Ovaries of Chrysanthemum Irradiated with High-Energy Photons and High-Energy Electrons Can Regenerate Plants with Novel Traits
Abstract
1. Introduction
1.1. Classical Mutation Breeding in Modern Horticulture
1.2. Mutagenic Treatment
1.3. The Impact of Explant in Mutation Breeding
2. Materials and Methods
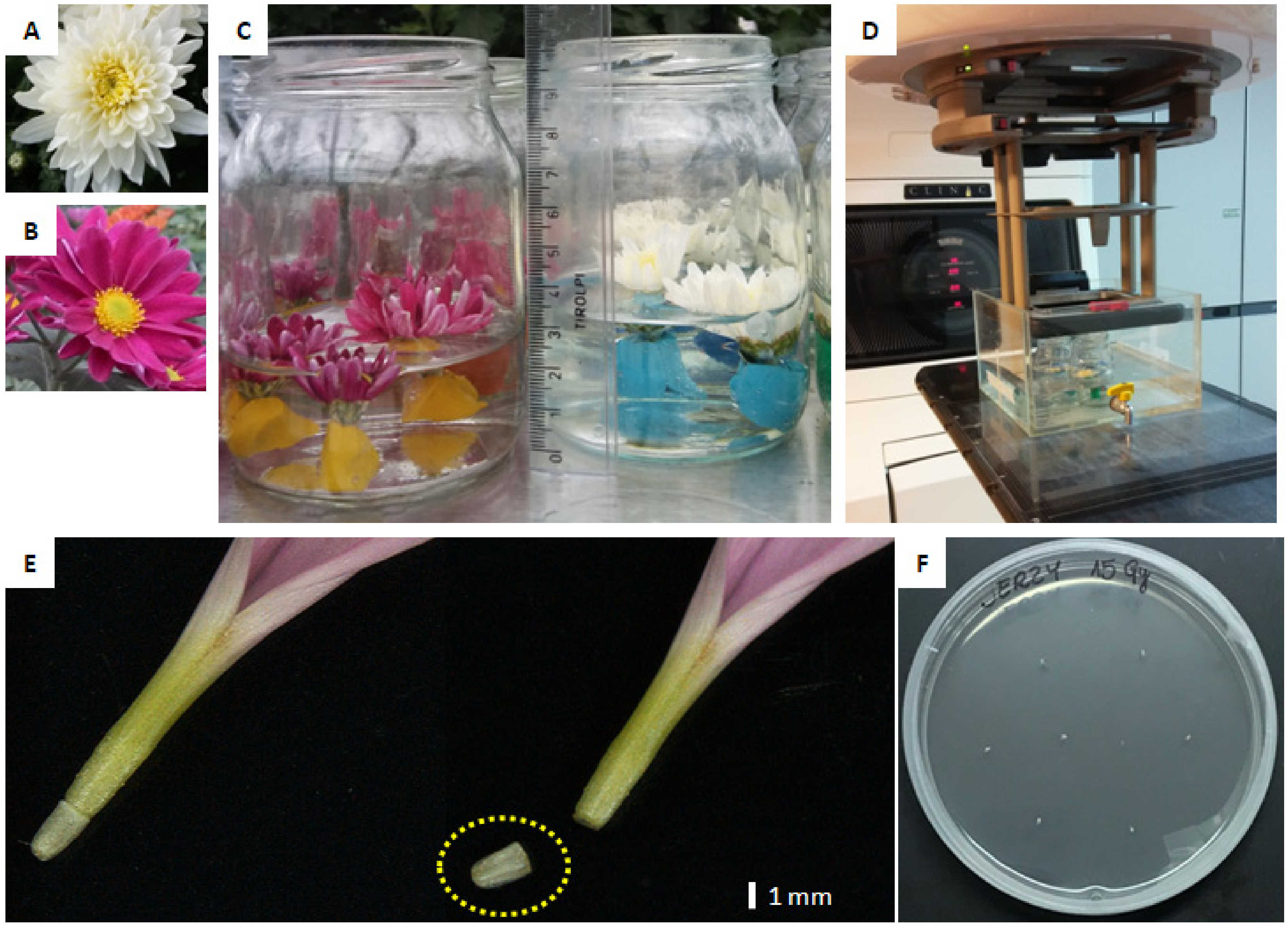
2.1. Plant Material
2.2. Irradiation Treatments
2.3. In Vitro Regeneration from Ovaries
2.4. Acclimatization and Greenhouse Cultivation of Regenerants
2.5. Flow Cytometric Measurements
2.6. Statistical Analyses
3. Results
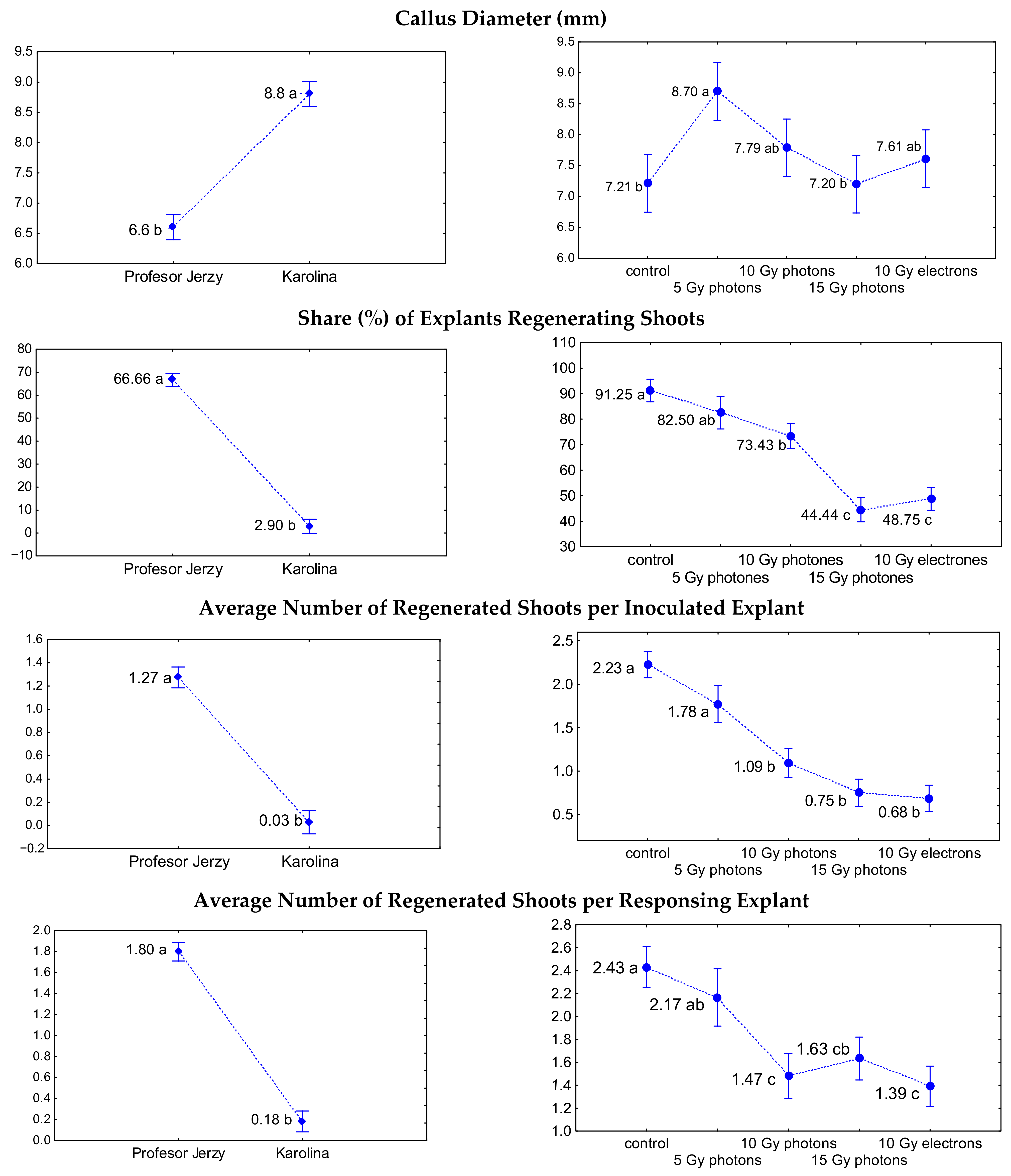
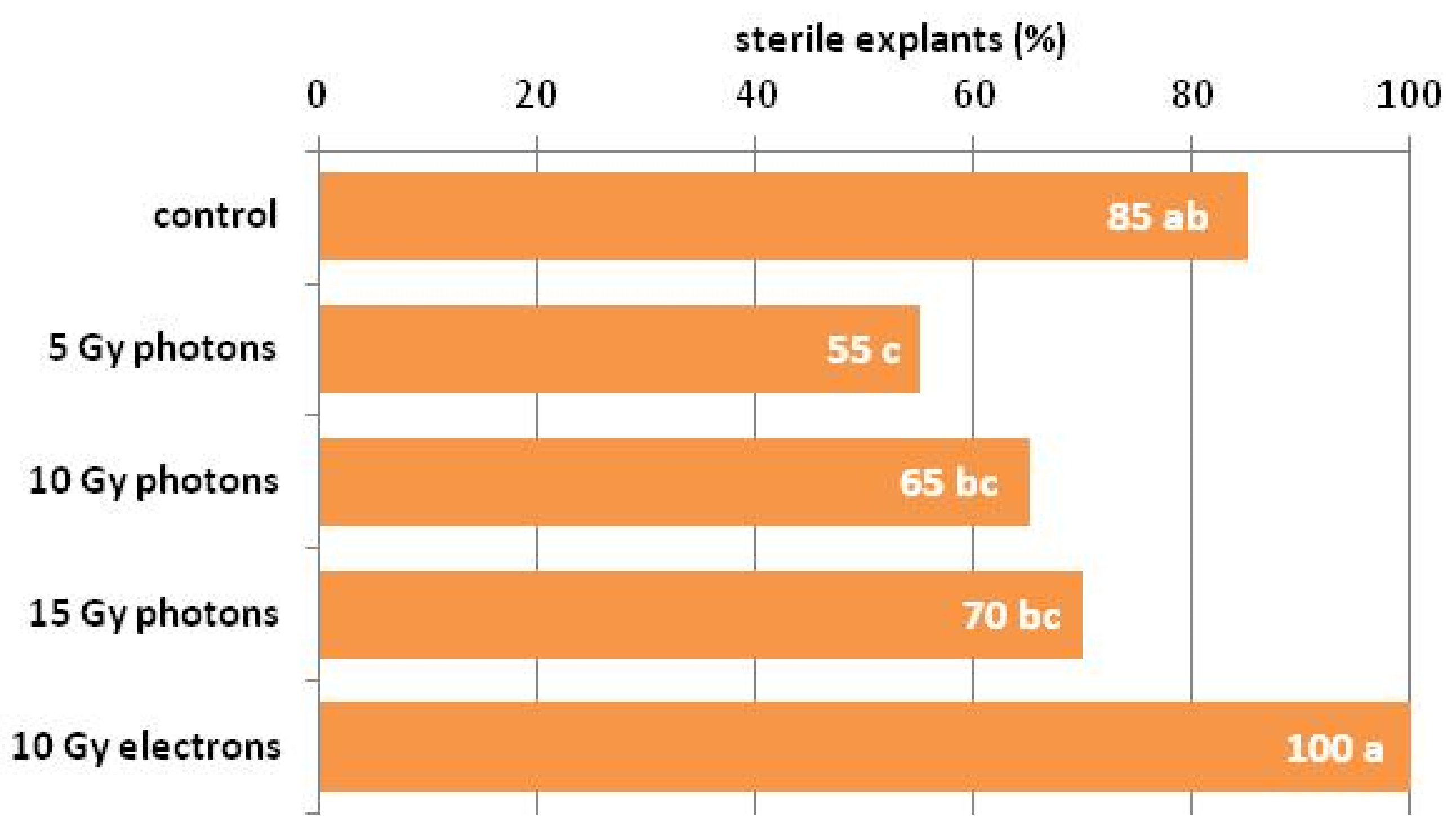
3.1. The Influence of Irradiation Dose and Type on In Vitro Regeneration from Ovaries
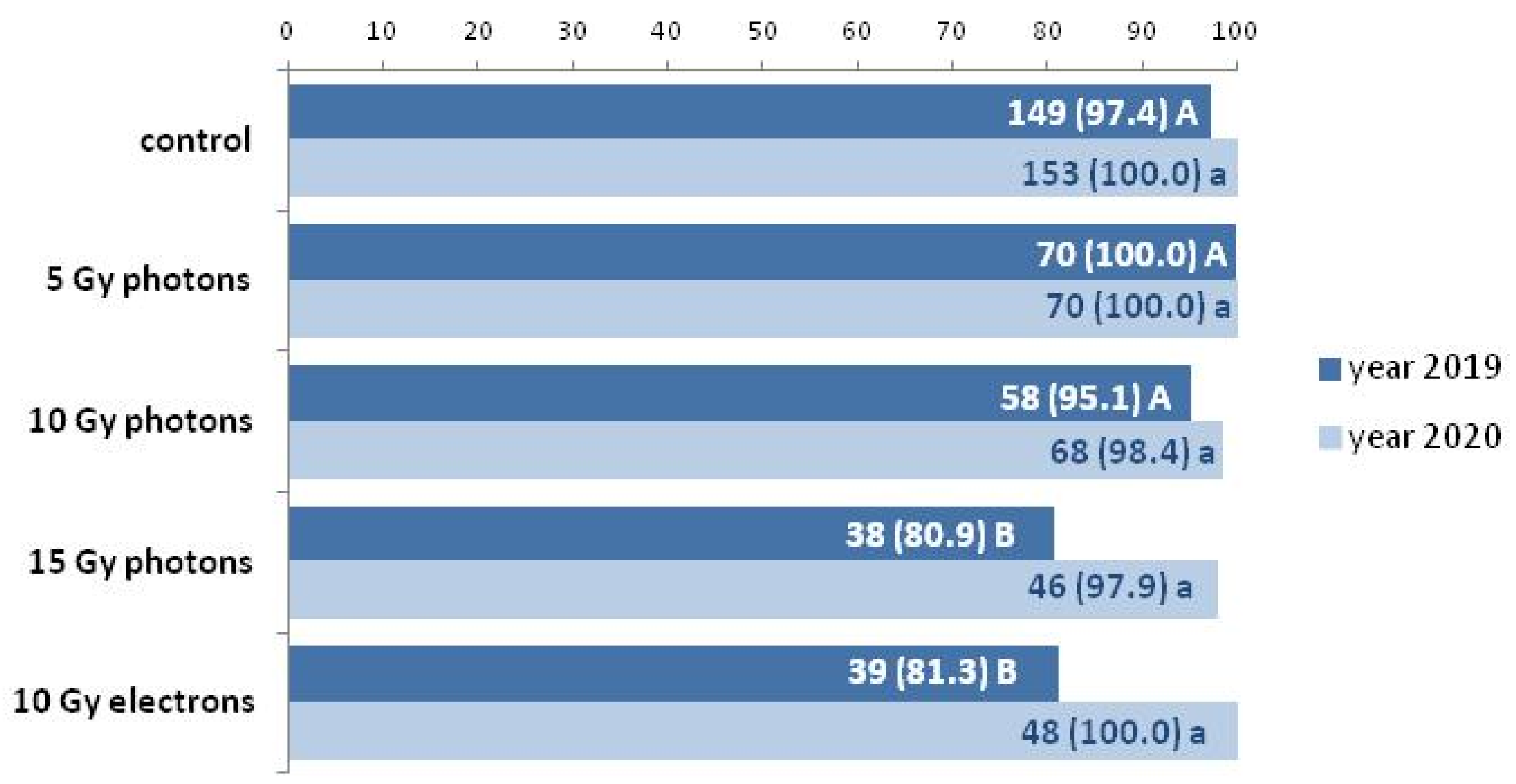
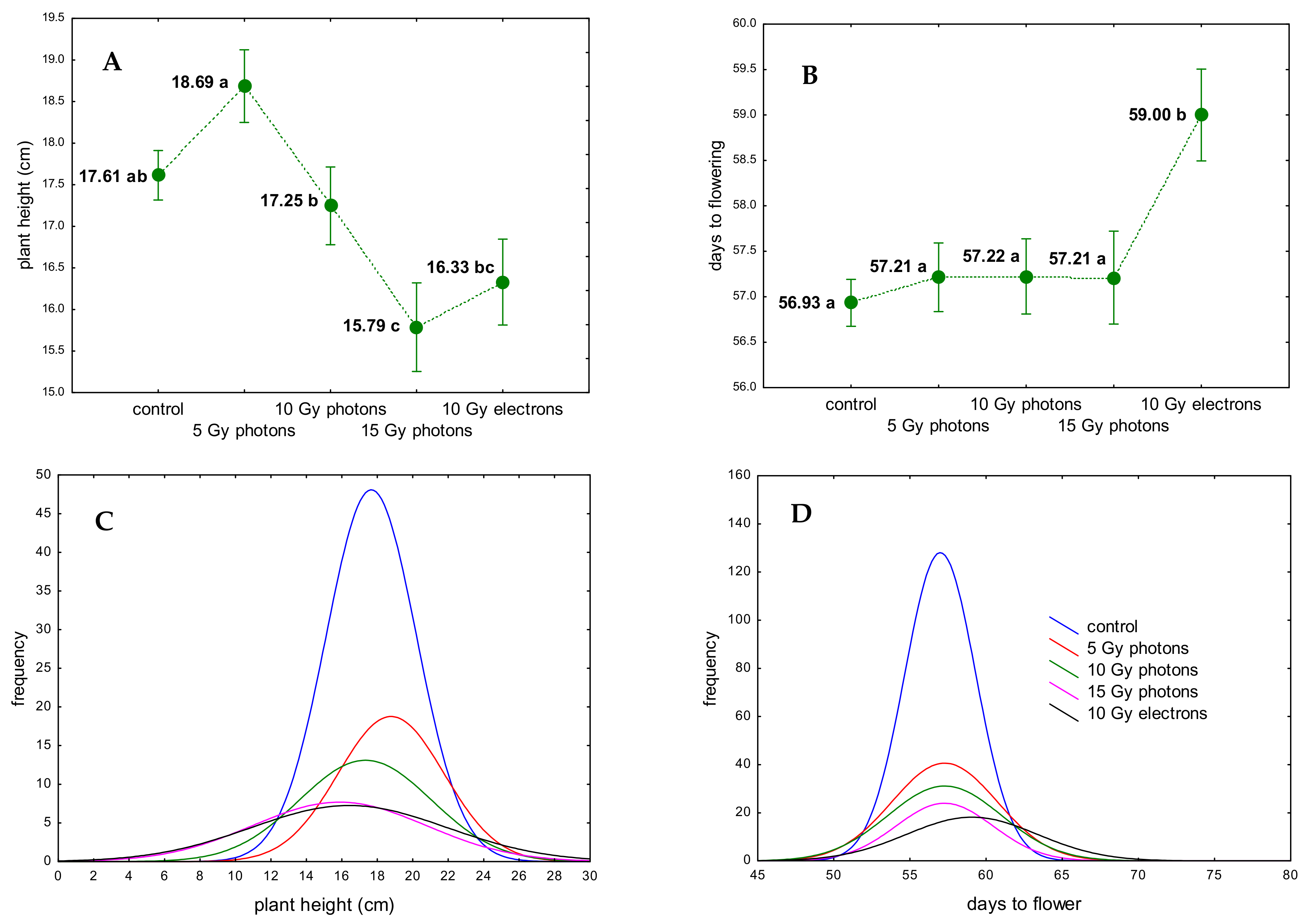
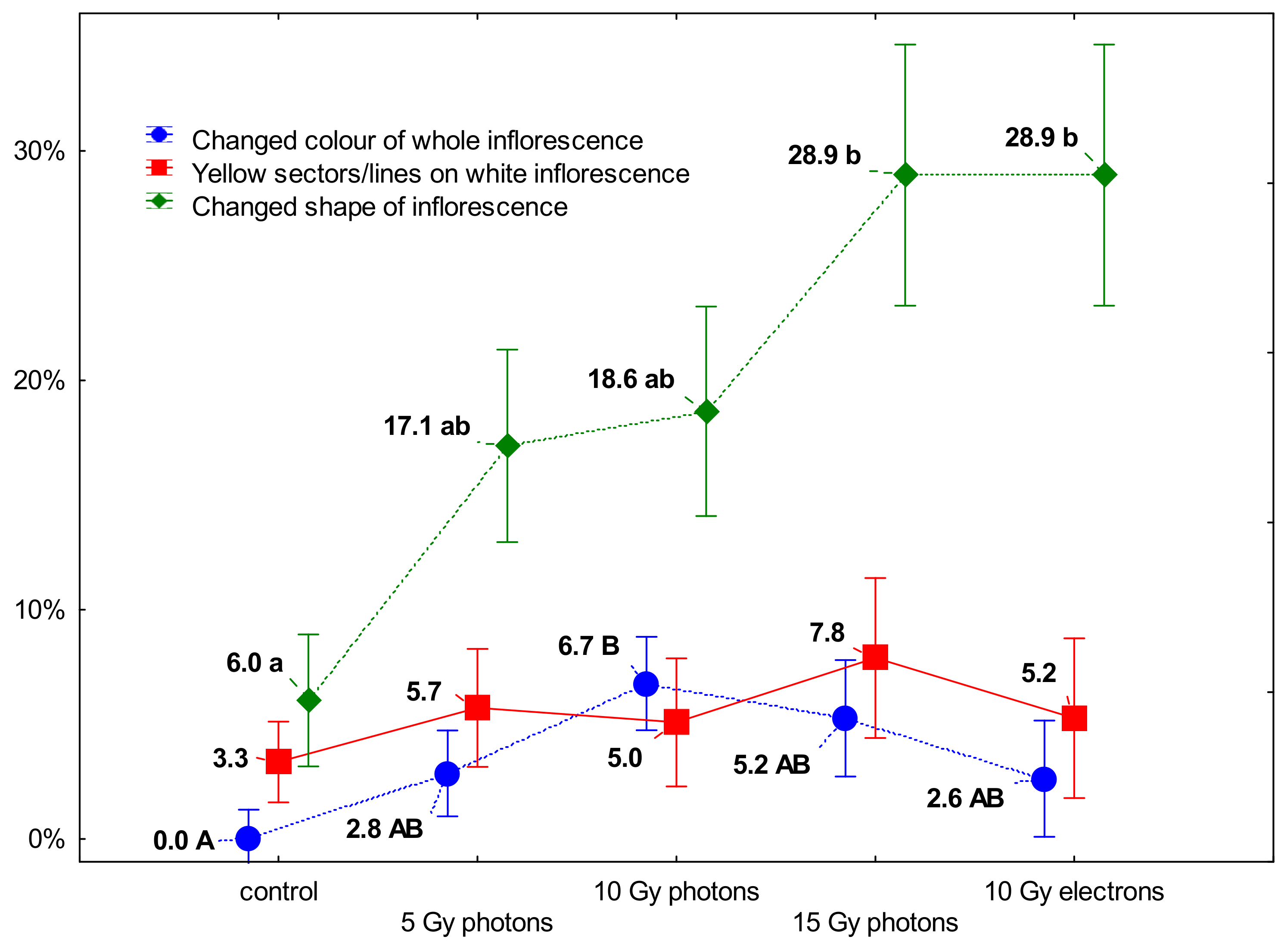
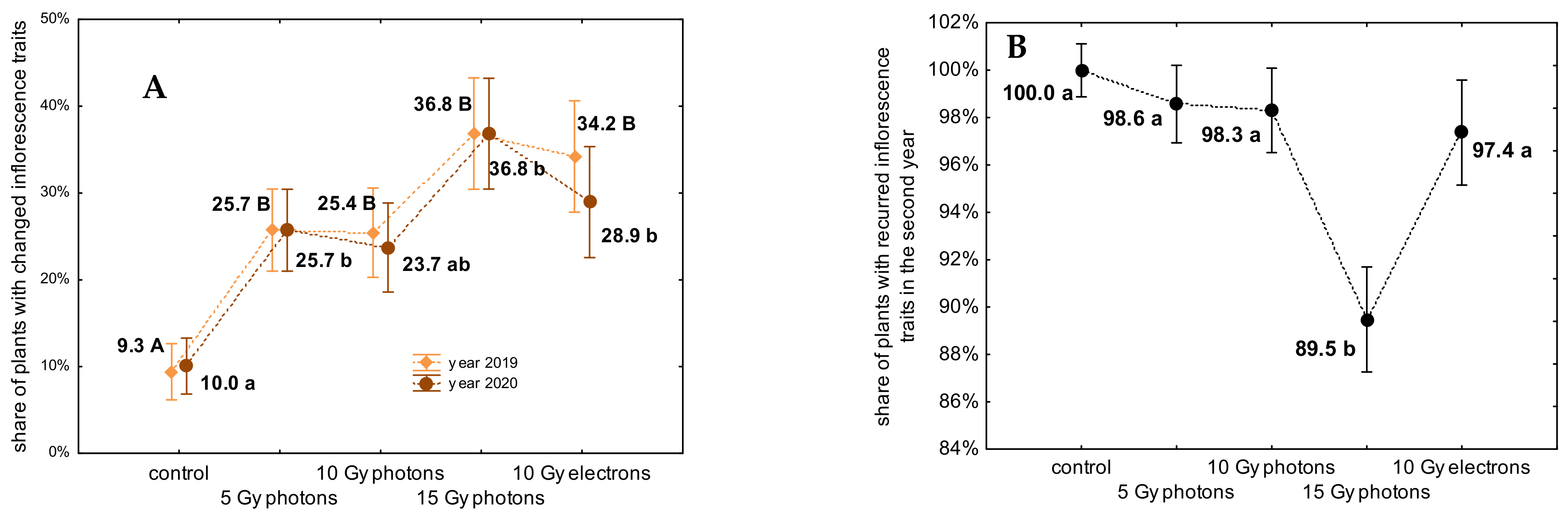
3.2. The Influence of Irradiation Dose and Type on Greenhouse Growth and Development of Ex Vitro Derived Chrysanthemum Plants
3.3. 2C DNA Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holme, I.B.; Gregersen, P.L.; Brinch-Pedersen, H. Induced Genetic Variation in Crop Plants by Random or Targeted Mutagenesis: Convergence and Differences. Front Plant Sci. 2019, 10, 1468. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.K. Induced mutations: Technological advancement for development of new ornamental varieties. Nucleus 2020, 63, 119–129. [Google Scholar] [CrossRef]
- Jo, Y.D.; Kim, J.-B. Frequency and Spectrum of Radiation-Induced Mutations Revealed by Whole-Genome Sequencing Analyses of Plants. Quantum Beam Sci. 2019, 3, 7. [Google Scholar] [CrossRef]
- Shelake, R.M.; Pramanik, D.; Kim, J.Y. Evolution of plant mutagenesis tools: A shifting paradigm from random to targeted genome editing. Plant Biotechnol. Rep. 2019, 13, 423–445. [Google Scholar] [CrossRef]
- Spaargaren, J.; Van Geest, G. Chrysanthemum. In Ornamental Crops, Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 11, pp. 319–348. [Google Scholar]
- Community Plant Variety Office (CPVO). Online Database for Registered Cultivars. Available online: https://cpvo.europa.eu/en/applications-and-examinations/cpvo-variety-finder (accessed on 22 May 2021).
- Protocol for Distinctness, Uniformity and Stability Test for Chrysanthemum × morifolium (Ramat.), Community Plant Variety Office (CPVO). 2008. Available online: https://cpvo.europa.eu/sites/default/files/documents/chrysanthemum_2.pdf (accessed on 22 May 2021).
- Kulus, D. Selected aspects of ornamental plants micropropagation in Poland and worldwide. Life Sci. 2015, 4, 1. [Google Scholar] [CrossRef]
- Latado, R.R.; Adames, A.H.; Neto, A.T. In vitro Mutation of Chrysanthemum (Dendranthema grandiflora Tzvelev) with Ethylmethanesulphonate (EMS) in Immature Floral Pedicels. Plant Cell Tiss. Organ Cult. 2004, 77, 103–106. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Sun, H.-Y.; Ji, R.-R.; Hu, X.-H.; Sui, J.-M.; Qiao, L.-X.; Chen, J.; Wang, J.-S. In vitro mutagenesis and directed screening for salt-tolerant mutants in peanut. Euphytica 2013, 193, 89–99. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Shimizu, A.; Hase, Y.; Tanaka, A.; Shikazono, N.; Degi, K.; Morishita, T. Effects of ion beam irradiation on mutation induction and nuclear DNA content in chrysanthemum. Breed. Sci. 2010, 60, 398–404. [Google Scholar] [CrossRef]
- Ulukapi, K.; Ozmen, S.F. Study of the effect of irradiation (60Co) on M1 plants of common bean (Phaseolus vulgaris L.) cultivars and determined of proper doses for mutation breeding. J. Rad. Res. App. Sci. 2018, 11, 157–161. [Google Scholar] [CrossRef]
- Ibrahim, R.; Ahmad, Z.; Salleh, S.; Hassan, A.A.; Ariffin, S. Mutation Breeding in Ornamentals. In Ornamental Crops. Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Dordrecht, The Netherlands, 2018; pp. 175–211. [Google Scholar] [CrossRef]
- Miler, N.; Kulus, D. Microwave treatment can induce chrysanthemum phenotypic and genetic changes. Sci. Hortic. 2018, 227, 223–233. [Google Scholar] [CrossRef]
- Slater, J.M. From X-rays to Ion Beams: A Short History of Radiation Therapy. In Ion Beam Therapy. Biological and Medical Physics, Biomedical Engineering; Linz, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–13. [Google Scholar] [CrossRef]
- Kokurewicz, K.; Brunetti, E.; Welsh, G.H.; Wiggins, S.M.; Boyd, M.; Sorensen, A.; Chalmers, A.J.; Schettino, G.; Subiel, A.; Desrosiers, C.; et al. Focused very high-energy electron beams as a novel radiotherapy modality for producing high-dose volumetric elements. Sci. Rep. 2019, 9, 10837. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.J. Sterilisation procedures for tissue allografts. In Standardisation in Cell and Tissue Engineering; Woodhead Publishing Series in Biomaterials; Salih, V., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 197–211. [Google Scholar] [CrossRef]
- Gerbi, B.J. Clinical Applications of High-Energy Electrons. In Technical Basis of Radiation Therapy. Medical Radiology (Radiation Oncology); Levitt, S.H., Purdy, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 135–164. [Google Scholar] [CrossRef]
- Kaul, A.; Kumar, S.; Ghani, M. In vitro mutagenesis and detection of variability among radiomutants of chrysanthemum using RAPD. Adv. Hortic. Sci. 2011, 25, 106–111. [Google Scholar]
- Van Harten, A.M. Mutation Breeding. Theory and Practical Applications; Cambridge University Press: Cambridge, UK, 1998; p. 353. [Google Scholar]
- Broertjes, C.; Roest, S.; Bokelmann, G.S. Mutation breeding of Chrysanthemum morifolium Ram. using in vivo and in vitro adventitious bud techniques. Euphytica 1976, 25, 11–19. [Google Scholar] [CrossRef]
- Cassells, A.C. Tissue Culture for Ornamental Breeding. In Breeding for Ornamentals: Classical and Molecular Approaches; Vainstein, A., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 139–153. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Lema-Rumińska, J.; Tymoszuk, A.; Kulpa, D. Regeneration from chrysanthemum flowers: A review. Acta Physiol. Plant. 2015, 37, 36. [Google Scholar] [CrossRef]
- Kang, E.; Lee, Y.; Sung, S.; Ha, B.; Kim, S.; Kim, D.; Kim, J.; Kang, S. Analysis of the genetic relationship of gamma-irradiated in vitro mutants derived from standard-type chrysanthemum cv. Migok. Hortic. Environ. Biotechnol. 2013, 54, 76–81. [Google Scholar] [CrossRef]
- Sun, C.-Q.; Chen, F.-D.; Teng, N.-J.; Liu, Z.-L.; Fang, W.-M.; Hou, X.-L. Factors affecting seed set in the crosses between Dendranthema grandiflorum (Ramat.) Kitamura and its wild species. Euphytica 2010, 171, 181–192. [Google Scholar] [CrossRef]
- Bohanec, B. Doubled Haploids via Gynogenesis. In Advances in Haploid Production in Higher Plants; Touraev, A., Forster, B.P., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 35–46. [Google Scholar] [CrossRef]
- Wang, H.; Dong, B.; Jiang, J.; Fang, W.; Guan, Z.; Liao, Y.; Chen, S.; Chen, F. Characterization of in vitro haploid and doubled haploid Chrysanthemum morifolium plants via unfertilized ovule culture for phenotypical traits and DNA methylation pattern. Front. Plant Sci. 2014, 5, 738. [Google Scholar] [CrossRef]
- Miler, N.; Muszczyk, P. Regeneration of callus and shoots from the ovules and ovaries of chrysanthemum in vitro. Acta Hortic. 2015, 1083, 103–106. [Google Scholar] [CrossRef]
- Miler, N.; Jedrzejczyk, I. Chrysanthemum plants regenerated from ovaries: A study on genetic and phenotypic variation. Turk. J. Bot. 2018, 42, 289–297. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jedrzejczyk, I.; Sliwinska, E. Leaves and seeds as materials for flowcytometric estimation of the genome size of 11 Rosaceae woody species containing DNA-staining inhibitors. J. Bot. 2010, 2010, 930895. [Google Scholar]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, J.; Sgorbati, S.; Lucretti, S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- Sliwinska, E. Flow cytometry—A modern method for exploring genome size and nuclear DNA synthesis in horticultural and medicinal plant species. Folia Hortic. 2018, 30, 103–128. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Britt, A.B.; Tripathi, L.; Sharma, S.; Upadhyaya, H.D.; Ortiz, R. Haploids: Constraints and opportunities in plant breeding. Biotechnol Adv. 2015, 33, 812–829. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Dobránszki, J. Dissecting the Concept of the Thin Cell Layer: Theoretical Basis and Practical Application of the Plant Growth Correction Factor to Apple, Cymbidium and Chrysanthemum. J. Plant Growth Regul. 2014, 33, 881–895. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Fukai, S. Chrysanthemum Organogenesis Through Thin Cell Layer Technology and Plant Growth Regulator Control. Asian J. Plant Sci. 2003, 2, 505–514. [Google Scholar] [CrossRef][Green Version]
- Jain, M.S. Mutation-assisted breeding for improving ornamental plants. Acta Hortic. 2006, 714, 85–98. [Google Scholar] [CrossRef]
- Datta, S.K.; Misra, P.; Mandal, A.K.A.; Chakrabarty, D. Direct shoot organogenesis from different explants of chrysanthemum, marigold, and tuberose. Israel J. Plant Sci. 2002, 50, 287–291. [Google Scholar] [CrossRef]
- Miler, N.; Zalewska, M. Somaclonal variation of chrysanthemum propagated in vitro from different explant types. Acta Sci. Pol. Hortorum Cultus 2014, 13, 69–82. [Google Scholar]
- Da Silva, J.A.T.; Kulus, D. Chrysanthemum biotechnology: Discoveries from the recent literature. Folia Hortic. 2014, 26, 67–77. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Datta, S.K. Management of chimera and in vitro mutagenesis for development of new flower colour/shape and chlorophyll variegated mutants in chrysanthemum. In Floriculture: Role of Tissue Culture and Molecular Techniques; Datta, S.K., Chakrabarty, D., Eds.; Pointer Publishers: Jaipur, India, 2010; pp. 157–164. [Google Scholar]
- Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R. Stochastic, Deterministic, and Heritable Effects (and Some Radiation Protection Basics). In Basic Radiotherapy Physics and Biology; Chang, D.S., Lasley, F.D., Das, I.J., Mendonca, M.S., Dynlacht, J.R., Eds.; Springer Science+Business Media: New York, NY, USA, 2021; pp. 337–348. [Google Scholar] [CrossRef]
- Valledor, L.; Hasbun, R.; Meijón, M.; Rodríguez, J.L.; Santamaría, E.; Viejo, M.; Berdasco, M.; Feito, I.; Fraga, M.F.; Canal, M.J.; et al. Involvement of DNA methylation in tree development and micropropagation. Plant Cell Tissue Organ Cult. 2007, 91, 75–86. [Google Scholar] [CrossRef]
- Park, S.Y.; Murthy, H.N.; Chakrabarthy, D.; Paek, K.Y. Detection of epigenetic variation in tissue-culture-derived plants of Doritaenopsis by methylation-sensitive amplification polymorphism (MSAP) analysis. Vitro Cell. Dev. Biol. Plant 2009, 45, 104–108. [Google Scholar] [CrossRef]
- Lu, C.; Li, Y.; Wang, J.; Qu, J.; Chen, Y.; Chen, X.; Huang, H.; Dai, S. Flower color classification and correlation between color space values with pigments in potted multiflora chrysanthemum. Sci. Hortic. 2021, 283, 110082. [Google Scholar] [CrossRef]
- Lema-Rumińska, J.; Zalewska, M. Changes in flower colour among Lady Group of Chrysanthemum × grandiflorum/Ramat./Kitam. as a result of mutation breeding. Folia Hortic. 2005, 17, 61–72. [Google Scholar]
- Zalewska, M.; Lema-Rumińska, J.; Miler, N. In vitro propagation using adventitious buds technique as a source of new variability in chrysanthemum. Sci. Hortic. 2007, 113, 70–73. [Google Scholar] [CrossRef]
- Kengkarj, P.; Smitamana, P.; Fujime, Y. Assessment of somaclonal variation in chrysanthemum (Dendranthema grandiflora Kitam.) using RAPD and morphological analysis. Plant Tissue Cult. Biotechnol. 2008, 18, 139–149. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Shimizu, A.; Degi, K.; Morishita, T. Effects of dose and dose rate of gamma ray irradiation on mutation induction and nuclear DNA content in chrysanthemum. Breed. Sci. 2008, 58, 331–335. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hase, Y.; Tanaka, A.; Shikazono, N.; Degi, K.; Shimizu, A.; Morishita, T. Mutagenic effects of ion beam irradiation on rice. Breed. Sci. 2009, 59, 169–177. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Van Houtven, W.; Bruznican, S.; Leus, L.; Van Huylenbroeck, J. Somaclonal Variation in Chrysanthemum × morifolium Protoplast Regenerants. Front. Plant. Sci. 2020, 11, 2104. [Google Scholar] [CrossRef]
- Winiecki, J. Principles of radiation therapy. Phys. Sci. Rev. 2020, in press. [Google Scholar] [CrossRef]





| Type of Irradiation | ||
|---|---|---|
| Characteristic | High-Energy Photons | High-Energy Electrons |
| Device | Vitalbeam v. 2.7 | Clinac 2300CD Silhouette |
| Beam energy * | 6 MV | 6 MeV |
| Total dose delivered [Gy] | 5; 10; 15 | 10 |
| Dose rate [Gy min−1] | 3.19 | 1.42 |
| Source-surface distance (SSD) [cm] | 100 | 100 |
| Depth [cm] | 2 | 2 |
| Inflorescence Color | Control | 5 Gy Photons | 10 Gy Photons | 15 Gy Photons | 10 Gy Electrons |
|---|---|---|---|---|---|
| Year 2019 | |||||
| White | 100 (149) | 97.1 (68) | 93.1 (54) | 94.7 (36) | 97.4 (38) |
| Dark yellow | - | 2.9 (2) | 3.4 (2) | 2.6 (1) | - |
| Light yellow | - | - | 1.7 (1) | 2.6 (1) | - |
| Pinkish | - | - | 1.7 (1) | - | 2.6 (1) |
| Year 2020 | |||||
| White | 100 (153) | 97.1 (68) | 93.3 (56) | 93.5 (43) | 96.0 (50) |
| Dark yellow | - | 2.9 (2) | 3.3 (2) | 2.2 (1) | - |
| Light yellow | - | - | - | - | - |
| Pinkish | - | - | 3.3 (2) | 4.3 (2) | 4.0 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miler, N.; Jedrzejczyk, I.; Jakubowski, S.; Winiecki, J. Ovaries of Chrysanthemum Irradiated with High-Energy Photons and High-Energy Electrons Can Regenerate Plants with Novel Traits. Agronomy 2021, 11, 1111. https://doi.org/10.3390/agronomy11061111
Miler N, Jedrzejczyk I, Jakubowski S, Winiecki J. Ovaries of Chrysanthemum Irradiated with High-Energy Photons and High-Energy Electrons Can Regenerate Plants with Novel Traits. Agronomy. 2021; 11(6):1111. https://doi.org/10.3390/agronomy11061111
Chicago/Turabian StyleMiler, Natalia, Iwona Jedrzejczyk, Seweryn Jakubowski, and Janusz Winiecki. 2021. "Ovaries of Chrysanthemum Irradiated with High-Energy Photons and High-Energy Electrons Can Regenerate Plants with Novel Traits" Agronomy 11, no. 6: 1111. https://doi.org/10.3390/agronomy11061111
APA StyleMiler, N., Jedrzejczyk, I., Jakubowski, S., & Winiecki, J. (2021). Ovaries of Chrysanthemum Irradiated with High-Energy Photons and High-Energy Electrons Can Regenerate Plants with Novel Traits. Agronomy, 11(6), 1111. https://doi.org/10.3390/agronomy11061111








