The Influence of Environmental Factors on Seed Germination of Polygonum perfoliatum L.: Implications for Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Evaluation of Seed Dormancy Status and Seed Dormancy Release
2.3. General Procedure for Germination Tests
2.4. Impact of Constant Temperatures on Seed Germination
2.5. Impact of Alternating Day/Night Temperatures on Seed Germination
2.6. Effect of Light/Dark on Seed Germination
2.7. Impact of pH on Seed Germination
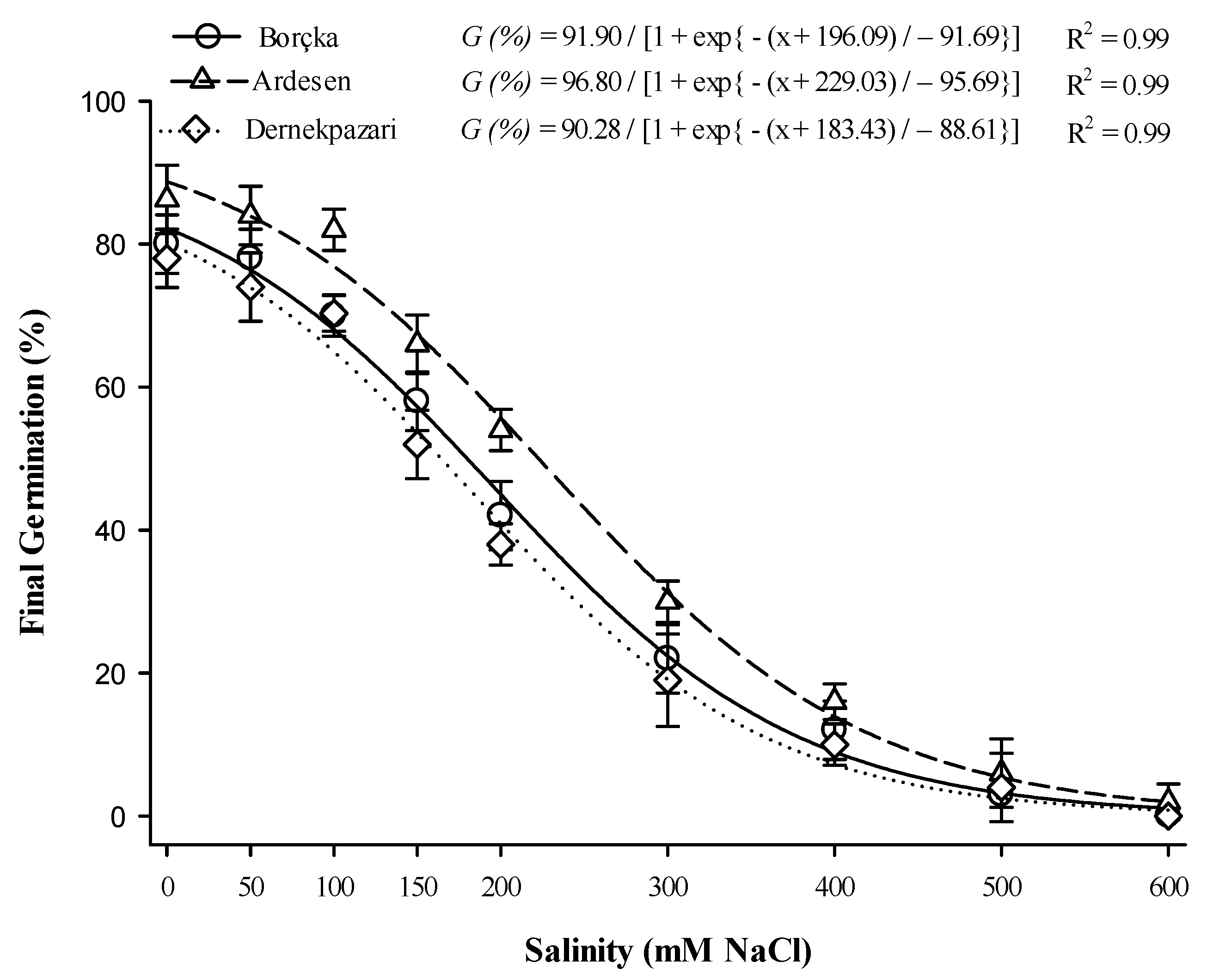
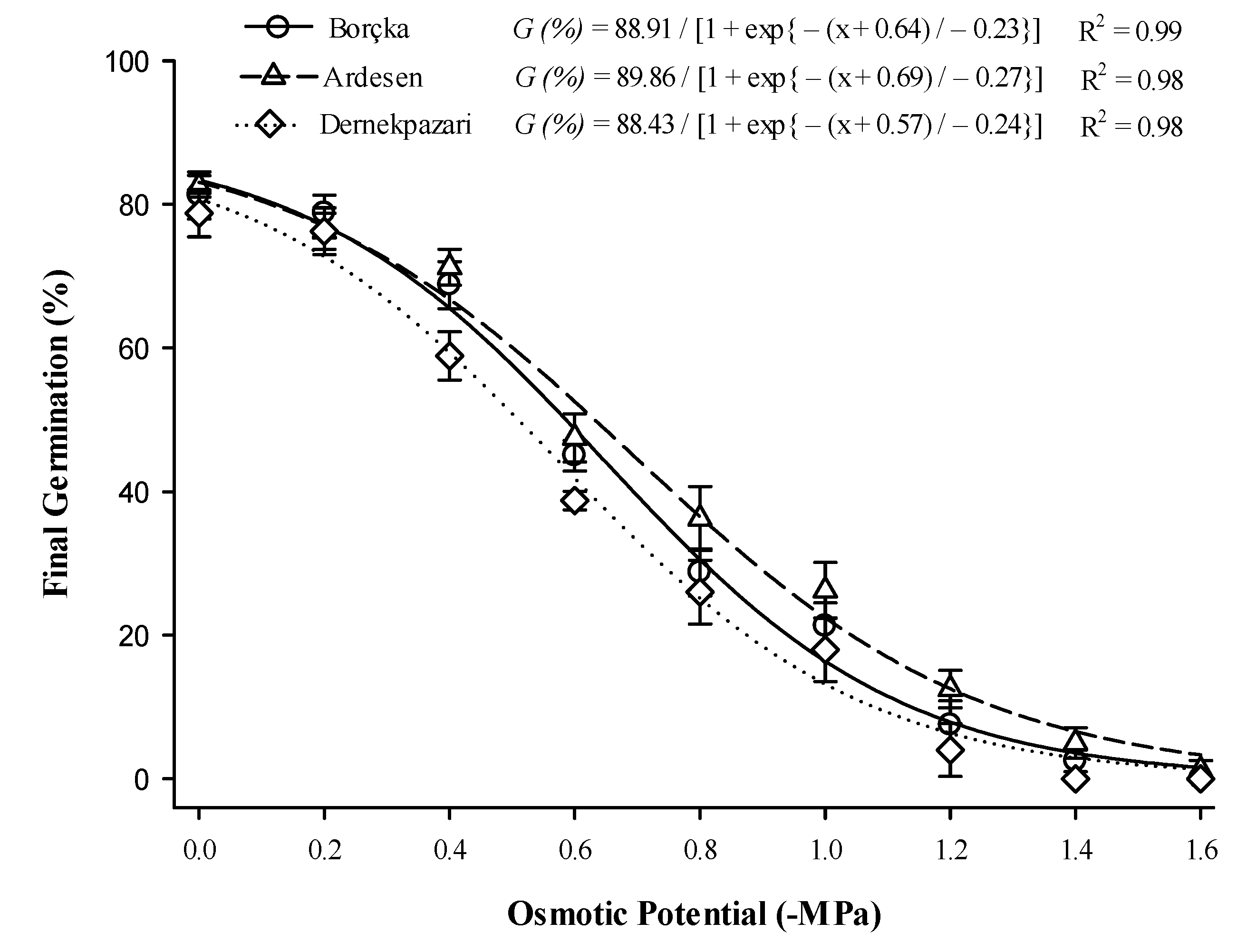
2.8. Impact of Osmotic and Salt Stress on Germination
2.9. Effect of Burial Depth on Seedling Emergence
2.10. Statistical Analysis
3. Results
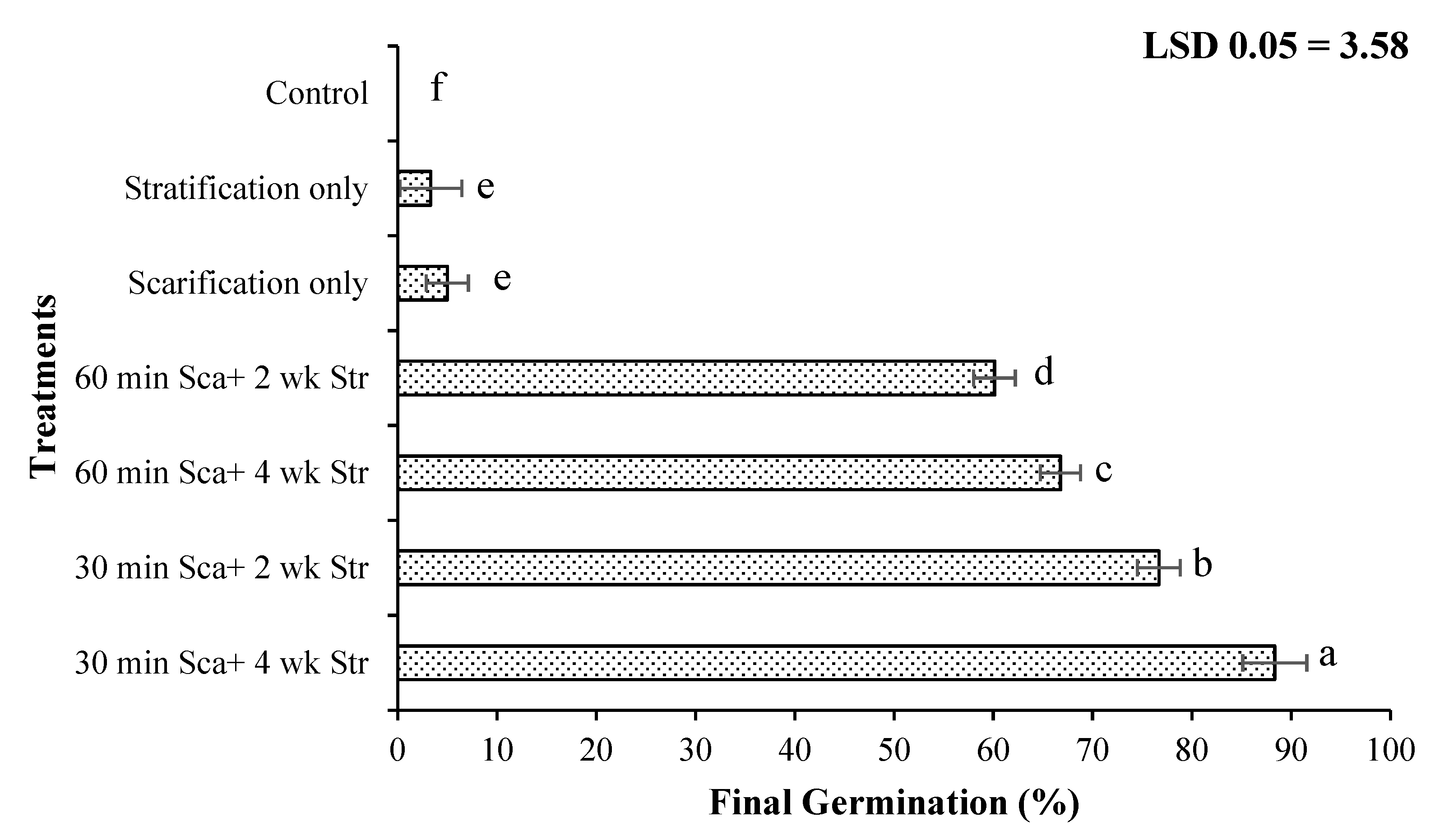
3.1. Seed Dormancy Release
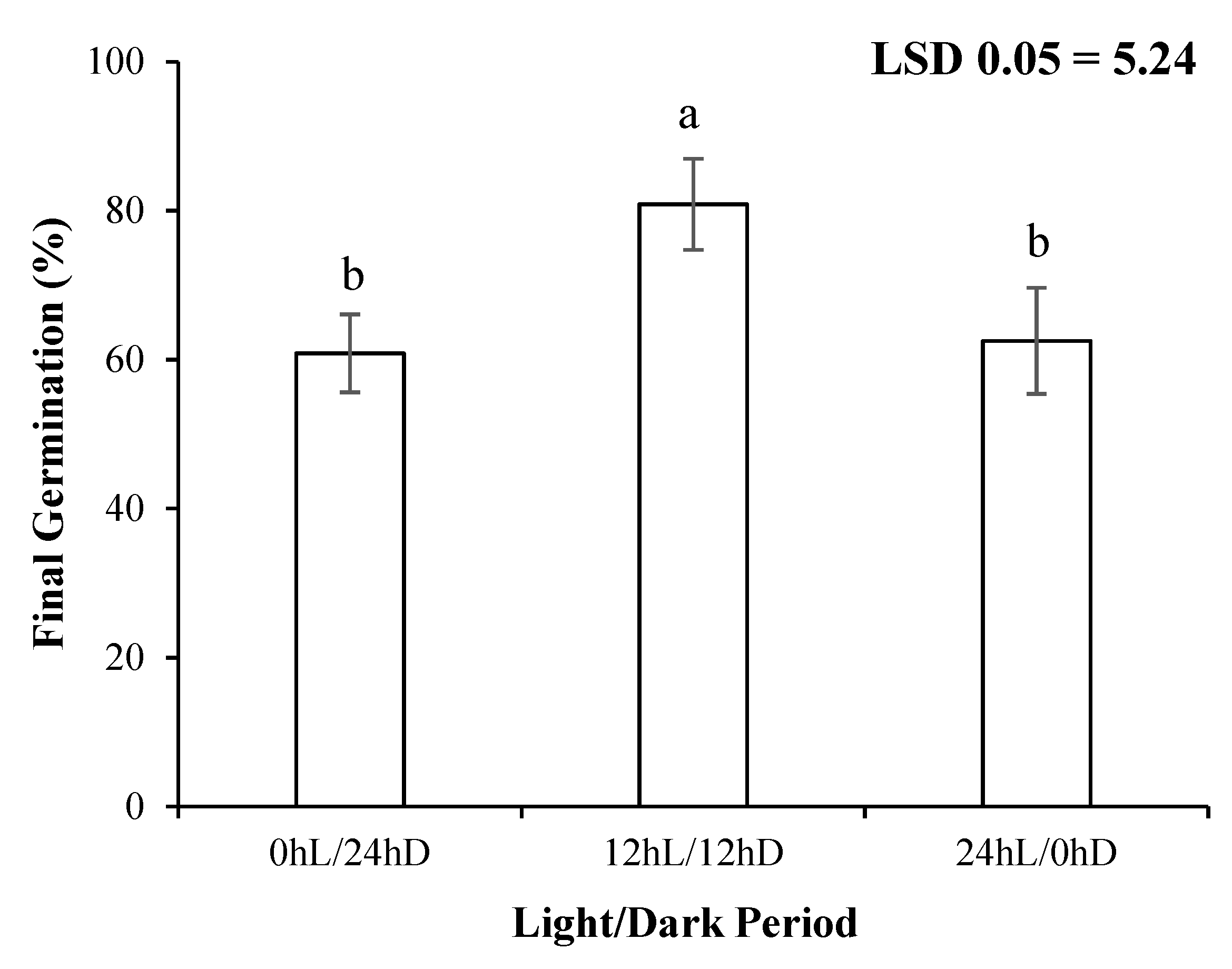
3.2. Light/Dark Regimes
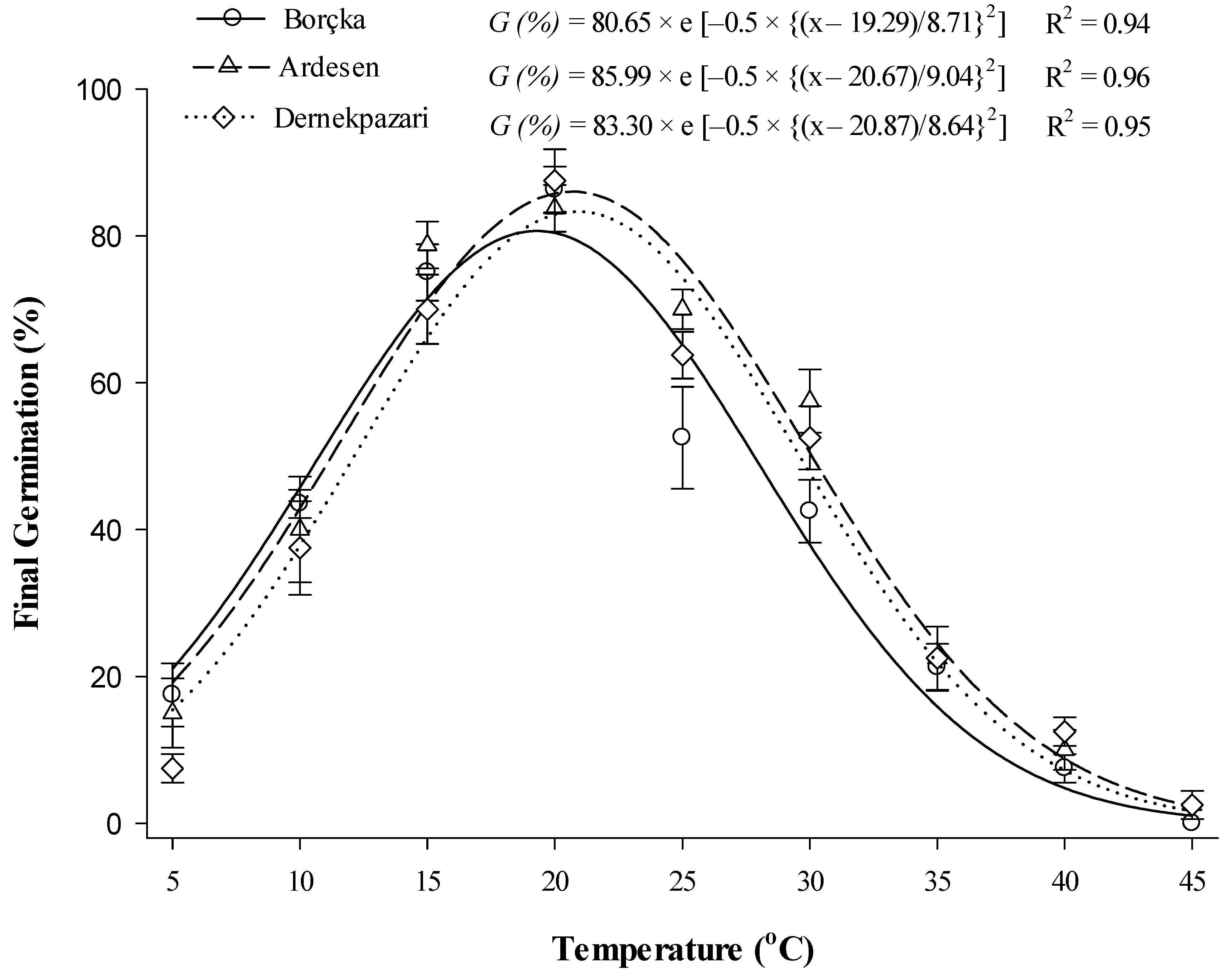
3.3. Constant Temperatures
3.4. Alternating Temperatures
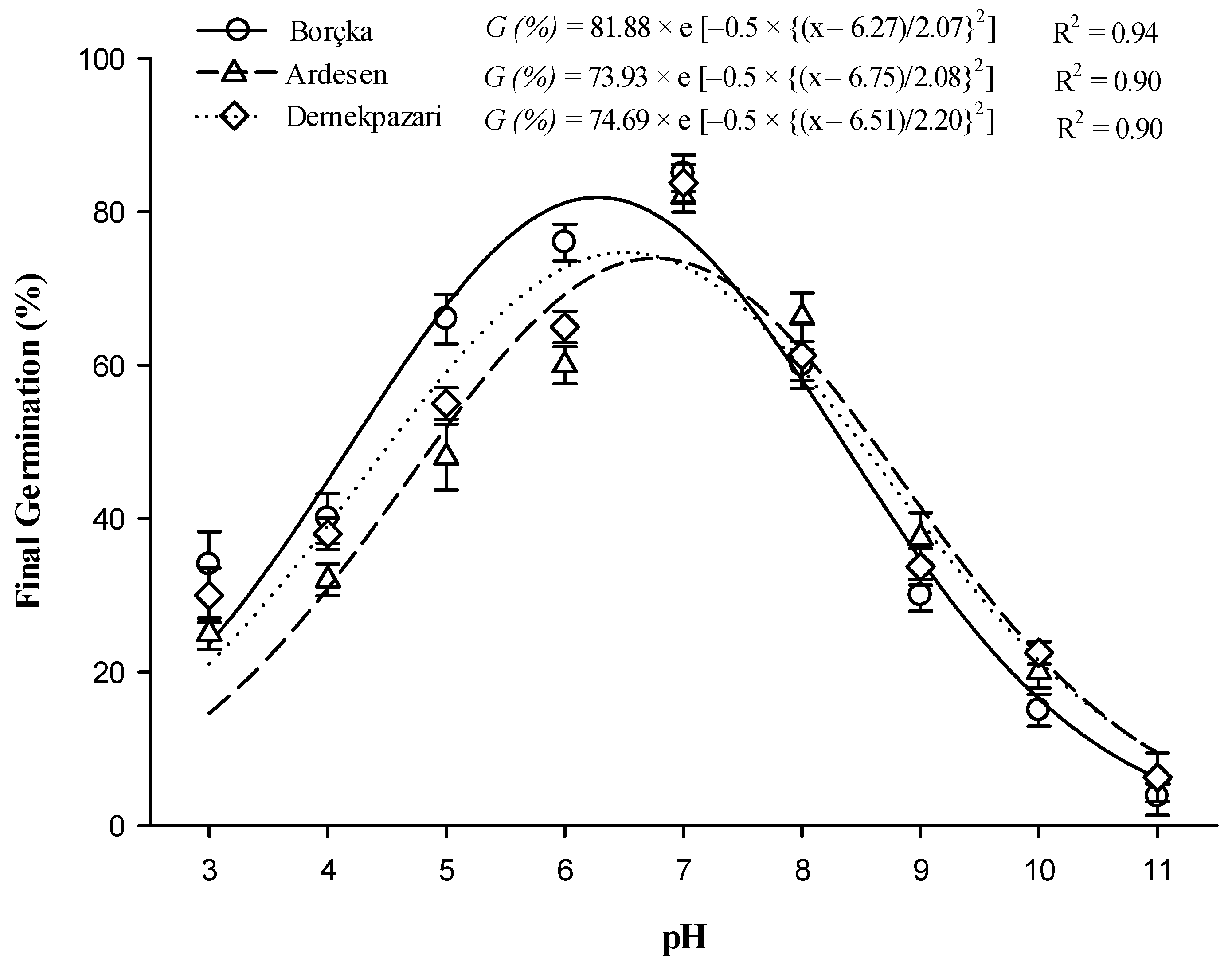
3.5. Soil Reaction/pH
3.6. Salinity and Osmotic Potential
3.7. Seedling Emergence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilà, M.; Basnou, C.; Pyšek, P.; Josefsson, M.; Genovesi, P.; Gollasch, S.; Nentwig, W.; Olenin, S.; Roques, A.; Roy, D.; et al. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. Bioscience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Sax, D.F.; Gaines, S.D. Species diversity: From global decreases to local increases. Trends Ecol. Evol. 2003, 18, 561–566. [Google Scholar] [CrossRef]
- Liu, Y.; van Kleunen, M. Responses of common and rare aliens and natives to nutrient availability and fluctuations. J. Ecol. 2017, 105, 1111–1122. [Google Scholar] [CrossRef]
- Jia, J.; Dai, Z.; Li, F.; Liu, Y. How will global environmental changes affect the growth of alien plants? Front. Plant Sci. 2016, 7, 1623. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, F.F.; Galloway, L.F. Adaptive divergence at the margin of an invaded range. Evolution 2013, 67, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, F.F.; Galloway, L.F. Evolution of marginal populations of an invasive vine increases the likelihood of future spread. New Phytol. 2016, 209, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Tad, S.; Onen, H.; Gunal, H.; Caldiran, U.; Ozaslan, C. Range expansion potential of two co-occurring invasive vines to marginal habitats in Turkey. Acta Oecol. 2017, 84, 23–33. [Google Scholar] [CrossRef]
- Travis, J.M.J.; Dytham, C. Dispersal evolution during invasions. Evol. Ecol. Res. 2002, 4, 1119–1129. [Google Scholar]
- Wolkovich, E.M.; Jonathan Davies, T.; Schaefer, H.; Cleland, E.E.; Cook, B.I.; Travers, S.E.; Willis, C.G.; Davis, C.C. Temperature-dependent shifts in phenology contribute to the success of exotic species with climate change. Am. J. Bot. 2013, 100, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Bykova, O.; Chuine, I.; Morin, X.; Higgins, S.I. Temperature dependence of the reproduction niche and its relevance for plant species distributions. J. Biogeogr. 2012, 39, 2191–2200. [Google Scholar] [CrossRef]
- Cochrane, A.; Yates, C.J.; Hoyle, G.L.; Nicotra, A.B. Will among-population variation in seed traits improve the chance of species persistence under climate change? Glob. Ecol. Biogeogr. 2015, 24, 12–24. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Bradford, K.J.; Huxman, T.E.; Venable, D.L. The contribution of germination functional traits to population dynamics of a desert plant community. Ecology 2016, 97, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; Volume 97, ISBN 0521653681. [Google Scholar]
- Ferreras, A.E.; Funes, G.; Galetto, L. The role of seed germination in the invasion process of Honey locust (Gleditsia triacanthos L., Fabaceae): Comparison with a native confamilial. Plant Species Biol. 2015, 30, 126–136. [Google Scholar] [CrossRef]
- Cochrane, A. Modelling seed germination response to temperature in Eucalyptus L’Her.(Myrtaceae) species in the context of global warming. Seed Sci. Res. 2017, 27, 99–109. [Google Scholar] [CrossRef]
- Awan, T.H.; Chauhan, B.S.; Sta Cruz, P.C. Influence of environmental factors on the germination of Urena lobata L. and its response to herbicides. PLoS ONE 2014, 9, e90305. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780124166837. [Google Scholar]
- Chauhan, B.S. Seed germination ecology of feather lovegrass [Eragrostis tenella (L.) Beauv. Ex Roemer & JA Schultes]. PLoS ONE 2013, 8, e79398. [Google Scholar]
- Chauhan, B.S. Germination biology of Hibiscus tridactylites in Australia and the implications for weed management. Sci. Rep. 2016, 6, 26006. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Skalicky, M.; Vachova, P.; Hajihashemi, S.; Jouini, L.; Zivcak, M.; Tlustos, P.; Brestic, M.; Hejnak, V.; Zoghlami Khelil, A. Comparing salt tolerance at seedling and germination stages in local populations of Medicago ciliaris L. to Medicago intertexta L. and Medicago scutellata L. Plants 2020, 9, 526. [Google Scholar] [CrossRef]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of Salt-tolerant rice landraces by seedling stage phenotyping and dissecting biochemical determinants of tolerance mechanism. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Tahjib-UI-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.G.M.; Polash, M.A.S.; Hossain, M.; Sohel, M.; Taher, A. Differential response of sugar beet to long-term mild to severe salinity in a soil–pot culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschlod, P.; Commander, L.E. Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Life cycles: Environmental influences and adaptations. In Plant Physiological Ecology; Springer: New York, NY, USA, 2008; pp. 375–402. [Google Scholar]
- Bajwa, A.A.; Chauhan, B.S.; Adkins, S.W. Germination ecology of two Australian biotypes of Ragweed Parthenium (Parthenium hysterophorus) relates to their invasiveness. Weed Sci. 2018, 66, 62–70. [Google Scholar] [CrossRef]
- Ye, J.; Wen, B. Seed germination in relation to the invasiveness in spiny amaranth and edible amaranth in Xishuangbanna, SW China. PLoS ONE 2017, 12, e0175948. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 35, 2867–2878. [Google Scholar] [CrossRef]
- Baskin, C.C.; Thompson, K.; Baskin, J.M. Mistakes in germination ecology and how to avoid them. Seed Sci. Res. 2006, 16, 165–168. [Google Scholar] [CrossRef]
- Rosbakh, S.; Poschlod, P. Initial temperature of seed germination as related to species occurrence along a temperature gradient. Funct. Ecol. 2015, 29, 5–14. [Google Scholar] [CrossRef]
- Saatkamp, A.; Affre, L.; Dutoit, T.; Poschlod, P. Germination traits explain soil seed persistence across species: The case of Mediterranean annual plants in cereal fields. Ann. Bot. 2011, 107, 415–426. [Google Scholar] [CrossRef]
- Guja, L.K.; Merritt, D.J.; Dixon, K.W. Buoyancy, salt tolerance and germination of coastal seeds: Implications for oceanic hydrochorous dispersal. Funct. Plant Biol. 2010, 37, 1175–1186. [Google Scholar] [CrossRef]
- Mollard, F.P.O.; Insausti, P. Geographic variation in the flood-induced fluctuating temperature requirement for germination in Setaria parviflora seeds. Plant Biol. 2011, 13, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S.; Gill, G.; Preston, C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006, 54, 854–860. [Google Scholar] [CrossRef]
- Hough-Goldstein, J.; Schiff, M.; Lake, E.; Butterworth, B. Impact of the biological control agent Rhinoncomimus latipes (Coleoptera: Curculionidae) on mile-a-minute weed, Persicaria perfoliata, in field cages. Biol. Control 2008, 46, 417–423. [Google Scholar] [CrossRef]
- Önen, H.; Özaslan, C.; Günal, H.; Akyol, N.; Caldiran, U. Expansion status of two invasive vines: Bur-Cucumber and Mile-a-Minute. In Proceedings of the 4th ESENIAS Workshop: International Workshop on IAS in Agricultural and Non-Agricultural Areas in ESENIAS Region, Çanakkale, Turkey, 16–17 December 2013; pp. 16–17. [Google Scholar]
- Ozaslan, C.; Onen, H.; Farooq, S. Mile-a-minute weed: A lurking peril for tea plantations in Black Sea Region. In Proceedings of the 2nd International Balkan Agriculture Congress, Tekirdag, Turkey, 16–18 May 2017; Namık Kemal University: Tekirdag, Turkey, 2017; p. 28. [Google Scholar]
- Onen, H.; Ozaslan, C.; Caldiran, U. Persicaria perfoliata. In Invasive Plants Catalogue of Turkey; Onen, H., Ed.; Tarımsal Araştırmalar ve Politikalar Genel Müdürlüğü Bitki Sağlığı Araştırmaları Daire Başkanlığı: Ankara, Turkey, 2015; pp. 410–423. ISBN 978-605-9175-05-0. [Google Scholar]
- Guener, A. A new record for the flora of Turkey and a new subspecies from Australia. Candollea 1984, 39, 345–348. [Google Scholar]
- Hough-Goldstein, J.; Lake, E.; Reardon, R.; Wu, Y. Biology and Biological Control of Mile-A-Minute Weed; University of Delaware: Newark, DE, USA, 2008. [Google Scholar]
- Johnson, C.F. Achene Germination Requirements, Temporal Viability and Germination when Stored under Natural Conditions, and Abundance in the Soil Seed Bank for the Exotic Invasive Mile-a Minute (Polygonum perfoliatum L.). Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 1996. [Google Scholar]
- Okay, J.A.G. Polygonum perfoliatum: A Study of Biological and Ecological Features Leading to the Formation of a Management Policy. Ph.D. Thesis, George Mason University, Fairfax, VA, USA, 1997. [Google Scholar]
- Colpetzer, K.; Hough-Goldstein, J. A rapid germination protocol for mile-a-minute weed, Polygonum perfoliatum L. Seed Sci. Technol. 2004, 32, 749–757. [Google Scholar] [CrossRef]
- Timson, J. Germination in Polygonum. New Phytol. 1965, 64, 179–186. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, C.; Wei, S.; Huang, H.; Liu, W. Factors affecting Tausch’s goatgrass (Aegilops tauschii Coss.) seed germination and seedling emergence. J. Agric. Sci. 2011, 4, 114. [Google Scholar] [CrossRef]
- Chachalis, D.; Reddy, K.N. Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci. 2000, 48, 212–216. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, B.; Dong, L.; Lou, Y. Seed Germination ecology of Catchweed Bedstraw (Galium aparine). Weed Sci. 2016, 64, 634–641. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biological Approach; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- IBM Corp.; IBM SPSS Inc. IBM SPSS Statistics for Windows; Version 20.0; IBM Corp.: Armonk, NY, USA, 2012; pp. 1–8. [Google Scholar]
- Batlla, D.; Luis Benech-Arnold, R. Predicting changes in dormancy level in weed seed soil banks: Implications for weed management. Crop Prot. 2007, 26, 189–197. [Google Scholar] [CrossRef]
- Batlla, D.; Kruk, B.C.; Benech-Arnold, R.L. Modelling Changes in Dormancy in Weed Soil Seed Banks: Implications for the Prediction of Weed Emergence; Handbook of Seed Physiology. Applications to Agriculture; Haworth Press Inc.: New York, NY, USA, 2004; pp. 245–264. [Google Scholar]
- Gioria, M.; Pyšek, P. Early bird catches the worm: Germination as a critical step in plant invasion. Biol. Invasions 2017, 19, 1055–1080. [Google Scholar] [CrossRef]
- Eslami, S. V Comparative germination and emergence ecology of two populations of common lambsquarters (Chenopodium album) from Iran and Denmark. Weed Sci. 2011, 59, 90–97. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, Y.S. Seed germination of Korean wild medicinal plants: Capsella bursa-pastoris, Persicaria perfoliata and Commelina communis. Korean Soc. Hortic. Sci. 1993, 34, 315–319. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds; Bewley, J., Black, M., Eds.; Springer: New York, NY, USA, 1994; pp. 1–33. [Google Scholar]
- Eyüpoğlu, F. Türkiye Topraklarının Verimlilik Durumu; TC Başbakanlık Köy Hizmetleri Genel Müdürlüğü: Ankara, Turkey, 1999; ISBN 9751921473. [Google Scholar]
- Sensoy, S.; Demircan, M.; Ulupinar, Y.; Balta, İ. Climate of Turkey; Republic of Turkey Ministry of Environment and Forestry: Ankara, Turkey, 2008; Volume 10. [Google Scholar]
- Han, G.; Chu, X.; Xing, Q.; Li, D.; Yu, J.; Luo, Y.; Wang, G.; Mao, P.; Rafique, R. Effects of episodic flooding on the net ecosystem CO2 exchange of a supratidal wetland in the Yellow River Delta. J. Geophys. Res. G Biogeosci. 2015, 120, 1506–1520. [Google Scholar] [CrossRef]


| Location | Clay | Sand | Silt | CaCO3 | OM | pH | EC | P |
|---|---|---|---|---|---|---|---|---|
| % | µS cm−1 | mg kg−1 | ||||||
| Borçka | 34.50 | 53.20 | 12.30 | 1.16 | 2.21 | 6.98 | 92.90 | 1.82 |
| Ardeşen | 32.00 | 54.00 | 14.00 | 3.54 | 3.94 | 7.02 | 458.50 | 7.26 |
| Dernekpazarı | 29.00 | 57.00 | 14.00 | 1.30 | 1.74 | 7.04 | 165.00 | 8.74 |
| Location | Lat | Long | Province | Altitude (m) | RH | Tave | Tmax | Tmin | Rainfall | PET |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (°C) | (mm) | ||||||||
| Borçka | 41.40 | 41.50 | Artvin | 173 | 61.82 | 12.18 | 18.35 | 11.52 | 1244.01 | 744.66 |
| Ardeşen | 41.15 | 41.01 | Rize | 6 | 62.29 | 13.25 | 17.62 | 10.53 | 1620.38 | 720.34 |
| Dernekpazarı | 40.91 | 40.28 | Trabzon | 190 | 68.55 | 13.45 | 18.17 | 10.15 | 1947.65 | 750.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, S.; Onen, H.; Tad, S.; Ozaslan, C.; Mahmoud, S.F.; Brestic, M.; Zivcak, M.; Skalicky, M.; El-Shehawi, A.M. The Influence of Environmental Factors on Seed Germination of Polygonum perfoliatum L.: Implications for Management. Agronomy 2021, 11, 1123. https://doi.org/10.3390/agronomy11061123
Farooq S, Onen H, Tad S, Ozaslan C, Mahmoud SF, Brestic M, Zivcak M, Skalicky M, El-Shehawi AM. The Influence of Environmental Factors on Seed Germination of Polygonum perfoliatum L.: Implications for Management. Agronomy. 2021; 11(6):1123. https://doi.org/10.3390/agronomy11061123
Chicago/Turabian StyleFarooq, Shahid, Huseyin Onen, Sonnur Tad, Cumali Ozaslan, Samy F. Mahmoud, Marian Brestic, Marek Zivcak, Milan Skalicky, and Ahmed M. El-Shehawi. 2021. "The Influence of Environmental Factors on Seed Germination of Polygonum perfoliatum L.: Implications for Management" Agronomy 11, no. 6: 1123. https://doi.org/10.3390/agronomy11061123
APA StyleFarooq, S., Onen, H., Tad, S., Ozaslan, C., Mahmoud, S. F., Brestic, M., Zivcak, M., Skalicky, M., & El-Shehawi, A. M. (2021). The Influence of Environmental Factors on Seed Germination of Polygonum perfoliatum L.: Implications for Management. Agronomy, 11(6), 1123. https://doi.org/10.3390/agronomy11061123










