Multi-Sensor Approach for Tropical Soil Fertility Analysis: Comparison of Individual and Combined Performance of VNIR, XRF, and LIBS Spectroscopies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Reference Analysis
2.2. Sample Preparation
2.3. VNIR Measurements and Spectra Pre-Processing
2.4. XRF Measurements and Spectra Pre-Processing
2.5. LIBS Measurements and Spectra Pre-Processing
2.6. Modeling
2.6.1. Single-Sensor Predictive Models
2.6.2. Data Fusion Approaches
3. Results
3.1. Characterisation of the Laboratory Measured Soil Properties
3.2. Prediction Performances of Single-Sensor and Data Fusion Approaches
4. Discussion
4.1. Individual Performance of VNIR, XRF, and LIBS Sensors: Coverage of Key Soil Fertility Attributes
4.2. Combined Performance of VNIR, XRF, and LIBS Sensors: Synergy for Predicting Key Soil Fertility Attributes
4.3. Operational Aspects Related to Sample Preparation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Attribute | Number of Intervals Selected | Spectral Ranges of the Selected Intervals (nm) |
|---|---|---|
| Clay | 3 | 220.84–222.21, 246.77–248.29, and 251.37–252.94 |
| OM ¹ | 1 | 295.08–296.92 |
| CEC ² | 52 | 201.31–272.37, 287.89–298.73, and 414.15–416.63 |
| pH | 3 | 220.84–222.21, 237.82–239.27, and 391.78–394.22 |
| V ³ | 57 | 201.31–272.37, 279.18–300.60, and 396.59–399.10 |
| ex-P 4 | 1 | 214.14–215.44 |
| ex-K 4 | 1 | 375.28–377.53 |
| ex-Ca 4 | 1 | 389.37–391.77 |
| ex-Mg 4 | 1 | 284.36–286.14 |
| Clay | OM ¹ | CEC ² | pH | V ³ | ex-P 4 | ex-K 4 | ex-Ca 4 | ex-Mg 4 | |
|---|---|---|---|---|---|---|---|---|---|
| R² | |||||||||
| VNIR | 0.93 | 0.86 | 0.51 | 0.19 | 0.80 | 0.07 | 0.74 | 0.68 | 0.52 |
| XRF | 0.92 | 0.74 | 0.88 | 0.34 | 0.95 | 0.01 | 0.95 | 0.96 | 0.89 |
| LIBS | 0.89 | 0.81 | 0.84 | 0.31 | 0.94 | 0.72 | 0.76 | 0.94 | 0.93 |
| VNIR + XRF – SF | 0.94 | 0.83 | 0.77 | 0.36 | 0.93 | 0.14 | 0.91 | 0.89 | 0.82 |
| VNIR + XRF – GR | 0.94 | 0.83 | 0.77 | 0.39 | 0.95 | 0.13 | 0.96 | 0.94 | 0.86 |
| VNIR + LIBS – SF | 0.95 | 0.84 | 0.85 | 0.45 | 0.93 | 0.32 | 0.75 | 0.93 | 0.89 |
| VNIR + LIBS – GR | 0.95 | 0.84 | 0.84 | 0.45 | 0.92 | 0.59 | 0.77 | 0.94 | 0.92 |
| XRF + LIBS – SF | 0.90 | 0.75 | 0.85 | 0.24 | 0.93 | 0.61 | 0.94 | 0.96 | 0.94 |
| XRF + LIBS – GR | 0.92 | 0.86 | 0.82 | 0.35 | 0.93 | 0.55 | 0.96 | 0.96 | 0.94 |
| VNIR + XRF + LIBS – SF | 0.95 | 0.84 | 0.85 | 0.46 | 0.94 | 0.31 | 0.83 | 0.94 | 0.92 |
| VNIR + XRF + LIBS – GR | 0.95 | 0.85 | 0.86 | 0.47 | 0.93 | 0.57 | 0.96 | 0.96 | 0.94 |
| RMSE | |||||||||
| vis-NIR | 27.32 | 2.10 | 18.66 | 0.34 | 10.38 | 12.05 | 1.20 | 10.98 | 8.85 |
| XRF | 29.40 | 3.01 | 10.19 | 0.33 | 5.60 | 13.27 | 0.53 | 4.09 | 4.28 |
| LIBS | 30.03 | 2.56 | 10.69 | 0.33 | 5.49 | 5.84 | 1.10 | 4.85 | 3.51 |
| VNIR + XRF – SF | 26.19 | 2.29 | 13.35 | 0.30 | 6.38 | 11.81 | 0.73 | 6.97 | 5.51 |
| VNIR + XRF – GR | 26.05 | 2.33 | 13.45 | 0.29 | 5.52 | 11.91 | 0.45 | 5.06 | 4.82 |
| VNIR + LIBS – SF | 23.57 | 2.30 | 10.26 | 0.28 | 6.34 | 9.97 | 1.16 | 5.32 | 4.48 |
| VNIR + LIBS – GR | 23.18 | 2.25 | 10.49 | 0.28 | 6.42 | 7.20 | 1.09 | 5.07 | 3.90 |
| XRF + LIBS – SF | 30.35 | 2.98 | 10.17 | 0.35 | 6.34 | 6.90 | 0.60 | 3.84 | 3.25 |
| XRF + LIBS – GR | 28.19 | 2.30 | 10.89 | 0.32 | 6.56 | 7.39 | 0.50 | 3.72 | 3.12 |
| VNIR + XRF + LIBS – SF | 22.88 | 2.27 | 10.04 | 0.28 | 6.04 | 10.17 | 0.93 | 4.96 | 3.70 |
| VNIR + XRF + LIBS – GR | 24.68 | 2.25 | 9.95 | 0.28 | 6.22 | 7.47 | 0.47 | 3.98 | 3.34 |
| VNIR | XRF | LIBS | VNIR + XRF | VNIR + LIBS | XRF + LIBS | VNIR + XRF + LIBS | |
|---|---|---|---|---|---|---|---|
| Clay | Excel. | Excel. | Excel. | Excel. | Excel. | Excel. | Excel. |
| OM ¹ | Good | Reason. | Good | Good | Good | Good | Good |
| CEC ² | Reason. | Good | Good | Reason. | Good | Good | Good |
| pH | Poor | Poor | Poor | Poor | Poor | Poor | Poor |
| V ³ | Good | Excel. | Excel. | Excel. | Excel. | Excel. | Excel. |
| ex-P 4 | Poor | Poor | Reason. | Poor | Reason. | Reason. | Reason. |
| ex-K 4 | Reason. | Excel. | Good | Excel. | Good | Excel. | Excel. |
| ex-Ca 4 | Reason. | Excel. | Excel. | Excel. | Excel. | Excel. | Excel. |
| ex-Mg 4 | Reason. | Good | Excel. | Good | Excel. | Excel. | Excel. |
References
- Gredilla, A.; de Vallejuelo, S.F.O.; Elejoste, N.; de Diego, A.; Madariaga, J.M. Non-destructive Spectroscopy combined with chemometrics as a tool for Green Chemical Analysis of environmental samples: A review. TrAC Trends Anal. Chem. 2016, 76, 30–39. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Dotto, A.C.; Bedin, L.G.; Sayão, V.M.; e Souza, A.B. Soil analytical quality control by traditional and spectroscopy techniques: Constructing the future of a hybrid laboratory for low environmental impact. Geoderma 2019, 337, 111–121. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Bouma, J. Soil sensing: A new paradigm for agriculture. Agric. Syst. 2016, 148, 71–74. [Google Scholar] [CrossRef]
- Molin, J.P.; Tavares, T.R. Sensor systems for mapping soil fertility attributes: Challenges, advances, and perspectives in brazilian tropical soils. Eng. Agric. 2019, 39, 126–147. [Google Scholar] [CrossRef] [Green Version]
- Nawar, S.; Corstanje, R.; Halcro, G.; Mulla, D.; Mouazen, A.M. Delineation of Soil Management Zones for Variable-Rate Fertilization: A Review, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 143. [Google Scholar] [CrossRef]
- Rossiter, D.G. Past, present & future of information technology in pedometrics. Geoderma 2018, 324, 131–137. [Google Scholar] [CrossRef]
- Kuang, B.; Mahmood, H.S.; Quraishi, M.Z.; Hoogmoed, W.B.; Mouazen, A.M.; van Henten, E.J. Sensing Soil Properties in the Laboratory, In Situ, and On-Line. Adv. Agron. 2012, 114, 155–223. [Google Scholar] [CrossRef]
- Mahmood, H.S.; Hoogmoed, W.B.; van Henten, E.J. Sensor data fusion to predict multiple soil properties. Precis. Agric. 2012, 13, 628–645. [Google Scholar] [CrossRef]
- Tavares, T.R.; Molin, J.P.; Hamed Javadi, S.; de Carvalho, H.W.P.; Mouazen, A.M. Combined use of vis-nir and xrf sensors for tropical soil fertility analysis: Assessing different data fusion approaches. Sensors 2021, 21, 148. [Google Scholar] [CrossRef]
- Xu, X.; Du, C.; Ma, F.; Shen, Y.; Wu, K.; Liang, D.; Zhou, J. Detection of soil organic matter from laser-induced breakdown spectroscopy (LIBS) and mid-infrared spectroscopy (FTIR-ATR) coupled with multivariate techniques. Geoderma 2019, 355. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, R.; Li, S.; Chen, S.; Jiang, Q.; Zhou, L.; Shi, Z. Multi-sensor fusion for the determination of several soil properties in the Yangtze River Delta, China. Eur. J. Soil Sci. 2019, 70, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. Adv. Agron. 2010, 107. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Krug, F.J.; Rocha, F.R.P. Métodos de Preparo de Amostras Para Análise Elementar; Sociedade Brasileira de Quimica: São Paulo, Brazil, 2016. [Google Scholar]
- Castanedo, F. A Review of Data Fusion Techniques. Sci. World J. 2013, 2013, 704504. [Google Scholar] [CrossRef] [PubMed]
- Abdul Munnaf, M.; Nawar, S.; Mouazen, A.M. Estimation of Secondary Soil Properties by Fusion of Laboratory and On-Line Measured Vis–NIR Spectra. Remote Sens. 2019, 11, 2819. [Google Scholar] [CrossRef] [Green Version]
- Romero, D.J.; Ben-Dor, E.; Demattê, J.A.M.; Souza, A.B.E.; Vicente, L.E.; Tavares, T.R.; Martello, M.; Strabeli, T.F.; da Silva Barros, P.P.; Fiorio, P.R.; et al. Internal soil standard method for the Brazilian soil spectral library: Performance and proximate analysis. Geoderma 2018, 312. [Google Scholar] [CrossRef]
- Tavares, T.R.; Molin, J.P.; Nunes, L.C.; Alves, E.E.N.; Melquiades, F.L.; de Carvalho, H.W.P.; Mouazen, A.M. Effect of x-ray tube configuration on measurement of key soil fertility attributes with XRF. Remote Sens. 2020, 12, 963. [Google Scholar] [CrossRef] [Green Version]
- Andrade, R.; Faria, W.M.; Silva, S.H.G.; Chakraborty, S.; Weindorf, D.C.; Mesquita, L.F.; Guilherme, L.R.G.; Curi, N. Prediction of soil fertility via portable X-ray fluorescence (pXRF) spectrometry and soil texture in the Brazilian Coastal Plains. Geoderma 2020, 357, 113960. [Google Scholar] [CrossRef]
- Riebe, D.; Erler, A.; Brinkmann, P.; Beitz, T.; Löhmannsröben, H.G.; Gebbers, R. Comparison of calibration approaches in laser-induced breakdown spectroscopy for proximal soil sensing in precision agriculture. Sensors 2019, 19, 5244. [Google Scholar] [CrossRef] [Green Version]
- Erler, A.; Riebe, D.; Beitz, T.; Löhmannsröben, H.-G.; Gebbers, R. Soil Nutrient Detection for Precision Agriculture Using Handheld Laser-Induced Breakdown Spectroscopy (LIBS) and Multivariate Regression Methods (PLSR, Lasso and GPR). Sensors 2020, 20, 418. [Google Scholar] [CrossRef] [Green Version]
- Wan, M.; Hu, W.; Qu, M.; Li, W.; Zhang, C.; Kang, J.; Hong, Y.; Chen, Y.; Huang, B. Rapid estimation of soil cation exchange capacity through sensor data fusion of portable XRF spectrometry and Vis-NIR spectroscopy. Geoderma 2020, 363, 114163. [Google Scholar] [CrossRef]
- Javadi, S.H.; Munnaf, M.A.; Mouazen, A.M. Fusion of Vis-NIR and XRF spectra for estimation of key soil attributes. Geoderma 2021, 385, 114851. [Google Scholar] [CrossRef]
- Zhang, Y.; Hartemink, A.E. Data fusion of vis–NIR and PXRF spectra to predict soil physical and chemical properties. Eur. J. Soil Sci. 2020, 71, 316–333. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Schad, P., van Huyssteen, C., Micheli, E., Eds.; FAO: Rome, Italy, 2015; ISBN 978-92-5-108369-7. [Google Scholar]
- EMBRAPA Solos. Brazilian Soil Classification System, 5th ed.; EMBRAPA: Brasilia, Brazil, 2018. [Google Scholar]
- Van Raij, B.; Andrade, J.C.; Cantarela, H.; Quaggio, J.A. Análise Química Para Avaliação de Solos Tropicais; IAC: Campinas, Brazil, 2001. [Google Scholar]
- Knadel, M.; Stenberg, B.; Deng, F.; Thomsen, A.; Greve, M.H. Comparing Predictive Abilities of Three Visible-Near Infrared Spectrophotometers for Soil Organic Carbon and Clay Determination. J. Near Infrared Spectrosc. 2013, 21, 67–80. [Google Scholar] [CrossRef]
- Debaene, G.; Niedźwiecki, J.; Pecio, A.; Żurek, A. Effect of the number of calibration samples on the prediction of several soil properties at the farm-scale. Geoderma 2014, 214-215, 114–125. [Google Scholar] [CrossRef]
- Tavares, T.R.; Nunes, L.C.; Alves, E.E.N.; de Almeida, E.; Maldaner, L.F.; Krug, F.J.; de Carvalho, H.W.P.; Molin, J.P. Simplifying sample preparation for soil fertility analysis by x-ray fluorescence spectrometry. Sensors 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouazen, A.M.; Maleki, M.R.; Cockx, L.; Van Meirvenne, M.; Van Holm, L.H.J.; Merckx, R.; De Baerdemaeker, J.; Ramon, H. Optimum three-point linkage set up for improving the quality of soil spectra and the accuracy of soil phosphorus measured using an on-line visible and near infrared sensor. Soil Tillage Res. 2009, 103, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Karoui, R.; De Baerdemaeker, J.; Ramon, H. Characterization of Soil Water Content Using Measured Visible and Near Infrared Spectra. Soil Sci. Soc. Am. J. 2006, 70, 1295–1302. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Inbar, Y.; Chen, Y. The reflectance spectra of organic matter in the visible near-infrared and short wave infrared region (400–2500 nm) during a controlled decomposition process. Remote Sens. Environ. 1997, 61, 1–15. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Nawar, S.; Mouazen, A.M. Predictive performance of mobile vis-near infrared spectroscopy for key soil properties at different geographical scales by using spiking and data mining techniques. Catena 2017, 151, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Tavares, T.R.; Mouazen, A.M.; Alves, E.E.N.; Dos Santos, F.R.; Melquiades, F.L.; De Carvalho, H.W.P.; Molin, J.P. Assessing soil key fertility attributes using a portable X-ray fluorescence: A simple method to overcome matrix effect. Agronomy 2020, 10, 787. [Google Scholar] [CrossRef]
- Nunes, L.C.; Rocha, F.R.P.; Krug, F.J. Slope ratio calibration for analysis of plant leaves by laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2019, 34, 2314–2324. [Google Scholar] [CrossRef]
- Nunes, L.C.; da Silva, G.A.; Trevizan, L.C.; Santos Júnior, D.; Poppi, R.J.; Krug, F.J. Simultaneous optimization by neuro-genetic approach for analysis of plant materials by laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 565–572. [Google Scholar] [CrossRef]
- Yu, K.Q.; Zhao, Y.R.; Liu, F.; He, Y. Laser-Induced Breakdown Spectroscopy Coupled with Multivariate Chemometrics for Variety Discrimination of Soil. Sci. Rep. 2016, 6, 27574. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.D.A.; Galvão, R.K.H.; De Araújo, M.C.U.; Véras, G.; Da Silva, E.C. The successive projections algorithm for interval selection in PLS. Microchem. J. 2013, 110, 202–208. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-Infrared Reflectance Spectroscopy–Principal Components Regression Analyses of Soil Properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, S.M.; Stockmann, U.; Holden, N.M.; McBratney, A.B.; Minasny, B. An assessment of model averaging to improve predictive power of portable vis-NIR and XRF for the determination of agronomic soil properties. Geoderma 2016, 279, 31–44. [Google Scholar] [CrossRef]
- Cardelli, V.; Weindorf, D.C.; Chakraborty, S.; Li, B.; De Feudis, M.; Cocco, S.; Agnelli, A.; Choudhury, A.; Ray, D.P.; Corti, G. Non-saturated soil organic horizon characterization via advanced proximal sensors. Geoderma 2017, 288, 130–142. [Google Scholar] [CrossRef]
- Granger, C.W.J.; Ramanathan, R. Improved methods of combining forecasts. J. Forecast. 1984, 3, 197–204. [Google Scholar] [CrossRef]
- Nawar, S.; Mouazen, A.M. Optimal sample selection for measurement of soil organic carbon using on-line vis-NIR spectroscopy. Comput. Electron. Agric. 2018, 151, 469–477. [Google Scholar] [CrossRef]
- Ben-Dor, E. Quantitative remote sensing of soil properties. Adv. Agron. 2002, 75, 173–243. [Google Scholar] [CrossRef]
- Fontes, M.P.F. Intemperismo de rochas e minerais. In Pedologia: Fundamentos; Ker, J.C., Curi, N., Schaefer, C.E.G.R., Vidal-Torrado, P., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2012; pp. 171–205. [Google Scholar]
- Schäefer, C.E.G.R.; Fabris, J.D.; Ker, J.C. Minerals in the clay fraction of Brazilian Latosols (Oxisols): A review. Clay Miner. 2008, 43, 137–154. [Google Scholar] [CrossRef]
- Coutinho, M.A.N.; Alari, F.d.O.; Ferreira, M.M.C.; do Amaral, L.R. Influence of soil sample preparation on the quantification of NPK content via spectroscopy. Geoderma 2019, 338, 401–409. [Google Scholar] [CrossRef]
- Lacerda, M.P.C.; Demattê, J.A.M.; Sato, M.V.; Fongaro, C.T.; Gallo, B.C.; Souza, A.B. Tropical Texture Determination by Proximal Sensing Using a Regional Spectral Library and Its Relationship with Soil Classification. Remote Sens. 2016, 8, 701. [Google Scholar] [CrossRef] [Green Version]
- Demattê, J.A.M.; Ramirez-Lopez, L.; Marques, K.P.P.; Rodella, A.A. Chemometric soil analysis on the determination of specific bands for the detection of magnesium and potassium by spectroscopy. Geoderma 2017, 288, 8–22. [Google Scholar] [CrossRef]
- Demattê, J.A.M.; Alves, M.R.; Gallo, B.C.; Fongaro, C.T.; Romero, D.J.; Sato, M.V. Hyperspectral remote sensing as an alternative to estimate soil attributes. Rev. Ciênc. Agron. 2015, 46, 223–232. [Google Scholar] [CrossRef]
- Silva, E.A.; Weindorf, D.C.; Silva, S.H.G.; Ribeiro, B.T.; Poggere, G.C.; Carvalho, T.S.; Gonçalves, M.G.M.; Guilherme, L.R.G.; Curi, N. Advances in Tropical Soil Characterization via Portable X-Ray Fluorescence Spectrometry. Pedosphere 2019, 29, 468–482. [Google Scholar] [CrossRef]
- Silva, S.H.G.; Teixeira, A.F.d.S.; de Menezes, M.D.; Guilherme, L.R.G.; Moreira, F.M.d.S.; Curi, N.; Silva, S.H.G.; Teixeira, A.F.d.S.; de Menezes, M.D.; Guilherme, L.R.G.; et al. Multiple linear regression and random forest to predict and map soil properties using data from portable X-ray fluorescence spectrometer (pXRF). Ciênc. Agrotecnol. 2017, 41, 648–664. [Google Scholar] [CrossRef]
- Santos, F.R.; de Oliveira, J.F.; Bona, E.; dos Santos, J.V.F.; Barboza, G.M.C.; Melquiades, F.L. EDXRF spectral data combined with PLSR to determine some soil fertility indicators. Microchem. J. 2020, 152, 104275. [Google Scholar] [CrossRef]
- de Lima, T.M.; Weindorf, D.C.; Curi, N.; Guilherme, L.R.G.G.; Lana, R.M.Q.Q.; Ribeiro, B.T. Elemental analysis of Cerrado agricultural soils via portable X-ray fluorescence spectrometry: Inferences for soil fertility assessment. Geoderma 2019, 353, 264–272. [Google Scholar] [CrossRef]
- Villas-Boas, P.R.; Romano, R.A.; de Menezes Franco, M.A.; Ferreira, E.C.; Ferreira, E.J.; Crestana, S.; Milori, D.M.B.P. Laser-induced breakdown spectroscopy to determine soil texture: A fast analytical technique. Geoderma 2016, 263, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.C.; Gomes Neto, J.A.; Milori, D.M.B.P.; Ferreira, E.J.; Anzano, J.M. Laser-induced breakdown spectroscopy: Extending its application to soil pH measurements. Spectrochim. Acta Part B At. Spectrosc. 2015, 110, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Jantzi, S.C.; Motto-Ros, V.; Trichard, F.; Markushin, Y.; Melikechi, N.; De Giacomo, A. Sample treatment and preparation for laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 115, 52–63. [Google Scholar] [CrossRef]
- Knadel, M.; Gislum, R.; Hermansen, C.; Peng, Y.; Moldrup, P.; de Jonge, L.W.; Greve, M.H. Comparing predictive ability of laser-induced breakdown spectroscopy to visible near-infrared spectroscopy for soil property determination. Biosyst. Eng. 2017, 156, 157–172. [Google Scholar] [CrossRef]
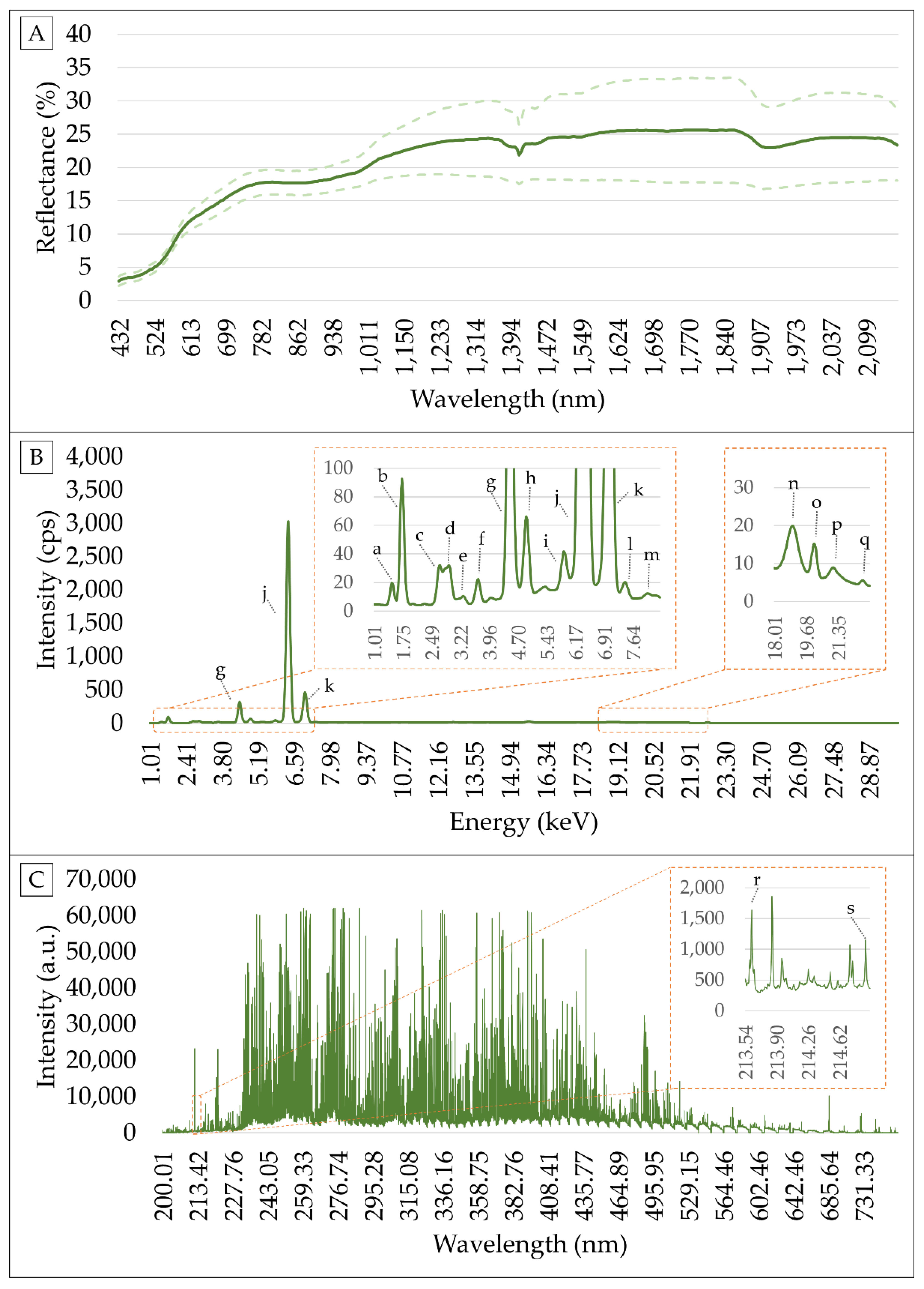

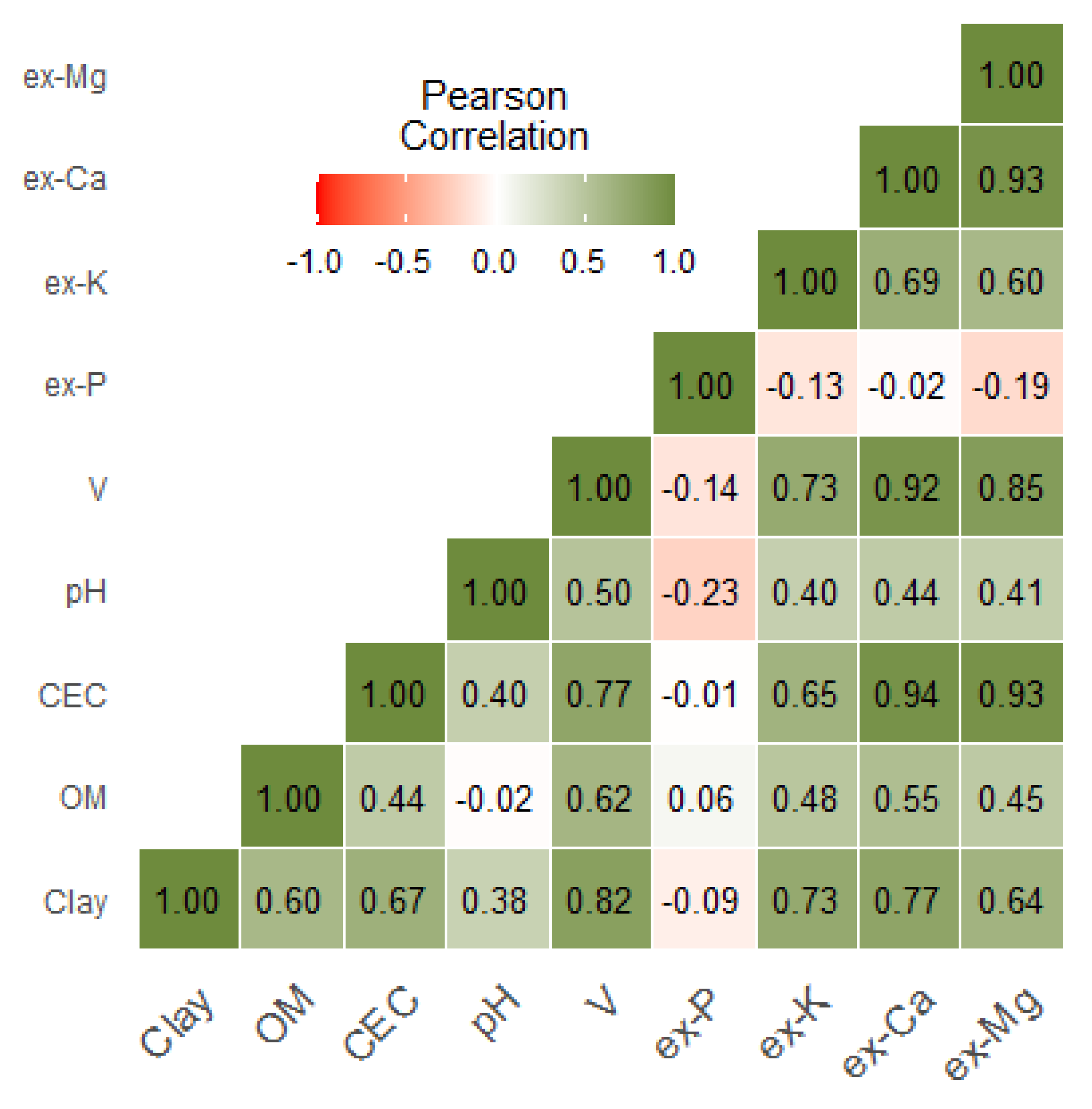
| Clay | OM | CEC | pH | V | ex-P | ex-K | ex-Ca | ex-Mg | |
|---|---|---|---|---|---|---|---|---|---|
| VNIR | 3.37 | 2.61 | 1.40 | 1.10 | 2.26 | 0.88 | 1.89 | 1.79 | 1.45 |
| XRF | 3.13 | 1.82 | 2.57 | 1.11 | 4.18 | 0.80 | 4.26 | 4.82 | 2.99 |
| LIBS | 3.06 | 2.14 | 2.45 | 1.12 | 4.27 | 1.82 | 2.05 | 4.07 | 3.65 |
| VNIR + XRF—SF | 3.51 | 2.39 | 1.96 | 1.22 | 3.67 | 0.90 | 3.09 | 2.83 | 2.33 |
| VNIR + XRF–GR | 3.53 | 2.35 | 1.94 | 1.26 | 4.24 | 0.89 | 4.98 | 3.90 | 2.66 |
| VNIR + LIBS–SF | 3.90 | 2.38 | 2.55 | 1.31 | 3.69 | 1.07 | 1.96 | 3.71 | 2.86 |
| VNIR + LIBS–GR | 3.97 | 2.43 | 2.49 | 1.31 | 3.65 | 1.48 | 2.08 | 3.89 | 3.28 |
| XRF + LIBS–SF | 3.03 | 1.84 | 2.57 | 1.05 | 3.69 | 1.54 | 3.78 | 5.14 | 3.94 |
| XRF + LIBS–GR | 3.26 | 2.38 | 2.40 | 1.14 | 3.57 | 1.44 | 4.52 | 5.30 | 4.11 |
| VNIR + XRF + LIBS–SF | 4.02 | 2.42 | 2.60 | 1.31 | 3.88 | 1.05 | 2.42 | 3.97 | 3.47 |
| VNIR + XRF + LIBS–GR | 3.73 | 2.44 | 2.63 | 1.32 | 3.77 | 1.43 | 4.83 | 4.96 | 3.83 |
| Clay | OM 1 | CEC 2 | pH | V 3 | ex-P 4 | ex-K 4 | ex-Ca 4 | ex-Mg 4 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Best single-sensor approach | RMSE | 27.32 | 2.10 | 10.19 | 0.33 | 5.49 | 5.84 | 0.53 | 4.09 | 4.28 | |
| Tech 5 | VNIR | VNIR | XRF | LIBS | LIBS | LIBS | XRF | XRF | LIBS | ||
| VNIR + XRF | SF | RMSE | 26.19 | 2.29 | 13.35 | 0.30 | 6.38 | 11.81 | 0.73 | 6.97 | 5.51 |
| RI (%) | 4.1 | −9.2 | −31.1 | 8.6 | −16.2 | −102.3 | −37.6 | −70.2 | −28.5 | ||
| GR | RMSE | 26.05 | 2.33 | 13.45 | 0.29 | 5.52 | 11.91 | 0.45 | 5.06 | 4.82 | |
| RI (%) | 4.7 | −10.7 | −32.0 | 11.2 | −0.7 | −104.1 | 14.4 | −23.5 | −12.4 | ||
| VNIR + LIBS | SF | RMSE | 23.57 | 2.30 | 10.26 | 0.28 | 6.34 | 9.97 | 1.16 | 5.32 | 4.48 |
| RI (%) | 13.7 | −9.5 | −0.7 | 14.5 | −15.6 | −70.7 | −117.4 | −30.0 | −4.5 | ||
| GR | RMSE | 23.18 | 2.25 | 10.49 | 0.28 | 6.42 | 7.20 | 1.09 | 5.07 | 3.90 | |
| RI (%) | 15.2 | −7.1 | −2.9 | 14.5 | −16.9 | −23.3 | −104.5 | −23.9 | 8.9 | ||
| XRF + LIBS | SF | RMSE | 30.35 | 2.98 | 10.17 | 0.35 | 6.34 | 6.90 | 0.60 | 3.84 | 3.25 |
| RI (%) | −11.1 | −41.8 | 0.2 | −6.5 | −15.6 | −18.2 | −12.6 | 6.2 | 24.2 | ||
| GR | RMSE | 28.19 | 2.30 | 10.89 | 0.32 | 6.56 | 7.39 | 0.50 | 3.72 | 3.12 | |
| RI (%) | −3.2 | −9.3 | −6.8 | 1.9 | −19.5 | −26.6 | 5.8 | 9.2 | 27.2 | ||
| VNIR + XRF + LIBS | SF | RMSE | 22.88 | 2.27 | 10.04 | 0.28 | 6.04 | 10.17 | 0.93 | 4.96 | 3.70 |
| RI (%) | 16.3 | −7.8 | 1.4 | 14.8 | −10.0 | −74.2 | −75.7 | −21.2 | 13.7 | ||
| GR | RMSE | 24.68 | 2.25 | 9.95 | 0.28 | 6.22 | 7.47 | 0.47 | 3.98 | 3.34 | |
| RI (%) | 9.7 | −6.9 | 2.3 | 15.3 | −13.3 | −28.0 | 11.9 | 2.9 | 22.0 | ||
| Best Single-Sensor Approach | Best Two-Sensor Approach | Three-Sensor Approach | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RMSE | Tech 5 | RMSE | Tech 5 | DF 6 | RI Comp. Single-Sensor 7 | RMSE | DF 6 | RI Comp. Single-Sensor 7 | RI Comp. Two-Sensor 8 | |
| Clay | 27.32 | VNIR 9 | 23.18 | VNIR + LIBS | GR 12 | 15.2 | 22.88 | SF 13 | 16.3 | 1.3 |
| OM 1 | 2.10 | VNIR | 2.33 | VNIR + XRF | GR | −10.7 | 2.25 | GR | −6.9 | 3.5 |
| CEC 2 | 10.19 | XRF 10 | 10.17 | XRF + LIBS | SF | 0.2 | 9.95 | GR | 2.3 | 2.2 |
| pH | 0.33 | LIBS 11 | 0.28 | VNIR + LIBS | GR | 14.5 | 0.28 | GR | 15.3 | 0.9 |
| V 3 | 5.49 | LIBS | 5.52 | VNIR + XRF | GR | −0.7 | 6.04 | SF | −10.0 | −8.5 |
| ex-P 4 | 5.84 | LIBS | 7.20 | VNIR + LIBS | GR | −23.3 | 7.47 | GR | −28.0 | −3.6 |
| ex-K 4 | 0.53 | XRF | 0.45 | VNIR + XRF | GR | 14.4 | 0.47 | GR | 11.9 | −2.9 |
| ex-Ca 4 | 4.09 | XRF | 3.72 | XRF + LIBS | GR | 9.2 | 3.98 | GR | 2.9 | −6.5 |
| ex-Mg 4 | 4.28 | LIBS | 3.12 | XRF + LIBS | GR | 27.2 | 3.34 | GR | 22.0 | −6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, T.R.; Molin, J.P.; Nunes, L.C.; Wei, M.C.F.; Krug, F.J.; de Carvalho, H.W.P.; Mouazen, A.M. Multi-Sensor Approach for Tropical Soil Fertility Analysis: Comparison of Individual and Combined Performance of VNIR, XRF, and LIBS Spectroscopies. Agronomy 2021, 11, 1028. https://doi.org/10.3390/agronomy11061028
Tavares TR, Molin JP, Nunes LC, Wei MCF, Krug FJ, de Carvalho HWP, Mouazen AM. Multi-Sensor Approach for Tropical Soil Fertility Analysis: Comparison of Individual and Combined Performance of VNIR, XRF, and LIBS Spectroscopies. Agronomy. 2021; 11(6):1028. https://doi.org/10.3390/agronomy11061028
Chicago/Turabian StyleTavares, Tiago Rodrigues, José Paulo Molin, Lidiane Cristina Nunes, Marcelo Chan Fu Wei, Francisco José Krug, Hudson Wallace Pereira de Carvalho, and Abdul Mounem Mouazen. 2021. "Multi-Sensor Approach for Tropical Soil Fertility Analysis: Comparison of Individual and Combined Performance of VNIR, XRF, and LIBS Spectroscopies" Agronomy 11, no. 6: 1028. https://doi.org/10.3390/agronomy11061028









