Advances in Mechanisms and Omics Pertaining to Fruit Cracking in Horticultural Plants
Abstract
:1. Introduction
2. Fruit Cracking Types and Occurrence Period
3. Factors Involved in Cracking
3.1. Physiological Factors
3.2. Genetic Factors
3.3. Environmental Factors
3.4. Postharvest Storage Factors
4. Mechanisms of Fruit Cracking
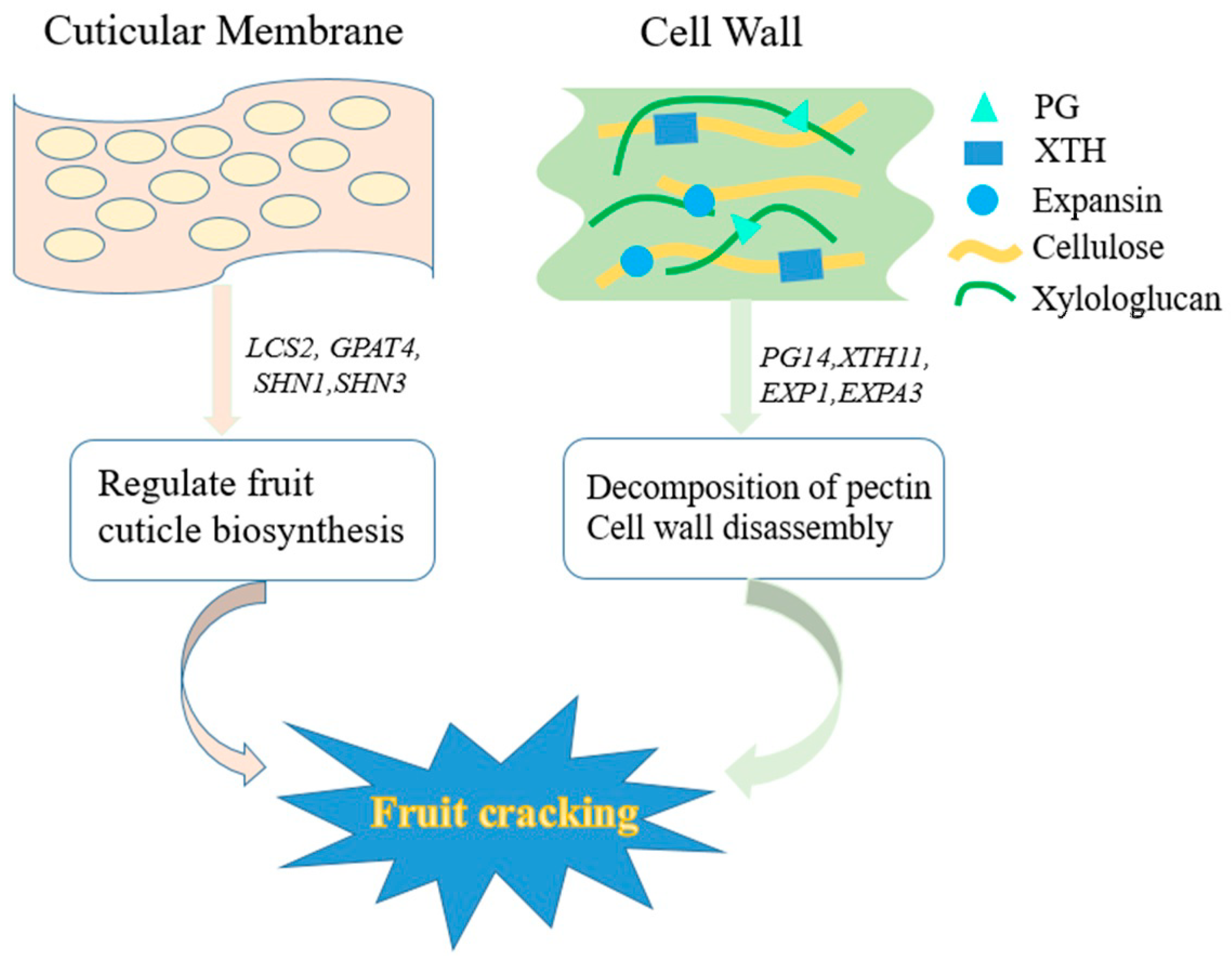
4.1. Cuticular Membrane
4.2. Cell Wall
4.2.1. Polygalacturonase Gene Family
4.2.2. Xylologlucan Endotransglucosylase/Hydrolase Gene Family
4.2.3. Expansin Gene Family
5. Studies on the Omics Regarding Fruit Cracking
6. Breeding and Conventional/Cultural Approaches to Reduce Fruit Cracking
6.1. Finding a Quantitative Trait Loci Marker
6.2. Plastic Rain Cover and Bagging
6.3. Mineral and Plant Growth Regulators Spray
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kertesz, Z.I.; Nebel, B.R. Observations on the cracking of cherries. Plant Physiol. 1935, 10, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, W.L.; Bollen, W.B. Control of Cracking of Fruit by Rain. Science 1947, 105, 334. [Google Scholar] [CrossRef]
- Thomidis, T.; Exadaktylou, E. Effect of a plastic rain shield on fruit cracking and cherry diseases in Greek orchards. Crop Prot. 2013, 52, 125–129. [Google Scholar] [CrossRef]
- Khalil, H.; Aly, S. Cracking and fruit quality of pomegranate (Punica granatum L.) as affected by pre-harvest sprays of some growth regulators and mineral nutrients. J. Hortic. Sci. Ornam. Plants 2013, 5, 71–76. [Google Scholar]
- Holland, D.; Bar-Ya’akov, I. Pomegranate (Punica Granatum L.) Breeding. In Advances in Plant Breeding Strategies, Fruits, Volume 3; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 601–647. [Google Scholar]
- Wang, J.; Wu, X.F.; Tang, Y.; Li, J.G.; Zhao, M.L. RNA-Seq Provides New Insights into the Molecular Events Involved in “Ball-Skin versus Bladder Effect” on Fruit Cracking in Litchi. Int. J. Mol. Sci. 2021, 22, 454. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.F.; Zhang, Y.F.; Bian, Y.H.; Liu, Z.Y.; Zhang, C.; Liu, X.; Wang, C.L. An Integrated Metabolic and Transcriptomic Analysis Reveals the Mechanism Through Which Fruit Bagging Alleviates Exocarp Semi-Russeting in Pear Fruit. Tree Physiol. 2020, in press. [Google Scholar] [CrossRef]
- Yuan, G.; Bian, S.; Han, X.; He, S.; Liu, K.; Zhang, C.; Cong, P. An Integrated Transcriptome and Proteome Analysis Reveals New Insights into Russeting of Bagging and Non-Bagging “Golden Delicious” Apple. Int. J. Mol. Sci. 2019, 20, 4462. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.H.; Qi, B.X.; Wang, X.Q.; Shen, L.Y.; Luo, J.; Zhang, Y.X. Proteomic analysis of the key mechanism of exocarp russet pigmentation of semi-russet pear under rainwater condition. Sci. Hortic. 2019, 254, 178–186. [Google Scholar]
- Niu, J.; Shi, Y.; Huang, K.; Zhong, Y.; Chen, J.; Sun, Z.; Luan, M.; Chen, J. Integrative transcriptome and proteome analyses provide new insights into different stages of Akebia trifoliata fruit cracking during ripening. Biotechnol. Biofuels 2020, 13, 149. [Google Scholar] [CrossRef]
- Joshi, M.; Baghel, R.S.; Fogelman, E.; Stern, R.A.; Ginzberg, I. Identification of candidate genes mediating apple fruit-cracking resistance following the application of gibberellic acids 4+7 and the cytokinin 6-benzyladenine. Plant Physiol. Biol. 2018, 127, 436–445. [Google Scholar]
- Chen, J.; Duan, Y.; Hu, Y.; Li, W.; Sun, D.; Hu, H.; Xie, J. Transcriptome analysis of atemoya pericarp elucidates the role of polysaccharide metabolism in fruit ripening and cracking after harvest. BMC Plant Biol. 2019, 19, 219. [Google Scholar] [CrossRef] [Green Version]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Ginzberg, I.; Stern, R.A. Control of Fruit Cracking by Shaping Skin Traits—Apple as a Model. Crit. Rev. Plant Sci. 2019, 38, 401–410. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, L.; Fan, X.; Zheng, T.; Dong, T.; Liu, C.; Fang, J. Anatomical characteristics associated with different degrees of berry cracking in grapes. Sci. Hortic. 2020, 261, 108992. [Google Scholar] [CrossRef]
- Li, N.; Song, Y.Q.; Li, J.; Chen, Y.Y.; Xue, X.F.; Li, L.L. Development of the cuticular membrane and biomechanical properties in Hupingzao (Ziziphus jujuba Mill. ‘Hupingzao’). Sci. Hortic. 2018, 229, 25–32. [Google Scholar] [CrossRef]
- Hou, L.; Chen, W.; Zhang, Z.; Pang, X.; Li, Y. Genome-wide association studies of fruit quality traits in jujube germplasm collections using genotyping-by-sequencing. Plant Genome 2020, 13, e20036. [Google Scholar] [CrossRef]
- Hosein-Beigi, M.; Zarei, A.; Rostaminia, M.; Erfani-Moghadam, J. Positive effects of foliar application of Ca, B and GA3 on the qualitative and quantitative traits of pomegranate (Punica granatum L.) cv. ‘Malase-Torshe-Saveh’. Sci. Hortic. 2019, 254, 40–47. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, A.K.; Meghwal, P.R. Fruit Cracking in Pomegranate, Extent, Cause, and Management—A Review. Int. J. Fruit Sci. 2020, 20, 1–20. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Ma, Z.; Chen, J.; Liu, Y. Analysis of the molecular basis of fruit cracking susceptibility in Litchi chinensis cv. Baitangying by transcriptome and quantitative proteome profiling. J. Plant Physiol. 2019, 234–235, 106–116. [Google Scholar] [CrossRef]
- Cronjé, P.J.R.; Stander, O.P.J.; Theronm, K.I. Fruit Splitting in Citrus. Hortic. Rev. 2013, 41, 177–200. [Google Scholar]
- Kwon, Y.; Han, H.H.; Park, H.S. The characteristics of cork and hypodermis tissues and cracking in Asian pear (Pyrus pyrifolia cv. Mansoo). Sci. Hortic. 2016, 199, 224–228. [Google Scholar] [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; De Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Sun, M.; Wu, Z.; Yu, L.; Yu, Q.; Tang, Y.; Jiang, F. LncRNA regulates tomato fruit cracking by coordinating gene expression via a hormone-redox-cell wall network. BMC Plant Biol. 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.K.; Tian, H.M.; Yan, C.S.; Jia, L.; Wang, Y.; Wang, M.X.; Jiang, C.J.; Li, Y.Y.; Jiang, J.Y.; Fang, L. RNA-seq analysis of watermelon (Citrullus lanatus) to identify genes involved in fruit cracking. Sci. Hortic. 2019, 248, 248–255. [Google Scholar] [CrossRef]
- Qi, Z.; Li, J.; Raza, M.A.; Zou, X.; Cao, L.; Rao, L.; Chen, L. Inheritance of fruit cracking resistance of melon (Cucumis melo L.) fitting E-0 genetic model using major gene plus polygene inheritance analysis. Sci. Hortic. 2015, 189, 168–174. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant 2014, 37, 1718. [Google Scholar] [CrossRef]
- Balbontin, C.; Ayala, H.; Rubilar, J.; Cote, J.; Figueroa, C.R. Transcriptional analysis of cell wall and cuticle related genes during fruit development of two sweet cherry cultivars with contrasting levels of cracking tolerance. Chil. J. Agric. Res. 2014, 74, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Saei, H.; Sharifani, M.M.; Dehghani, A.; Seifi, E.; Akbarpour, V. Description of biomechanical forces and physiological parameters of fruit cracking in pomegranate. Sci. Hortic. 2014, 178, 224–230. [Google Scholar] [CrossRef]
- Konarska, A. The structure of the fruit peel in two varieties of Malus domestica Borkh. (Rosaceae) before and after storage. Protoplasma 2013, 250, 701–714. [Google Scholar] [CrossRef] [Green Version]
- Yazici, K.; Ercisli, S. Characterization of hybrid pomegranate genotypes based on sunburn and cracking traits related to maturation time. J. Appl. Bot. Food Qual. 2017, 90, 132–139. [Google Scholar]
- Galindo, A.; Rodríguez, P.; Collado-González, J.; Cruz, Z.N.; Torrecillas, E.; Ondoño, S.; Corell, M.; Moriana, A.; Torrecillas, A. Rainfall intensifies fruit peel cracking in water stressed pomegranate trees. Agric. For. Meteorol. 2014, 194, 29–35. [Google Scholar] [CrossRef]
- Knoche, M.; Khanal, B.P.; Stopar, M. Russeting and Microcracking of ‘Golden Delicious’ Apple Fruit Concomitantly Decline Due to Gibberellin A 4+7 Application. J. Am. Soc. Hortic. Sci. 2011, 136, 159–164. [Google Scholar] [CrossRef]
- Simon, G. Review on rain induced fruit cracking of sweet cherries (Prunus avium L.), its causes and the possibilities of prevention. Int. J. Hortic. Sci. 2006, 12, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, J. Citrus Fruit-Cracking, Causes and Occurrence. Hortic. Plant J. 2017, 3, 255–260. [Google Scholar] [CrossRef]
- Dorais, M.; Demers, D.A.; Papadopoulos, A.; Ieperen, W. Greenhouse Tomato Fruit Cuticle Cracking. Hortic. Rev. 2010, 30, 163–184. [Google Scholar]
- Ginzberg, I.; Fogelman, E.; Rosenthal, L.; Stern, R.A. Maintenance of high epidermal cell density and reduced calyx-end cracking in developing ‘Pink Lady’ apples treated with a combination of cytokinin 6-benzyladenine and gibberellins A(4) + A(7). Sci. Hortic. 2014, 165, 324–330. [Google Scholar] [CrossRef]
- Kasai, S.; Hayama, H.; Kashimura, Y.; Kudo, S.; Osanai, Y. Relationship between fruit cracking and expression of the expansin gene MdEXPA3 in ‘Fuji’ apples (Malus domestica Borkh.). Sci. Hortic. 2008, 116, 194–198. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, Y.Q.; Zhao, J.; Liu, M.J. Screening and Evaluation of Germplasms with High Resistance to Fruit Cracking in Ziziphus jujuba Mill. Sci. Agric. Sin. 2013, 46, 4968–4976. (In Chinese) [Google Scholar]
- Li, X.; Chen, X.; Wen, X.; Zhao, Y.; Ma, H. Correlation Analysis between the Sugar Components and Fruit Cracking in Easily Cracked and Resistant Jujube. Mol. Plant Breed. 2020, 18, 6180–6186. [Google Scholar]
- Cuartero, J.; Palomares, G.; Balasch, S.; Nuez, F. Tomato fruit cracking under plastic-house and in the open air. II. General and specific combining abilities. BMC Infect. Dis. 1981, 15, 1–13. [Google Scholar]
- Beyer, M.; Hahn, R.; Peschel, S.; Harz, M.; Knoche, M. Analysing fruit shape in sweet cherry (Prunus avium L.). Sci. Hortic. 2002, 96, 139–150. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Harrigan, G. Metabolome Analyses: Strategies for Systems Biology; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Polat, A.A.; Caliskan, O.; Kamiloglu, O. Determination of pomological characteristics of some pomegranate cultivars in drtyol (turkey) conditions. Acta Hortic. 2012, 401–405. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Sato, I.; Ishiguro, M. Influences of Epidermal Cell Sizes and Flesh Firmness on Cracking Susceptibility in Sweet Cherry (Prunus avium L.) Cultivars and Selections. J. Jpn. Soc. Hortic. Sci. 2002, 71, 738–746. [Google Scholar] [CrossRef]
- Simon, G.; Hrotkó, K.; Magyar, L. Fruit Quality of Sweet Cherry Cultivars Grafted on Four Different Rootstocks. Int. J. Hortic. Sci. 2004, 10, 365–370. [Google Scholar] [CrossRef]
- Greco, P.; Palasciano, M.; Mariani, R.; Pacifico, A.; Godini, A. Susceptibility to cracking of thirty sweet cherry cultivars. Acta Hortic. 2008, 379–382. [Google Scholar] [CrossRef]
- Beyer, M.; Peschel, S.; Knoche, M.; Knrgen, M. Studies on Water Transport Through the Sweet Cherry Fruit Surface, IV. Regions of Preferential Uptake. HortScience 2002, 37, 637–641. [Google Scholar] [CrossRef]
- Peña, M.E.; Artés-Hernández, F.; Aguayo, E.; Martínez-Hernández, G.B.; Gómez, P.A. Effect of sustained deficit irrigation on physicochemical properties, bioactive compounds and postharvest life of pomegranate fruit (cv. ‘Mollar de Elche’). Postharvest Biol. Technol. 2013, 86, 171–180. [Google Scholar] [CrossRef]
- Yılmaz, C.; Özgüven, A.I. The effects of some plant nutrients, gibberellic acid and pinolene treatments on the yield, fruit quality and cracking in pomegranate. Acta Hortic. 2009, 818, 205–212. [Google Scholar] [CrossRef]
- Wang, S.Y.; Camp, M.J. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 2000, 85, 183–199. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Functional characterisation of lenticels, micro-cracks, wax patterns, peel tissue fractions and water loss of pomegranate fruit (cv. Wonderful) during storage. Postharvest Biol. Technol. 2021, 178, 111539. [Google Scholar] [CrossRef]
- Wang, Y.; Long, L.E. Physiological and biochemical changes relating to postharvest splitting of sweet cherries affected by calcium application in hydrocooling water. Food Chem. 2015, 181, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Santos, M.; Glinska, S.; Gapinska, M.; Matos, M.; Carnide, V.; Schouten, R.; Silva, A.P.; Goncalves, B. Effects of exogenous compound sprays on cherry cracking, skin properties and gene expression. J. Sci. Food Agric. 2020, 100, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Philippe, G.; Sørensen, I.; Jiao, C.; Sun, X.; Rose, J.K. Cutin and suberin, assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 2020, 55, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lian, Q.; Wang, L.; Shan, Y.; Jiang, Y. Chemical composition of the cuticular membrane in guava fruit (Psidium guajava L.) affects barrier property to transpiration. Plant Physiol. Biochem. 2020, 155, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Fich, E.A.; Segerson, N.A.; Rose, J.K. The Plant Polyester Cutin, Biosynthesis, Structure, and Biological Roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef]
- Samuels, A.; Kunst, L.; Jetter, R. Sealing Plant Surfaces, Cuticular Wax Formation by Epidermal Cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [Green Version]
- Qiao, P.; Bourgault, R.; Mohammadi, M.; Matschi, S.; Philippe, G.; Smith, L.G.; Gore, M.A.; Molina, I.; Scanlon, M.J. Transcriptomic network analyses shed light on the regulation of cuticle development in maize leaves. Proc. Natl. Acad. Sci. USA 2020, 117, 12464–12471. [Google Scholar] [CrossRef]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger, its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles, an overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef]
- Peschel, S.; Franke, R.; Schreiber, L.; Knoche, M. Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 2007, 68, 1017–1025. [Google Scholar] [CrossRef]
- Knoche, M.; Lang, A. Ongoing Growth Challenges Fruit Skin Integrity. Crit. Rev. Plant Sci. 2017, 36, 190–215. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef]
- Shi, J.X.; Adato, A.; Alkan, N.; He, Y.; Lashbrooke, J.; Matas, A.J.; Meir, S.; Malitsky, S.; Isaacson, T.; Prusky, D. The tomato SlSHINE3 transcription factor regulates fruit cuticle formation and epidermal patterning. New Phytol. 2013, 197, 468–480. [Google Scholar] [CrossRef]
- Lashbrooke, J.; Aharoni, A.; Costa, F. Genome investigation suggests MdSHN3, an APETALA2-domain transcription factor gene, to be a positive regulator of apple fruit cuticle formation and an inhibitor of russet development. J. Exp. Bot. 2015, 66, 6579–6589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkio, M.; Jonas, U.; Sprink, T.; van Nocker, S.; Knoche, M. Identification of putative candidate genes involved in cuticle formation in Prunus avium (sweet cherry) fruit. Ann. Bot. 2012, 110, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobson, L.; Lindgren, L.O.; Verdier, G.; Laanemets, K.; Brosche, M.; Beisson, F.; Kollist, H. BODYGUARD is required for the biosynthesis of cutin in Arabidopsis. New Phytol. 2016, 211, 614–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, J.; Bres, C.; Mauxion, J.P.; Tai, F.W.; Martin, L.B.; Fich, E.A.; Joubes, J.; Rose, J.K.; Domergue, F.; Rothan, C. The Glycerol-3-Phosphate Acyltransferase GPAT6 from Tomato Plays a Central Role in Fruit Cutin Biosynthesis. Plant Physiol. 2016, 171, 894–913. [Google Scholar] [CrossRef] [PubMed]
- Espana, L.; Heredia-Guerrero, J.A.; Segado, P.; Benitez, J.J.; Heredia, A.; Dominguez, E. Biomechanical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes in the relative amounts of its components. New Phytol. 2014, 202, 790–802. [Google Scholar] [CrossRef] [Green Version]
- Alkio, M.; Jonas, U.; Declercq, M.; Van Nocker, S.; Knoche, M. Transcriptional dynamics of the developing sweet cherry (Prunus avium L.) fruit, sequencing, annotation and expression profiling of exocarp-associated genes. Hortic. Res. 2014, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.X.; Malitsky, S.; De Oliveira, S.; Branigan, C.; Franke, R.B.; Schreiber, L.; Aharoni, A. SHINE transcription factors act redundantly to pattern the archetypal surface of Arabidopsis flower organs. PLoS Genet. 2011, 7, e1001388. [Google Scholar] [CrossRef] [Green Version]
- Kannangara, R.; Branigan, C.; Liu, Y.; Penfield, T.; Rao, V.; Mouille, G.; Höfte, H.; Pauly, M.; Riechmann, J.L.; Broun, P. The Transcription Factor WIN1/SHN1 Regulates Cutin Biosynthesis in Arabidopsis thaliana. Plant Cell 2007, 19, 1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; van Arkel, G.; Pereira, A. The SHINE Clade of AP2 Domain Transcription Factors Activates Wax Biosynthesis, Alters Cuticle Properties, and Confers Drought Tolerance when Overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.T.; Kieber, J.J. Dynamic Construction, Perception, and Remodeling of Plant Cell Walls. Annu. Rev. Plant Biol. 2020, 71, 39–69. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Kirui, A.; Huang, S.; Wang, L.; Barnes, W.J.; Kiemle, S.; Zheng, Y.; Rui, Y.; Ruan, M.; Qi, S. Mutations in the Pectin Methyltransferase QUASIMODO2 Influence Cellulose Biosynthesis and Wall Integrity in Arabidopsis thaliana. Plant Cell 2020, 32, 3576–3597. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef]
- Miedes, E.; Lorences, E.P. Xyloglucan endotransglucosylase/hydrolases (XTHs) during tomato fruit growth and ripening. J. Plant Physiol. 2009, 166, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Gine-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguilo-Aguayo, I.; Lopez, M.L.; Larrigaudiere, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Dautt-Castro, M.; Lopez-Virgen, A.G.; Ochoa-Leyva, A.; Contreras-Vergara, C.A.; Sortillon-Sortillon, A.P.; Martinez-Tellez, M.A.; Gonzalez-Aguilar, G.A.; Casas-Flores, J.S.; Sanudo-Barajas, A.; Kuhn, D.N.; et al. Genome-Wide Identification of Mango (Mangifera indica L.) Polygalacturonases, Expression Analysis of Family Members and Total Enzyme Activity during Fruit Ripening. Front. Plant Sci. 2019, 10, 969. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Yu, Y.; Cui, J.; Lyu, M.; Xu, L.; Cao, J. A comparative analysis of the evolution, expression, and cis-regulatory element of polygalacturonase genes in grasses and dicots. Funct. Integr. Genom. 2016, 16, 641–656. [Google Scholar] [CrossRef]
- Chen, H.; Shao, H.; Fan, S.; Ma, J.; Zhang, D.; Han, M. Identification and Phylogenetic Analysis of the POLYGALACTURONASE Gene Family in Apple. Hortic. Plant J. 2016, 2, 241–252. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Yan, X.; Han, M.; Li, J.; Li, F.; Li, F.; Zhang, D.; Zhao, C. Identification and Expression Analysis of Polygalacturonase Family Members during Peach Fruit Softening. Int. J. Mol. Sci. 2016, 17, 1933. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Wang, H.; Li, Y.; Zhu, B.; Zang, Y.; He, Y.; Cao, J.; Zhu, Z.; Yu, Y. Genome-Wide Identification and Analysis of Polygalacturonase Genes in Solanum lycopersicum. Int. J. Mol. Sci. 2018, 19, 2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Ma, M.; Zhang, H.; Zhang, S.; Qian, M.; Zhang, Z.; Luo, W.; Fan, J.; Liu, Z.; Wang, L. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019, 19, 587. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH Family of Enzymes Involved in Xyloglucan Endotransglucosylation and Endohydrolysis: Current Perspectives and a New Unifying Nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ding, A.; Kong, Y. Genome-Wide Identification and Expression Profiling Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Tobacco (Nicotiana tabacum L.). Genes 2018, 9, 273. [Google Scholar]
- Song, L.; Valliyodan, B.; Prince, S.; Wan, J.; Nguyen, H.T. Characterization of the XTH Gene Family, New Insight to the Roles in Soybean Flooding Tolerance. Int. J. Mol. Sci. 2018, 19, 2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Z.; Li, T.; Gao, H.; Zhu, H.; Duan, X. Integrated Transcriptomic, Proteomic, and Metabolomics Analysis Reveals Peel Ripening of Harvested Banana under Natural Condition. Biomolecules 2019, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Saladié, M.; Rose, J.K.; Cosgrove, D.J.; Catalá, C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J. 2006, 47, 282–295. [Google Scholar] [CrossRef]
- Munoz-Bertomeu, J.; Miedes, E.; Lorences, E.P. Expression of xyloglucan endotransglucosylase/hydrolase (XTH) genes and XET activity in ethylene treated apple and tomato fruits. J. Plant Physiol. 2013, 170, 1194–1201. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, N.; Jiang, S.; Xu, H.; Wang, Y.; Wang, C.; Li, M.; Liu, J.; Qu, C.; Liu, W. Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family during Apple Fruit Ripening and Softening. J. Agric. Food Chem. 2017, 65, 429–434. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Y.; Jiang, Y.; Li, J.; Liu, H.; Duan, X.; Song, L. Differential expression of litchi XET genes in relation to fruit growth. Plant Physiol. Biochem. 2006, 44, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jones, L.; McQueen-Mason, S. Expansins and cell growth. Curr. Opin. Plant Biol. 2003, 6, 603–610. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef]
- Brummell, A.; Howie, W.J.; Ma, C.; Dunsmuir, P. Postharvest fruit quality of transgenic tomatoes suppressed in expression of a ripening-related expansin. Postharvest Biol. Technol. 2002, 25, 209–220. [Google Scholar] [CrossRef]
- Wakasa, Y.; Hatsuyama, Y.; Takahashi, A.; Sato, T.; Niizeki, M.; Harada, T. Divergent Expression of six Expansin Genes during Apple Fruit Ontogeny. Eur. J. Hortic. Sci. 2003, 68, 253–259. [Google Scholar]
- Yong, W.; Lu, W.J.; Li, J.G.; Jiang, Y.M. Differential expression of two expansin genes in developing fruit of cracking-susceptible and resistant litchi cultivars. J. Am. Soc. Hortic. Sci. 2006, 131, 118–121. [Google Scholar] [CrossRef]
- Liao, N.; Hu, Z.; Li, Y.; Hao, J.; Chen, S.; Xue, Q.; Ma, Y.; Zhang, K.; Mahmoud, A.; Ali, A.; et al. Ethylene-responsive factor 4 is associated with the desirable rind hardness trait conferring cracking resistance in fresh fruits of watermelon. Plant Biotechnol. J. 2020, 18, 1066–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, P.; Akinmoju, T.A.; Nimmakayala, P.; Lopez-Ortiz, C.; Garcia-Lozano, M.; Thompson, B.J.; Stommel, J.; Reddy, U.K. Integrated Metabolomic and Transcriptomic Analysis to Characterize Cutin Biosynthesis between Low- and High-Cutin Genotypes of Capsicum chinense Jacq. Int. J. Mol. Sci. 2020, 21, 1397. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Darley, C.P.; Ongaro, V.; Fleming, A.; Schipper, O.; Baldauf, S.L.; McQueen-Mason, S.J. Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiol. 2002, 128, 854–864. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Yu, J.; Zhao, M.; Wang, M.; Yang, G. Transcriptome analysis of metabolisms related to fruit cracking during ripening of a cracking-susceptible grape berry cv. Xiangfei (Vitis vinifera L.). Genes Genom. 2020, 42, 639–650. [Google Scholar] [CrossRef]
- Wasinger, V.C.; Cordwell, S.J.; Cerpa-Poljak, A.; Yan, J.X.; Humphery-Smith, I. Progress with gene-product mapping of the Mollicutes, Mycoplasma genitalium. Electrophoresis 1995, 16, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Mann, M. Proteomics to study genes and genomes. Nature 2000, 405, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.C.; Robledo, F.; Schreiber, L.; Zeisler, V.; Lang, E.; Carrasco, B.; Silva, H. Association between the concentration of n -alkanes and tolerance to cracking in commercial varieties of sweet cherry fruits. Sci. Hortic. 2015, 197, 57–65. [Google Scholar] [CrossRef]
- Capel, C.; Yuste-Lisbona, F.; Lopez-Casado, G.; Angosto, T.; Cuartero, J.; Lozano, R.; Capel, J. Multi-environment QTL mapping reveals genetic architecture of fruit cracking in a tomato RIL Solanum lycopersicum × S. pimpinellifolium population. Theor. Appl. Genet. 2017, 130, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, Z.; Liu, J.; Qiu, B.; Gao, P. QTL mapping of pericarp and fruit-related traits in melon (Cucumis melo L.) using SNP-derived CAPS markers. Sci. Hortic. 2020, 265, 109243. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pal, R.K.; Sagar, V.R.; Parmanick, K.K.; Paul, V.; Gupta, V.K.; Kumar, K.; Rana, M.R. Impact of pre-harvest fruit-bagging with different coloured bags on peel colour and the incidence of insect pests, disease and storage disorders in ‘Royal Delicious’ apple. J. Hortic. Sci. Biotechnol. 2015, 89, 613–618. [Google Scholar] [CrossRef]
- Chen, C.S.; Zhang, D.; Wang, Y.Q.; Li, P.M.; Ma, F.W. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, C.; Zhang, X.; Wang, C.; Yang, S. Preharvest bagging and postharvest calcium treatment affects superficial scald incidence and calcium nutrition during storage of ‘Chili’ pear (Pyrus bretschneideri) fruit. Postharvest Biol. Technol. 2020, 163, 111149. [Google Scholar] [CrossRef]
- Grinan, I.; Morales, D.; Galindo, A.; Torrecillas, A.; Perez-Lopez, D.; Moriana, A.; Collado-Gonzalez, J.; Carbonell-Barrachina, A.A.; Hernandez, F. Effect of preharvest fruit bagging on fruit quality characteristics and incidence of fruit physiopathies in fully irrigated and water stressed pomegranate trees. J. Sci. Food Agric. 2019, 99, 1425–1433. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Yin, Y.L.; Feng, L.J.; Zhao, X.Q.; Hou, L.F.; Zhang, Y.X. Evaluation of pomegranate bagging and fruit cracking in Shandong, China. Acta Hortic. 2012, 125–129. (In Chinese) [Google Scholar] [CrossRef]
- Asrey, R.; Kumar, K.; Sharma, R.R.; Meena, N.K. Fruit bagging and bag color affects physico-chemical, nutraceutical quality and consumer acceptability of pomegranate (Punica granatum L.) arils. J. Food Sci. Technol. 2020, 57, 1469–1476. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Abadía, J.; Val, J.; Davarynejad, G.; Aran, M.; Khorassani, R. Foliar calcium fertilization reduces fruit cracking in pomegranate (Punica granatum cv. Ardestani). Sci. Hortic. 2018, 230, 86–91. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Karamanoli, K.; Lazaridou, A.; Matsi, T.; Molassiotis, A. Metabolomic and physico-chemical approach unravel dynamic regulation of calcium in sweet cherry fruit physiology. Plant Physiol. Biochem. 2017, 116, 68–79. [Google Scholar] [CrossRef] [PubMed]

| Species | Fruit Cracking Types | Occurrence Period | Reference |
|---|---|---|---|
| Cherry | Apical end cracking, deep side cracking, stem end cracking | Early stage of fruit development or when rain happens near harvest | [2,34] |
| Grape | Longitudinal cracking, ring cracking, Cuticular cracking, ring and longitudinal cracking | Growth and development | [15] |
| Citrus | Flavedo-splitting, inner-cracking and albedo-splitting (fruit creasing or pitting) | Cell enlargement or fruit maturity | [35] |
| Tomato | Radial and concentric cracking, circular cracking | Last phase of fruit growth | [36] |
| Apple | Calyx-end cracking, internal ring cracking and stem-end splitting | Pre-harvest period, the phase of rapid fruit growth | [37,38] |
| Jujube | Longitudinal cracking, transversal cracking, longitudinal + transversal cracking, irregular cracking | Fruit maturity | [39] |
| Gene Family | Species | Gene | Gene Function | Reference |
|---|---|---|---|---|
| AP2/ERF | Watermelon | ERF4 | Rind hardness | [99] |
| Peppers | SHN1 | Contributing to the high cutin content | [100] | |
| Apple | SHN3 | Cuticle formation | [66] | |
| PG | Atemoya | PG | Pectin degradation | [12] |
| Mango | PG21-1, PG14, PG17 | Fruit ripening | [80] | |
| XTH | Tomato | XTH5, XTH8 | Fruit mature | [91] |
| Apple | XTH2, XTH10, XTH11 | Fruit mature | [91] | |
| Lichi | XET1 | Fruit cracking | [93] | |
| Watermelon | XET1, XET2 | Fruit cracking | [25] | |
| Expansins | Tomato | EXP1 | Cell wall disassembly | [24] |
| Lichi | EXP2 | Cracking-resistant | [98] | |
| Apple | EXPA3 | Fruit cracking | [38] | |
| Apple | EXPA4 | Cell-wall modification/loosening | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Guo, L.; Zhao, X.; Zhao, Y.; Hao, Z.; Luo, H.; Yuan, Z. Advances in Mechanisms and Omics Pertaining to Fruit Cracking in Horticultural Plants. Agronomy 2021, 11, 1045. https://doi.org/10.3390/agronomy11061045
Wang Y, Guo L, Zhao X, Zhao Y, Hao Z, Luo H, Yuan Z. Advances in Mechanisms and Omics Pertaining to Fruit Cracking in Horticultural Plants. Agronomy. 2021; 11(6):1045. https://doi.org/10.3390/agronomy11061045
Chicago/Turabian StyleWang, Yuying, Linhui Guo, Xueqing Zhao, Yujie Zhao, Zhaoxiang Hao, Hua Luo, and Zhaohe Yuan. 2021. "Advances in Mechanisms and Omics Pertaining to Fruit Cracking in Horticultural Plants" Agronomy 11, no. 6: 1045. https://doi.org/10.3390/agronomy11061045






