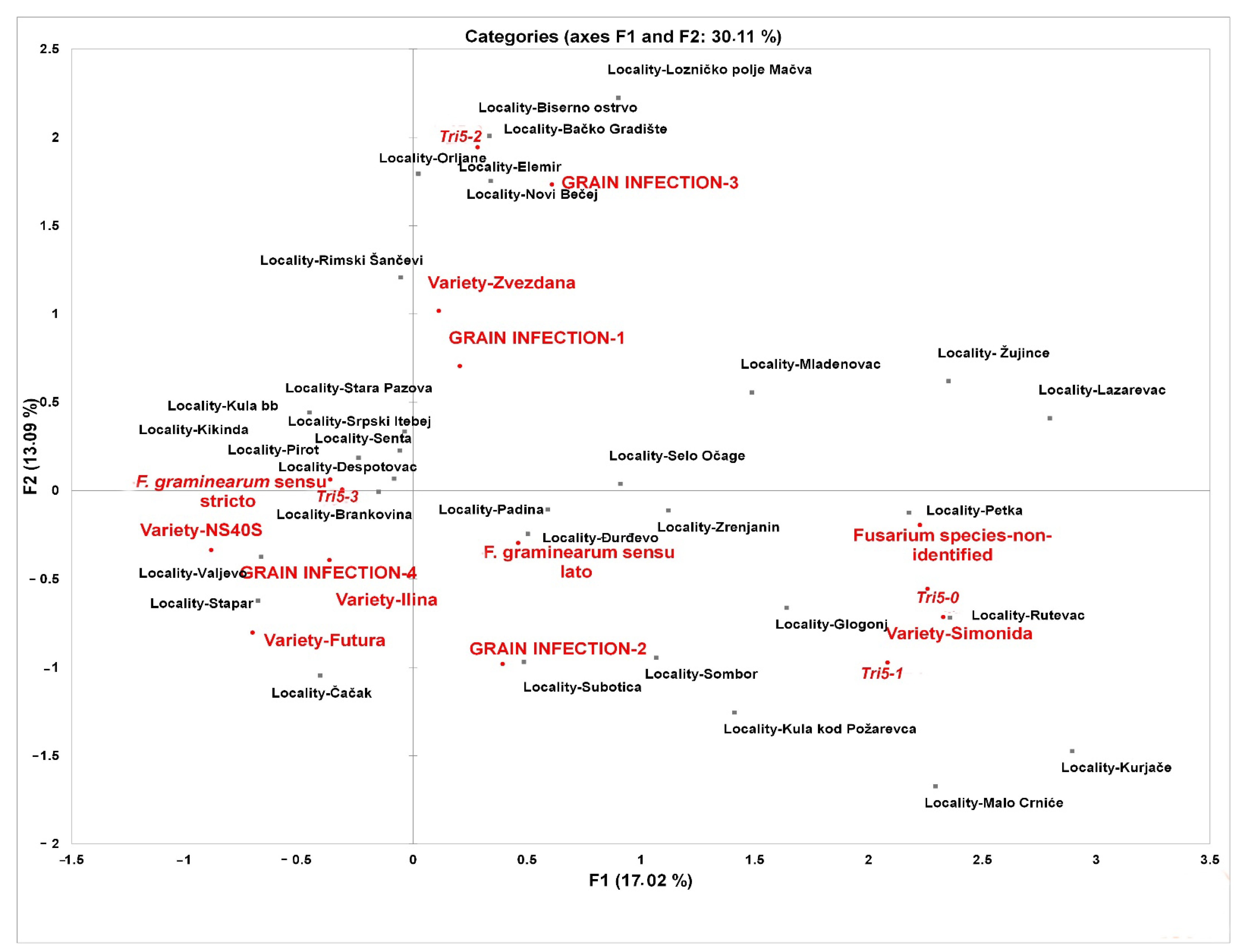
3.2. Geographic Distribution of FGSC Members and Their Association with Winter Wheat Grain Infection in Serbia
4. Discussion
4.1. Effectiveness of Species- and Trichothecene-Specific Primers for the Characterization of the F. graminearum Population in Serbia
4.2. Geographic Distribution of FGSC Members and Their Association with Winter Wheat Grain Infection in Serbia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.-L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European Database of Fusarium graminearum and F. culmorum Trichothecene Genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Aoki, T.; Ward, T.J.; Kistler, H.C.; O’Donnell, K. Systematics, Phylogeny and Trichothecene Mycotoxin Potential of Fusarium Head Blight Cereal Pathogens. Mycotoxins 2012, 62, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Van Der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, J. Population Analysis of the Fusarium graminearum Species Complex from Wheat in China Show a Shift to More Aggressive Isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, K.; Ward, T.J.; Geiser, D.M.; Kistler, H.C.; Aoki, T. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 2004, 41, 600–623. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, D. Global distribution of Fusarium graminearum, F. asiaticum and F. boothii from wheat in relation to climate. Eur. J. Plant Pathol. 2014, 139, 161–173. [Google Scholar] [CrossRef]
- De Kuppler, A.M.; Steiner, U.; Sulyok, M.; Krska, R.; Oerke, E.-C. Genotyping and phenotyping of Fusarium graminearum isolates from Germany related to their mycotoxin biosynthesis. Int. J. Food Microbiol. 2011, 151, 78–86. [Google Scholar] [CrossRef]
- Harris, L.J.; Desjardins, A.E.; Plattner, R.D.; Nicholson, P.; Butler, G.; Young, J.C.; Weston, G.; Proctor, R.H.; Hohn, T.M. Possible Role of Trichothecene Mycotoxins in Virulence of Fusarium graminearum on Maize. Plant Dis. 1999, 83, 954–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Jestoi, M.; Parikka, P.; Hietaniemi, V.; Gagkaeva, T.; Sarlin, T.; Haikara, A.; Laaksonen, S.; Rizzo, A. Real-time PCR detection and quantification of Fusarium poae, F. graminearum, F. sporotrichioides, and F. langsethiae in cereal grains in Finland and Russia. Arch. Phytopathol. Plant Prot. 2018, 41, 243–260. [Google Scholar] [CrossRef]
- Mesterházy, A.; Lehoczki-Krsjak, S.; Varga, M.; Szabó-Hevér, Á.; Tóth, B.; Lemmens, M. Breeding for FHB Resistance via Fusarium Damaged Kernels and Deoxynivalenol Accumulation as Well as Inoculation Methods in Winter Wheat. Agric. Sci. 2015, 6, 970–1002. [Google Scholar] [CrossRef] [Green Version]
- Villafana, R.T.; Ramdass, A.C.; Rampersad, S.N. TRI Genotyping and Chemotyping: A Balance of Power. Toxins 2020, 12, 64. [Google Scholar] [CrossRef] [Green Version]
- Krnjaja, V.; Stanković, S.; Obradović, A.; Petrović, T.; Mandić, V.; Bijelić, Z.; Božić, M. Trichothecene Genotypes of Fusarium graminearum Populations Isolated from Winter Wheat Crops in Serbia. Toxins 2018, 10, 460. [Google Scholar] [CrossRef] [Green Version]
- Župunski, V.; Jevtić, R.; Lalošević, M.; Orbović, B. Diversity of trichothecene genotypes of Fusarium graminearum sensu stricto from winter wheat in Serbia. Eur. J. Plant Pathol. 2019, 155, 461–473. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Kissoudis, C.; Van De Wiel, C.; Visser, R.G.F.; Van Der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S.; et al. Antagonistic Interaction between Systemic Acquired Resistance and the Abscisic Acid–Mediated Abiotic Stress Response in Arabidopsis. Plant Cell 2008, 20, 1678–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Župunski, V.; Jevtić, R.; Lalošević, M.; Jocković, B.; Župunski, L.; Skenderović, N. Effect of cultivation practices on diversity in susceptibility reactions of winter wheat genotypes to Fusarium head blight. Eur. J. Agron. 2021, 125, 126250. [Google Scholar] [CrossRef]
- ISTA (2018) International Rules for Seed Testing; The International Seed Testing Association: Bassersdorf, Switzerland, 2018; pp. 1–12. [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi. Mycologia 1972, 64, 930. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, P.; Simpson, D.; Weston, G.; Rezanoor, H.; Lees, A.; Parry, D.; Joyce, D. Detection and quantification ofFusarium culmorumandFusarium graminearumin cereals using PCR assays. Physiol. Mol. Plant Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Carter, J.; Rezanoor, H.; Holden, D.; Desjardins, A.; Plattner, R.; Nicholson, P. Variation in Pathogenicity Associated with the Genetic Diversity of Fusarium graminearum. Eur. J. Plant Pathol. 2002, 108, 573–583. [Google Scholar] [CrossRef]
- Chandler, E.A.; Simpson, D.R.; Thomsett, M.A.; Nicholson, P. Development of PCR assays to Tri7 and Tri13 trichothecene biosynthetic genes, and characterisation of chemotypes of Fusarium graminearum, Fusarium culmorum and Fusarium cerealis. Physiol. Mol. Plant Pathol. 2003, 62, 355–367. [Google Scholar] [CrossRef]
- Wang, C.-L.; Cheng, Y.-H. Identification and trichothecene genotypes of Fusarium graminearum species complex from wheat in Taiwan. Bot. Stud. 2017, 58, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, P.; Coates, M.E.; Walsh, K.; Turner, J.A.; Nicholson, P. Determination of deoxynivalenol- and nivalenol-producing chemotypes of Fusarium graminearum isolated from wheat crops in England and Wales. Plant Pathol. 2004, 53, 643–652. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Gagkaeva, T. Molecular chemotyping of Fusarium graminearum, F. culmorum, and F. cerealis isolates from Finland and Russia. In Molecular Identification of Fungi; Gherbawy, Y., Voigt, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 159–177. [Google Scholar]
- Amarasinghe, C.; Wang, J.-H.; Liao, Y.-C.; Fernando, W.D. Difference in TRI13 Gene Sequences between the 3-Acetyldeoxynivalenol Producing Fusarium graminearum Chemotypes from Canada and China. Int. J. Mol. Sci. 2011, 12, 6164–6175. [Google Scholar] [CrossRef] [Green Version]
- Demeke, T.; Clear, R.M.; Patrick, S.K.; Gaba, D. Species-specific PCR-based assays for the detection of Fusarium species and a comparison with the whole seed agar plate method and trichothecene analysis. Int. J. Food Microbiol. 2005, 103, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Vázquez, C.; Patiño, B.; González-Jaén, M.T. PCR detection assays for the trichothecene-producing species Fusarium graminearum, Fusarium culmorum, Fusarium poae, Fusarium equiseti and Fusarium sporotrichioides. Syst. Appl. Microbiol. 2005, 28, 562–568. [Google Scholar] [CrossRef]
- Tok, F.M.; Arslan, M. Distribution and genetic chemotyping ofFusarium graminearumandFusarium culmorumpopulations in wheat fields in the eastern Mediterranean region of Turkey. Biotechnol. Biotechnol. Equip. 2015, 30, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Sanoubar, R.; Bauer, A.; Seigner, L. Detection, Identification and Quantification of Fusarium graminearum and Fusarium culmorum in Wheat Kernels by PCR Techniques. J. Plant Pathol. Microbiol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Logrieco, A. Occurrence and Toxigenicity of Fusarium proliferation from Preharvest Maize Ear Rot, and Associated Mycotoxins, in Italy. Plant Dis. 1995, 79, 723–727. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Sour. Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Lenc, L.; Czecholiński, G.; Wyczling, D.; Turów, T.; Kaźmierczak, A. Fusarium head blight (FHB) and Fusarium spp. on grain of spring wheat cultivars grown in Poland. J. Plant Prot. Res. 2015, 55, 266–277. [Google Scholar] [CrossRef]
- Minitab 17 Statistical Software; Computer Software; Minitab Inc.: State College, PA, USA, 2010; Available online: www.minitab.com (accessed on 1 December 2020).
- XLSTAT. Available online: http://www.xlstat.com/ (accessed on 18 February 2013).
- Nielsen, L.K.; Jensen, J.D.; Nielsen, G.C.; Spliid, N.H.; Thomsen, I.K.; Justesen, A.F.; Collinge, D.B.; Jørgensen, L.N. Fusarium Head Blight of Cereals in Denmark: Species Complex and Related Mycotoxins. Phytopathology 2011, 101, 960–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and other selected Fusarium toxins in Swedish oats—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Parry, D.W.; Nicholson, P.; A Thomsett, M.; A Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blightin wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Burlakoti, R.; Tamburic-Ilincic, L.; Limay-Rios, V. Comparative population structure and trichothecene mycotoxin profiling of Fusarium graminearum from Corn and Wheat in Ontario, Central Canada. Plant Pathol. 2016, 66, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Waalwijk, C.; Kastelein, P.; De Vries, I.; Kerényi, Z.; Van Der Lee, T.; Hesselink, T.; Kohl, J.; Kema, G. Major Changes in Fusarium spp. in Wheat in the Netherlands. Eur. J. Plant Pathol. 2003, 109, 743–754. [Google Scholar] [CrossRef]
- Jurgenson, J.E.; Bowden, R.L.; Zeller, K.A.; Leslie, J.F.; Alexander, N.J.; Plattner, R.D. A genetic map of Gibberella zeae (Fusarium graminearum). Genetics 2002, 160, 1451–1460. [Google Scholar]
- Boutigny, A.-L.; Ward, T.J.; Van Coller, G.J.; Flett, B.; Lamprecht, S.C.; O’Donnell, K.; Viljoen, A. Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genet. Biol. 2011, 48, 914–920. [Google Scholar] [CrossRef]
- Lee, T.; Oh, D.-W.; Kim, H.-S.; Lee, J.; Kim, Y.-H.; Yun, S.-H.; Lee, Y.-W. Identification of Deoxynivalenol- and Nivalenol-Producing Chemotypes of Gibberella zeae by Using PCR. Appl. Environ. Microbiol. 2001, 67, 2966–2972. [Google Scholar] [CrossRef] [Green Version]
- Ward, T.J.; Bielawski, J.P.; Kistler, H.C.; Sullivan, E.; O’Donnell, K. Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc. Natl. Acad. Sci. USA 2002, 99, 9278–9283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, M.; Klix, M.B.; Verreet, J.-A. Estimating mycotoxin contents of Fusarium-damaged winter wheat kernels. Int. J. Food Microbiol. 2007, 119, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Cowger, C.; Arrellano, C. Plump Kernels with High Deoxynivalenol Linked to Late Gibberella zeae Infection and Marginal Disease Conditions in Winter Wheat. Phytopathology 2010, 100, 719–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Dreisigacker, S.; Singh, R.P.; Singh, P.K. Genetics for low correlation between Fusarium head blight disease and deoxynivalenol (DON) content in a bread wheat mapping population. Theor. Appl. Genet. 2019, 132, 2401–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Chang, I.-Y.; Kim, H.; Yun, S.-H.; Leslie, J.F.; Lee, Y.-W. Genetic Diversity and Fitness of Fusarium graminearum Populations from Rice in Korea. Appl. Environ. Microbiol. 2009, 75, 3289–3295. [Google Scholar] [CrossRef] [Green Version]
- Wegulo, S.N.; Bockus, W.W.; Nopsa, J.H.; De Wolf, E.D.; Eskridge, K.M.; Peiris, K.H.S.; Dowell, F.E. Effects of Integrating Cultivar Resistance and Fungicide Application on Fusarium Head Blight and Deoxynivalenol in Winter Wheat. Plant Dis. 2011, 95, 554–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Steier, I.; Köppen, R.; Siegel, D.; Proske, M.; Korn, U.; Koch, M. Cocultivation of phytopathogenic Fusarium and Alternaria strains affects fungal growth and mycotoxin production. J. Appl. Microbiol. 2012, 113, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, R.; Župunski, V.; Lalošević, M.; Živanov, S.T. Colonization of winter wheat grain with Fusarium and Alternaria species and influence on pest control management. J. Gen. Plant Pathol. 2019, 85, 273–281. [Google Scholar] [CrossRef]

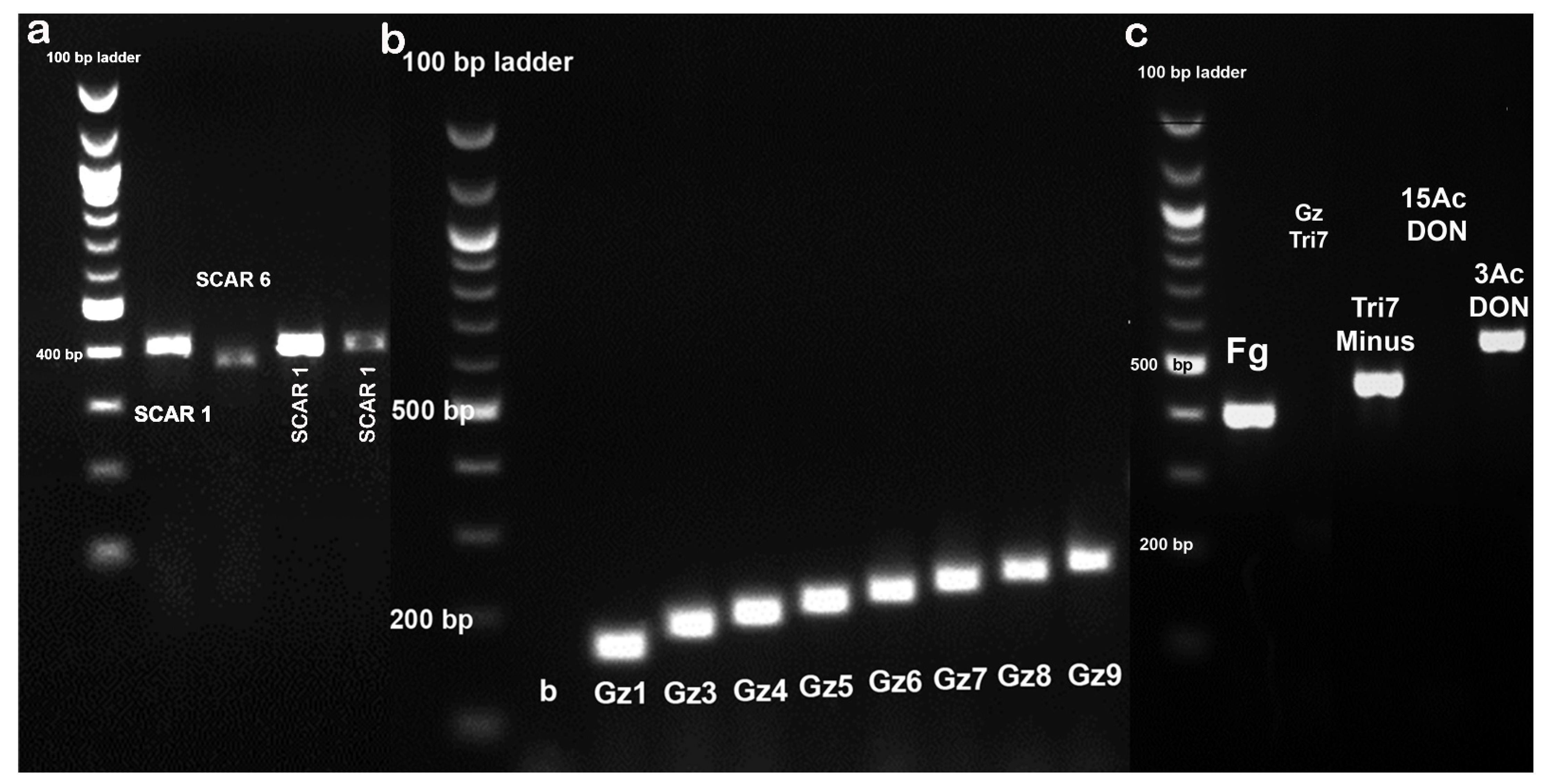

| Primer Set | Nucleotide Sequence (5′-3′) | Size of Amplified PCR Fragment (bp) | PCR Conditions |
|---|---|---|---|
| Fg16F Fg16R | CTCCGGATATGTTGCGTCAA GGTAGGTATCCGACATGGCAA | 420 (SCAR 1) 400 (SCAR 6) | Initial Denaturation: 95 °C, 3 min 38 Cycles: 95 °C, 30 s 62 °C, 20 s (Fg16F/R) 60 °C, 20 s (Fp82F/R) 72 °C, 45 s Final Extension: 72 °C, 5 min |
| Fp82F Fp82R | CAAGCAAACAGGCTCTTCACC TGTTCCACCTCAGTGACAGGTT | 220 | |
| Fgr-F Fgc-R | GTTGATGGGTAAAAGTGTG CTCTCATATACCCTCCG | 500 | Initial Denaturation: 94 °C, 85 s 25 Cycles: 95 °C, 35 s 53 °C, 30 s 72 °C, 30 s Final Extension: 72 °C, 5 min |
| Primer Set | Nucleotide Sequence (5′-3′) | Size of Amplified PCR Fragment (bp) | PCR Conditions |
|---|---|---|---|
| GzTri7f1/ GzTri7r1 | GGCTTTACGACTCCTCAACAATGG AGAGCCCTGCGAAAG(C/T)ACTGGTGC | 173–250 | Initial Denaturation: 94 °C, 2 min 30 Cycles: 94 °C, 30 s 60 °C, 1 min 72 °C, 2 min Final Extension: 72 °C, 10 min |
| Tri303F/ Tri303R | GATGGCCGCAAGTGGA GCCGGACTGCCCTATTG | 586 | |
| Tri315F/ Tri315R | CTCGCTGAAGTTGGACGTAA GTCTATGCTCTCAACGGACAAC | 864 | |
| Tri5-F Tri5-R | AGCGACTACAGGCTTCCCTC AAACCATCCAGTTCTCCATCTG | 544 | Initial Denaturation: 95 °C, 3 min 38 Cycles: 95 °C, 30 s 62 °C, 20 s 72 °C, 45 s Final Extension: 72 °C, 5 min |
| Minus Tri7F/ Minus Tri7R | TGGATGAATGACTTGAGTTGACA AAAGCCTTCATTCACAGCC | 483 | Initial Denaturation: 94 °C, 5 min 30 Cycles: 94 °C, 1 min 60 °C, 1 min 72 °C, 1 min Final Extension: 72 °C, 10 min |
| Tri7 | Tri5 | Tri3 | |||||
|---|---|---|---|---|---|---|---|
| FGSC | Number of Isolates | Genotype Type | GzTri7f1/r1 | MinusTri7f/r | Tri5F/R | Tri303F/R (3-AcDON) | Tri315F/R (15-AcDON) |
| F. graminearum s. stricto | 163 | 1 | + | - | + | - | + |
| 1 | 2 | - | + | + | + | - | |
| 3 | 3 | + | - | - | - | + | |
| F. graminearum s. lato | 10 | 1 | + | - | + | - | + |
| 3 | 3 | + | - | - | - | + | |
| 1 | 4 | - | - | - | - | + | |
| 4 | 5 | + | - | + | - | - | |
| 1 | 6 | + | - | - | - | - | |
| 1 | 7 | - | - | + | - | - | |
| Nonidentified | 12 | 1 | + | - | + | - | + |
| 5 | 3 | + | - | - | - | + | |
| 1 | 4 | - | - | - | - | + | |
| 3 | 5 | + | - | + | - | - | |
| 2 | 6 | + | - | - | - | - | |
| Effectiveness of primer pairs | 98.5% | 100% | 92.4% | 100% | 95.2% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Župunski, V.; Jevtić, R.; Lalošević, M.; Mikić, S.; Orbović, B. The Applicability of Species- and Trichothecene-Specific Primers in Monitoring the Fusarium graminearum Species Complex and Its Impact on the Surveillance of Fusarium Head Blight in Winter Wheat in Serbia. Agronomy 2021, 11, 778. https://doi.org/10.3390/agronomy11040778
Župunski V, Jevtić R, Lalošević M, Mikić S, Orbović B. The Applicability of Species- and Trichothecene-Specific Primers in Monitoring the Fusarium graminearum Species Complex and Its Impact on the Surveillance of Fusarium Head Blight in Winter Wheat in Serbia. Agronomy. 2021; 11(4):778. https://doi.org/10.3390/agronomy11040778
Chicago/Turabian StyleŽupunski, Vesna, Radivoje Jevtić, Mirjana Lalošević, Sanja Mikić, and Branka Orbović. 2021. "The Applicability of Species- and Trichothecene-Specific Primers in Monitoring the Fusarium graminearum Species Complex and Its Impact on the Surveillance of Fusarium Head Blight in Winter Wheat in Serbia" Agronomy 11, no. 4: 778. https://doi.org/10.3390/agronomy11040778