Changes in Microstructure, Germination, Sprout Growth, Phytochemical and Microbial Quality of Ultrasonication Treated Adzuki Bean Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Materials
2.2. Ultrasonication Treatment
2.3. Seed Hydration and Germination Determinations
2.4. α-Amylase Activity Determinations
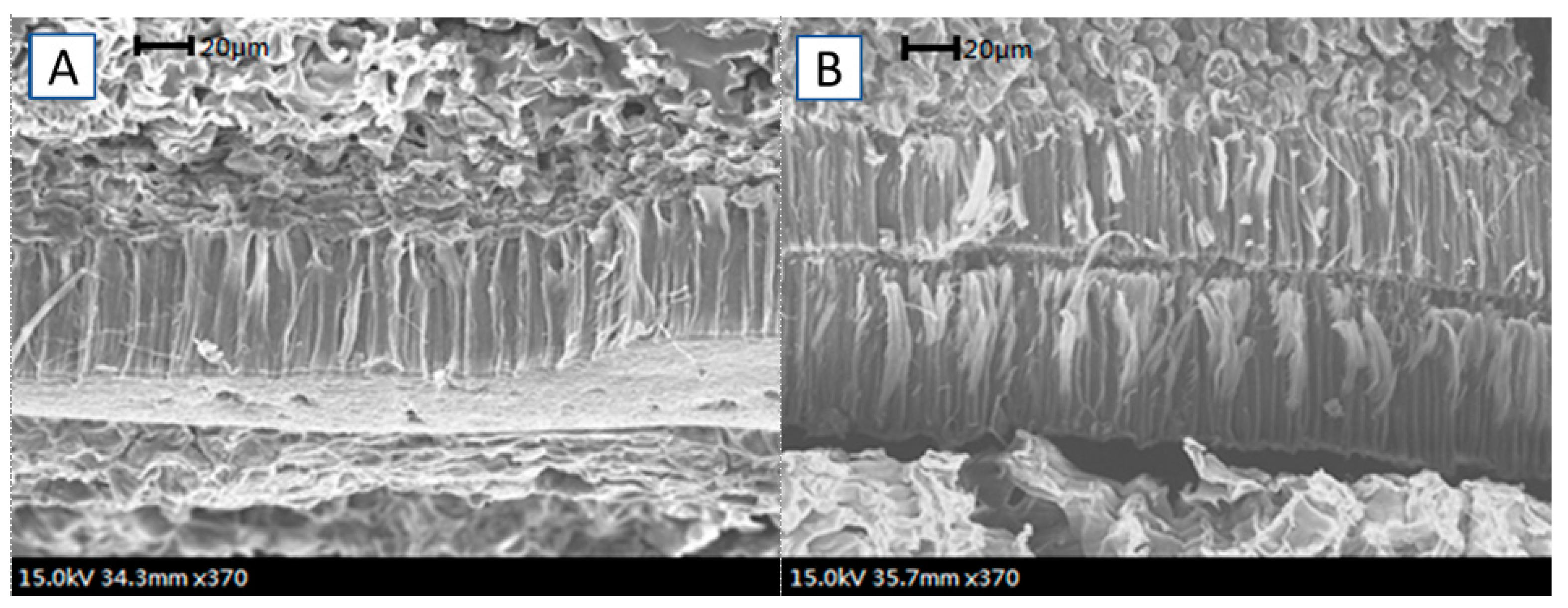
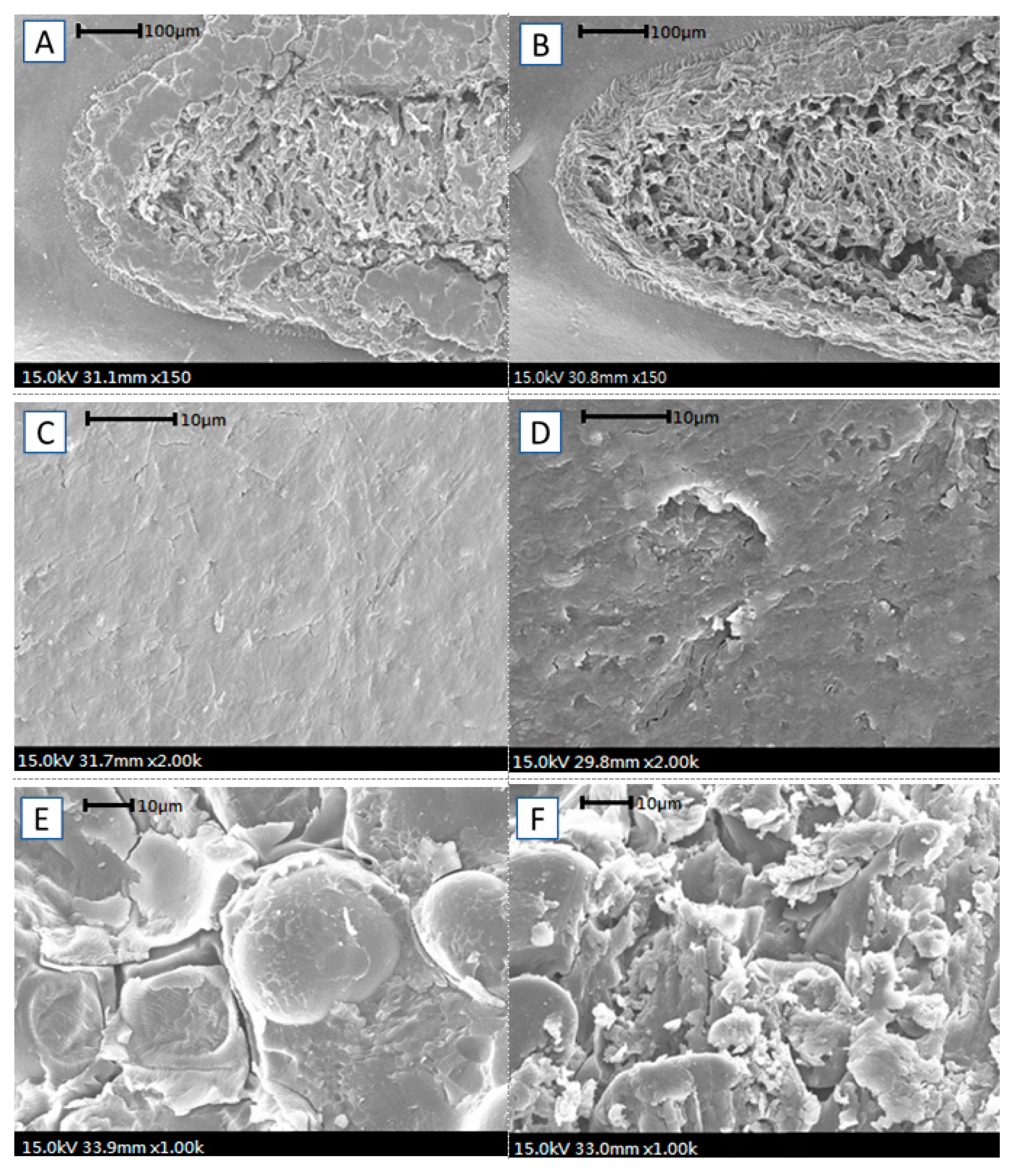
2.5. Scanning Electron Microscopy Examinations
2.6. Phytochemicals and Anti-Oxidative Activities Determinations
2.7. Data Analysis
3. Results and Discussion
3.1. Ultrasonication Treatment on Seed Hydration
3.2. Ultrasonication Treatment on Seed Germination and α-Amylase Activity
3.3. Effects of Ultrasonication Treatments on Sprouts Growths and Yields
3.4. Ultrasonication Treatment on Sprout Phytochemicals
3.5. Ultrasonication Treatment on Antioxidative Potentials
3.6. Effects of Ultrasonication Treatments on Microbial Loads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Cai, Z.; Xu, B. Characterization and quantifcation of favonoids and saponins in adzuki bean (Vigna angularis L.) by HPLC-DAD-ESI-MSN analysis. Chem. Cent. J. 2017, 11, 93–110. [Google Scholar] [CrossRef]
- Adav, U.; Singh, N.; Kaur, A.; Thakur, S. Physico-chemical, hydration, cooking, textural and pasting properties of different adzuki bean (Vigna angularis) accessions. J. Food Sci. Technol. 2018, 55, 802–810. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dzikia, U.; Jakubczyka, A.; Bochnaka, J.; Sikoraa, M.; Suliburskab, J. Nutritional quality of fresh and stored legumes sprouts-effect of Lactobacillus plantarum 299v enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef]
- Kramer, C.; Soltani1, N.; Swanton, C.J.; Robinson, D.E.; Sikkema, P.H. Control of volunteer adzuki bean (Vigna angularis) with pre- and postemergence herbicides in corn (Zea mays). Can. J. Plant Sci. 2010, 90, 925–932. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef]
- Isemura, T.; Kaga, A.; Konishi, S.; Ando, T.; Tomooka, N.; Han, O.K.; Vaughan, D.A. Genome dissection of traits related to domestication in azuki bean (Vigna angularis) and comparison with other warm-season legumes. Ann. Bot. 2007, 100, 1053–1071. [Google Scholar] [CrossRef]
- Chiu, K.Y. Ultrasonication-enhanced microbial safety of sprouts produced from selected crop species. J. Appl. Bot. Food Qual. 2015, 88, 120–126. [Google Scholar] [CrossRef]
- Yusaf, T.; Al-Juboori, R.A. Alternative methods of microorganism disruption for agricultural applications. Appl. Energy 2014, 114, 909–923. [Google Scholar] [CrossRef]
- Ravikumar, M.; Suthar, H.; Desai, C.; Gowda, S.A.J. Ultrasonication: An advanced technology for food preservation. Int. J. Pure Appl. Biosci. 2017, 5, 363–371. [Google Scholar] [CrossRef]
- Bhargavaa, N.; Mora, R.S.; Kumarb, K.; Sharanagata, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Kumara, S.; Gautama, S. A combination process to ensure microbiological safety, extend storage lifeand reduce anti-nutritional factors in legume sprouts. Food Biosci. 2019, 27, 18–29. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Sung, J.M. Use of ultrasonication to enhance pea seed germination andmicrobial quality of pea sprouts. Int. J. Food Sci. Technol. 2014, 49, 1699–1706. [Google Scholar] [CrossRef]
- Hayta, M.; Isçimen, E.M. Optimization of ultrasound-assisted antioxidant compoundsextraction from germinated chickpea using response surface methodology. LWT Food Sci. Technol. 2017, 77, 208–216. [Google Scholar] [CrossRef]
- Yang, Y.; Meier, F.; Lo, J.A.; Yuan, W.; Sze, V.L.P.; Chung, H.-J.; Yuk, H.-G. Overview of recent events in the microbiological safety of sprouts and new intervention technologies. Compr. Rev. Food Sci. Saf. 2013, 12, 265–279. [Google Scholar] [CrossRef]
- Miano, C.A.; da Costa Pereira, J.; Castanha, N.; da MattaJúnior, M.D.; Augusto, E.D. Enhancing mung bean hydration using the ultrasound technology: Description of mechanisms and impact on its germination and main components. Sci. Rep. 2016, 6, 388996. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Eteghadipour, M. Impacts of ultrasonic waves on seeds: A mini-review. Agric Res. Technol. 2017, 6, 1–5. [Google Scholar] [CrossRef]
- Kim, H.J.; Feng, H.; Kushad, M.M.; Fan, X. Effects of ultrasound, irradiation, and acidic electrolyzed water on germination of alfalfa and broccoli seeds and Escherichia coli O157:H7. J. Food Sci. 2006, 71, M168–M173. [Google Scholar] [CrossRef]
- Osman, A.M. The advantages of using natural substrate-based methods in assessing the roles and synergistic and competitive intractions of barley malt starch-degrading enzymes. J. Inst. Brew. 2002, 108, 204–214. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, S.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Nguimbou, R.M.; Boudjeko, T.; Njintang, N.Y.; Himeda, M.; Scher, J.; Mbofung, C.M.F. Mucilage chemical profile and antioxidant properties of giant swamp taro tubers. J. Food Sci. Technol. 2014, 51, 3559–3567. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Vador, N.; Vador, B.; Hole, R. Simple spectrophotometric methods for standardizing aaurvedic formulation. Indian J. Pharm. Sci. 2012, 74, 161–163. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; pp. 133–181. Available online: https://www.springer.com/gp/book/9781461446927 (accessed on 3 May 2021).
- Miano, A.C.; Augusto, P.E.D. From the sigmoidal to the downward concave shape behavior during the hydration of grains: Effect of the initial moisture content on adzuki beans (Vigna angularis). Food Bioprod. Process. 2015, 96, 43–51. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Chen, Y.p.; Liu, Q.; Yue, X.Z. Ultrasonic vibration seeds showed improved resistance to cadmium and lead in wheat seedling. Environ. Sci. Pollut. Res. 2013, 20, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Duangjai, T.; Areeya, T.; Apinan, P.; Aujana, Y. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Świeca, M.; Herok, A.; Piwowarczyk, K.; Sikora, M.; Ostanek, P.; Gawlik-Dziki, U.; Kapusta, I.; Czyż, J. Potentially bioaccessible phenolics from mung bean and adzuki bean sprouts enriched with probiotic-antioxidant properties and effect on the motility and survival of AGS human gastric carcinoma cells. Molecules 2020, 25, 2963. [Google Scholar] [CrossRef] [PubMed]
- Borges-Martínez, E.; Tzayhri Gallardo-Veláazouez, T.; Anaberta Cardadormartínez, A.; Moguel-Concha, D.; Osorio-Revilla, G.; Ruiz-Ruiz, J.C.; Martínez, C.J. Phenolic compounds profile and antioxidant activity of pea (Pisum sativum L.) and black bean (Phaseolus vulgaris L.) sprouts. Food Sci. Technol 2021. [Google Scholar] [CrossRef]
- Singh, B.; Pal Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Flores, D.; Garcia-Patino, M.; Serna-Guerrero, D.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Characterization and quantification of saponins and flavonoids in sprouts, seed coats and cotyledons of germinated black beans. Food Chem. 2012, 134, 1312–1319. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Wang, B.; Li, S.; Guo, F.; Hua, H.B.; Zhao, Y.; Yu, Z. Comparison of phenolic compounds, antioxidant and antidiabetic activities between selected edible beans and their different growth. J. Funct. Foods 2017, 35, 694–702. [Google Scholar] [CrossRef]
- Soedarjo, M.; Suhartina, S.; Nugrahaeni, N.; Wijanarko, A.; Putri, D.A.; Fatmawati, S. The Antioxidant Activities and Phenolic Content of Improved Soybean Seeds Varieties of Different Grain Sizes. IPTEK J. Technol. Sci. 2020, 31, 83–90. [Google Scholar] [CrossRef]
- Singh, B.; Singh, N.; Thakur, S.; Kaur1, A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J. Food Sci. Technol. 2017, 54, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Sikin, A.M.; Zoellner, C.; Rizvi, S.S.H. Current intervention strategies for the microbial safety of sprouts. J. Food Prot. 2013, 76, 2099–2123. [Google Scholar] [CrossRef]

| Ultrasonication Treatment | Germination | Mean Germination Time | Hypocotyl Length | Radicle Length | Sprout Fresh Weight | |
|---|---|---|---|---|---|---|
| Variety | (kHz) | (%) | (days) | (cm) | (cm) | (g 10 g−1 seeds) |
| KH8 | 0 | 66.1 ± 4.1 | 4.43 ± 0.1 | 5.43 ± 0.0 | 6.72 ± 0.1 | 212.4 ± 4.4 |
| 28 | 87.2 ± 2.3 | 3.48 ± 0.2 | 5.92 ± 0.0 | 7.62 ± 0.0 | 238.6 ± 9.8 | |
| 40 | 97.2 ± 2.4 | 2.43 ± 0.1 | 7.25 ± 0.0 | 8.46 ± 0.1 | 331.5 ± 6.8 | |
| 80 | 92.3 ± 2.2 | 2.63 ± 0.2 | 6.73 ± 0.1 | 7.64 ± 0.1 | 283.4 ± 5.8 | |
| KH10 | 0 | 34.5 ± 3.4 | 5.65 ± 0.7 | 2.42 ± 0.0 | 1.24 ± 0.0 | 167.6 ± 3.4 |
| 28 | 78.5 ± 2.2 | 4.63 ± 0.2 | 3.84 ± 0.0 | 2.83 ± 0.0 | 211.4 ± 4.3 | |
| 40 | 94.9 ± 1.5 | 3.12 ± 0.1 | 5.56 ± 0.0 | 6.25 ± 0.0 | 253.9 ± 5.2 | |
| 80 | 94.6 ± 1.6 | 2.78 ± 0.1 | 6.84 ± 0.1 | 7.47 ± 0.1 | 274.8 ± 6.7 | |
| LSD0.05 | 3.16 | 0.14 | 0.30 | 0.10 | 13.13 |
| Ultrasonication Treatment | Total Polyphenols | Total Flavonoids | Total Sapanins | FRAP | DPPH | Total Polyphenols | |
|---|---|---|---|---|---|---|---|
| Variety | (kHz) | (mg gallic acid g−1 F.W.) | (mg CAE g−1 F.W.) | (mg g−1 F.W.) | (mmol Trolox 100 g−1 F.W.) | (% inhibition at 100 ug mL−1) | (mg gallic acid g−1 F.W.) |
| KH8 | 0 | 4.34 ± 0.04 | 1.13 ± 0.01 | 12.43 ± 0.10 | 8.22 ± 0.07 | 60.01 ± 1.2 | 4.34 ± 0.04 |
| 28 | 6.62 ± 0.04 | 1.65 ± 0.01 | 15.10 ± 0.09 | 11.53 ± 0.09 | 62.38 ± 2.6 | 6.62 ± 0.04 | |
| 40 | 8.44 ± 0.06 | 1.60 ± 0.01 | 17.22 ± 0.11 | 13.93 ± 0.10 | 65.68 ± 1.2 | 8.44 ± 0.06 | |
| 80 | 5.89 ± 0.05 | 1.44 ± 0.02 | 15.49 ± 0.13 | 12.29 ± 0.06 | 61.01 ± 1.3 | 5.89 ± 0.05 | |
| KH10 | 0 | 3.76 ± 0.03 | 1.49 ± 0.01 | 10.33 ± 0.08 | 7.06 ± 0.05 | 58.37 ± 1.2 | 3.76 ± 0.03 |
| 28 | 7.04 ± 0.06 | 2.64 ± 0.02 | 15.53 ± 0.13 | 12.46 ± 0.10 | 62.66 ± 1.3 | 7.04 ± 0.06 | |
| 40 | 8.89 ± 0.06 | 2.48 ± 0.02 | 18.45 ± 0.12 | 14.66 ± 0.09 | 64.50 ± 1.1 | 8.89 ± 0.06 | |
| 80 | 8.86 ± 0.07 | 2.04 ± 0.03 | 19.40 ± 0.16 | 15.70 ± 0.13 | 68.79 ± 1.7 | 8.86 ± 0.07 | |
| LSD0.05 | 0.28 | 0.13 | 1.01 | 0.41 | 3.45 | 0.28 |
| Ultrasonication Treatment | Total Aerobic Bacterial Count | Total Coliforms Count | Total Mould Count | |
|---|---|---|---|---|
| Variety | (kHz) | (log10 CFU g−1 fresh weight) | ||
| KH8 | 0 | 9.22 ± 1.74 | 6.29 ± 0.87 | 7.46 ± 0.43 |
| 28 | 4.78 ± 0.63 | 2.38 ± 0.14 | 3.61 ± 0.23 | |
| 40 | 3.79 ± 0.18 | 1.48 ± 0.08 | 2.76 ± 0.03 | |
| 80 | 3.28 ± 0.07 | 0.10 ± 0.14 | 2.15 ± 0.11 | |
| KH10 | 0 | 11.81 ± 1.83 | 7.35 ± 0.48 | 8.12 ± 0.46 |
| 28 | 5.88 ± 0.74 | 3.69 ± 0.09 | 4.91 ± 0.34 | |
| 40 | 4.13 ± 0.23 | 2.59 ± 0.24 | 2.69 ± 0.13 | |
| 80 | 3.93 ± 0.17 | 2.37 ± 0.33 | 2.33 ± 0.24 | |
| LSD0.05 | 0.22 | 0.15 | 0.18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, K.-Y. Changes in Microstructure, Germination, Sprout Growth, Phytochemical and Microbial Quality of Ultrasonication Treated Adzuki Bean Seeds. Agronomy 2021, 11, 1093. https://doi.org/10.3390/agronomy11061093
Chiu K-Y. Changes in Microstructure, Germination, Sprout Growth, Phytochemical and Microbial Quality of Ultrasonication Treated Adzuki Bean Seeds. Agronomy. 2021; 11(6):1093. https://doi.org/10.3390/agronomy11061093
Chicago/Turabian StyleChiu, Kai-Ying. 2021. "Changes in Microstructure, Germination, Sprout Growth, Phytochemical and Microbial Quality of Ultrasonication Treated Adzuki Bean Seeds" Agronomy 11, no. 6: 1093. https://doi.org/10.3390/agronomy11061093
APA StyleChiu, K.-Y. (2021). Changes in Microstructure, Germination, Sprout Growth, Phytochemical and Microbial Quality of Ultrasonication Treated Adzuki Bean Seeds. Agronomy, 11(6), 1093. https://doi.org/10.3390/agronomy11061093





