Potassium and Elemental Sulfur as Factors Determining Nitrogen Management Indices of Soil and Faba Bean (Vicia faba L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil and Meteorological Conditions
2.3. Plant Sampling and Chemical Analysis
| Year | Characteristics | April | May | June | July | Total/ Average 1 |
|---|---|---|---|---|---|---|
| 2012 | Precipitation | 22.9 | 77.2 | 163.0 | 197.6 | 460.7 |
| Temperature | 8.8 | 14.8 | 16 | 19.2 | 14.7 | |
| SHC 2 | 0.87 | 1.68 | 3.40 | 3.32 | - | |
| 2013 | Precipitation | 15.4 | 69.8 | 125.3 | 67.3 | 277.8 |
| Temperature | 8.0 | 14.4 | 17.3 | 20.1 | 15.0 | |
| SHC | 0.64 | 1.56 | 2.41 | 1.08 | - | |
| 2014 | Precipitation | 46.3 | 73.5 | 42.0 | 83.1 | 244.9 |
| Temperature | 10.5 | 13.1 | 16.1 | 21.5 | 15.3 | |
| SHC | 1.47 | 1.81 | 0.87 | 1.25 | - | |
| 1961–2011 | Precipitation | 37.2 | 57.1 | 64.1 | 81.2 | 239.6 |
| Temperature | 8.0 | 13.2 | 16.5 | 18.2 | 14.0 | |
| SHC | 1.55 | 1.40 | 1.29 | 1.44 | - |
2.4. Soil Sampling and Analysis
2.5. Indices of Faba Bean N Management
2.6. Indices of Soil N Balance
2.7. Statistical Analysis
3. Results
3.1. Yield and Plant Dry Matter
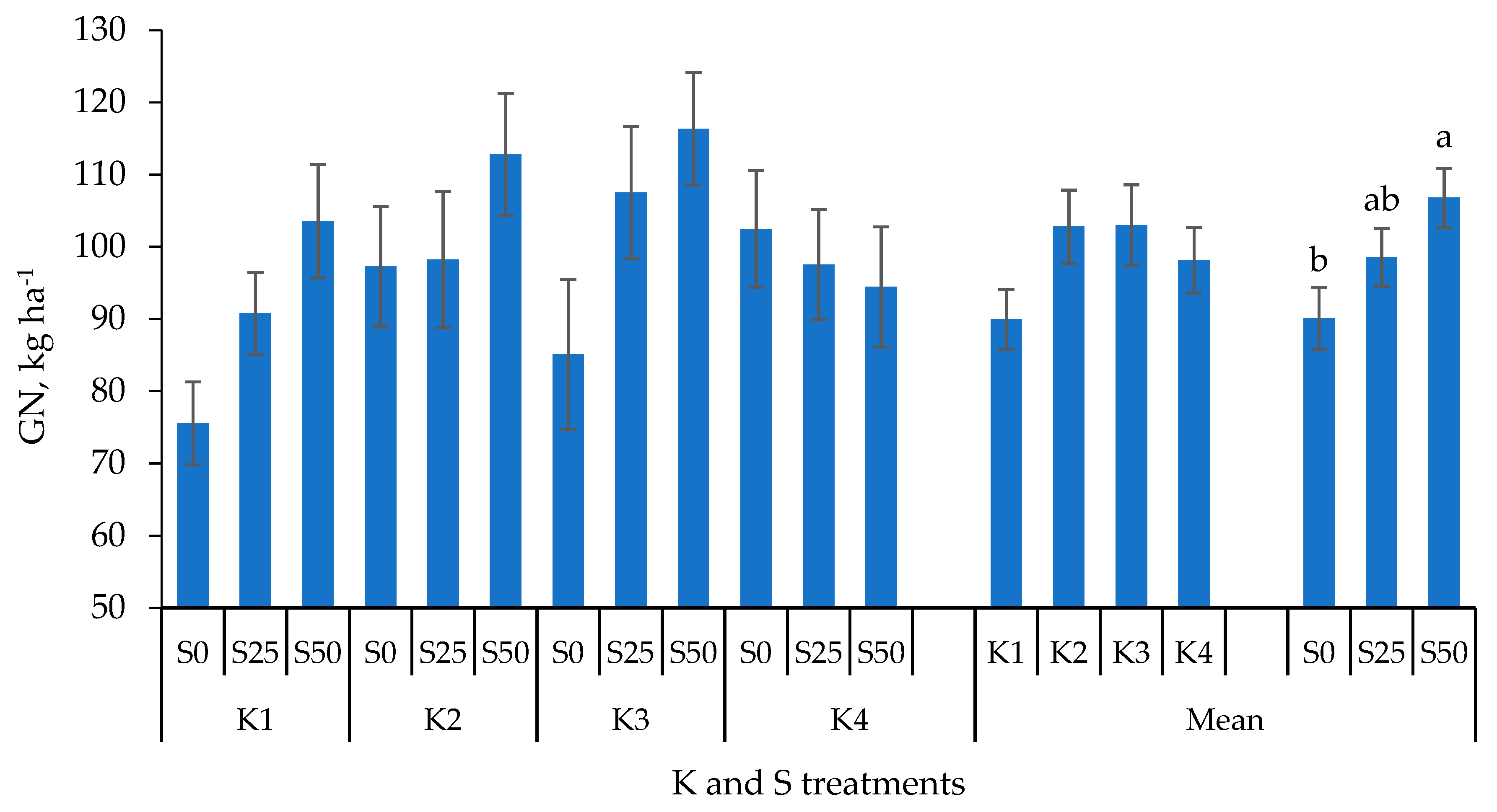
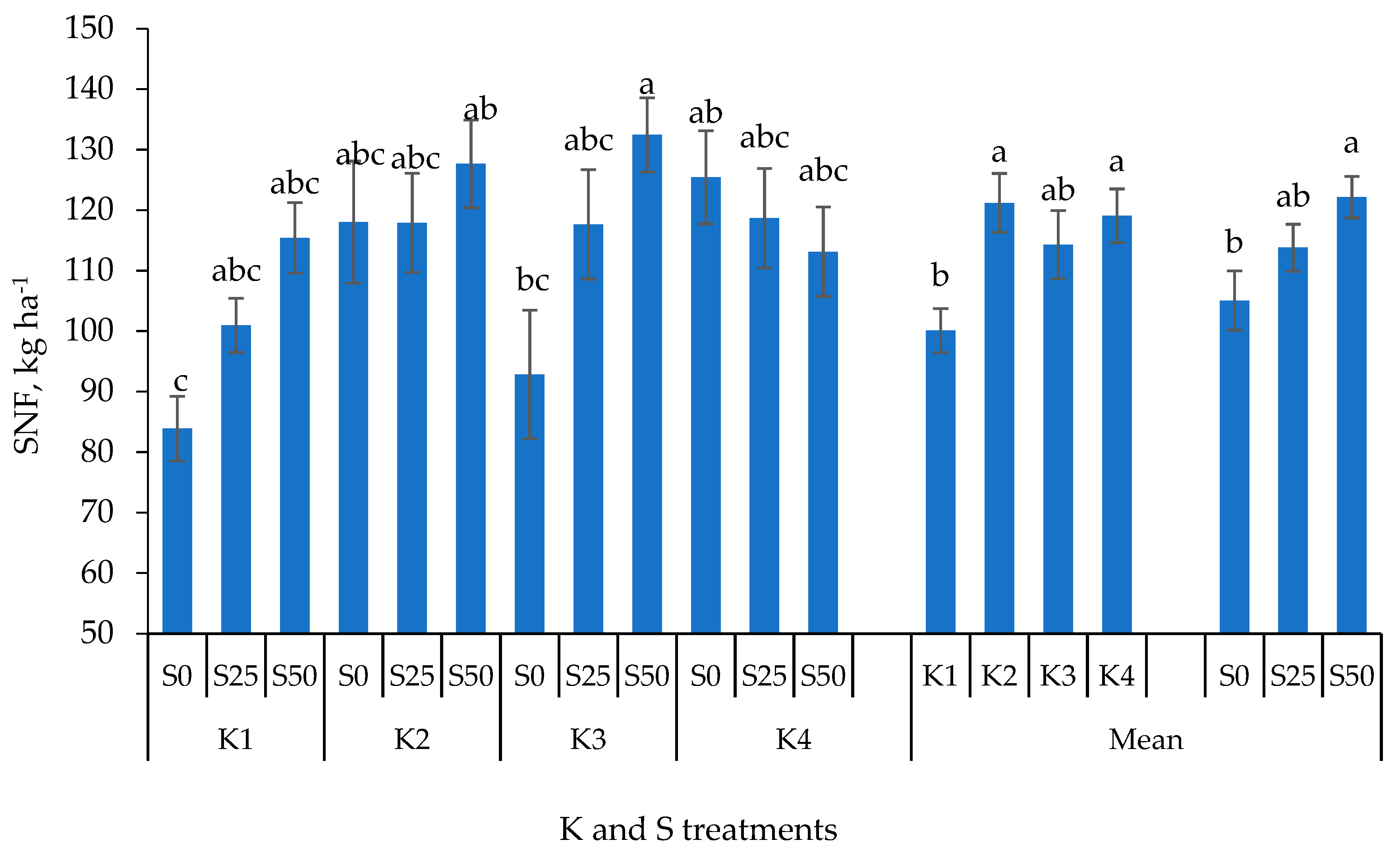
3.2. Yield Components
3.3. Indices of Plant N Management
3.4. Indices of Soil N Management
3.5. Relationships between Plant and Soil Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Adjesiwor, A.T. Nitrogen Fixation and Transfer in Agricultural Production Systems. In Nitrogen in Agriculture—Updates; Amanullah, Fahad, S., Eds.; IntechOpen Limeted: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.S.; Hauggaard-Nielsen, H. How can increased use of biological N2 fixation in agriculture benefit the environment? Plant Soil 2003, 252, 177–186. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crop Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Köpke, U.; Nemecek, T. Ecological services of Faba bean. Field Crop Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Kennedy, A.C. Grain legumes in Northern Great Plains: Impacts on selected biological soil processes. Agron. J. 2007, 99, 1700–1709. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; SMaskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peples, M.B.; Hauggaard-Nielson, H. Faba bean in cropping systems. Field Crop Res. 2010, 115, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Landry, E.J.; Coyne, C.J.; McGee, R.J.; Hu, J. Adaptation of autum-n-sown faba bean germplasm to southeastern Washington. Agron. J. 2016, 108, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Rochester, I.J.; Peoples, M.B. Growing vetches (Vicia villosa Roth) in irrigated cotton systems: Inputs of fixed N, N fertilizer savings and cotton productivity. Plant Soil 2005, 271, 251–264. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. The comparison of nitrogen use and leaching in sole cropped versus intercropped pea and barley. Nutr. Cycl. Agroecosyst. 2003, 65, 289–300. [Google Scholar] [CrossRef]
- Peoples, M.B.; Bowman, A.M.; Gault, R.R.; Herridge, D.F.; McCallum, M.H.; McCormick, K.M.; Norton, R.M.; Rochester, I.J.; Scammell, G.J.; Schwenke, G.D. Factors regulating the contributions of fixed nitrogen by pasture and crop legumes to different farming systems of eastern. Australia Plant Soil 2001, 228, 29–41. [Google Scholar] [CrossRef]
- FAOSTAT. Broad Beans, Horse Beans, Dry. Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/faostat/en (accessed on 18 February 2021).
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Barker, A.V.; Zandvakili, O.R.; Liu, L. Agronomy, Nutritional Value, and Medicinal Application of Faba Bean (Vicia faba L.). Hortic. Plant J. 2019, 5, 170–182. [Google Scholar] [CrossRef]
- GUS—Central Statistical Office. Statistical Yearbook of the Republic of Poland. 2018. Available online: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-the-republic-of-poland-2018,2,19.html (accessed on 18 February 2021).
- Pszczółkowska, A.; Okorski, A.; Fordonski, G.; Kotecki, A.; Kozak, M.; Dzienis, G. Effect of Weather Conditions on Yield and Health Status of Faba Bean Seeds in Poland. Agronomy 2020, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- López-Bellido, F.J.; López-Bellido, L.; López-Bellido, R.J. Competition, growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 2005, 23, 359–378. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Nahar, K.; Hossain, M.; Mahmud, J.A.; Hossen, M.; Masud, A.A.C.; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Kurdali, F.; Al-Ain, F.; Al-Shamma, M. Nodulation, dry matter production, and N2 fixation by faba bean and chickpea as affected by soil moisture and potassium fertilizer. J. Plant Nutr. 2002, 25, 355–368. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Taha, A.A.; Omar, M.M.; Hadeer, K. Effect of different sources and levels of potassium on growth, yield and chemical composition of faba bean. J. Soil Sci. Agric. Eng. Mansoura Univ. 2016, 7, 243–248. [Google Scholar] [CrossRef]
- FAO. Fertilizer Use by Crop in Poland; The Food and Agriculture Organization (FAO): Rome, Italy, 2003; Available online: http://www.fao.org/tempref/agl/agll/docs/fertusepoland.pdf (accessed on 23 March 2021).
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, S.; Schulz, M.; Scherer, H. Influence of sulphur supply on glucose and ATP concentrations of inoculated broad beans (Vicia faba minor L.). Biol. Fertil. Soils 2006, 42, 324–329. [Google Scholar] [CrossRef]
- Głowacka, A.; Gruszecki, T.; Szostak, B.; Michałek, S. The response of common bean to sulphur and molybdenum fertilization. Int. J. Agron. 2019, 2019, 3830712. [Google Scholar] [CrossRef]
- Scherer, H.W.; Pacyna, S.; Manthey, N.; Schulz, M. Sulphur supply to peas (Pisum Sativum L.) influences symbiotic N2 fixation. Plant Soil Environ. 2006, 52, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Habtemichial, K.H.; Singh, B.R.; Aune, J.B. Wheat response to N2 fixed by faba bean (Vicia faba L.) as affected by sulfur fertilization and rhizobial inoculation in semi-arid Northern Ethiopia. J. Plant Nutr. Soil Sci. 2007, 170, 412–418. [Google Scholar] [CrossRef]
- Cazzato, E.; Tufarelli, V.; Ceci, E.; Stellacci, A.M.; Laudadio, V. Quality, yield and nitrogen fixation of faba bean seeds as affected by sulphur fertilization. Acta Agric. Scand. Sect. B 2012, 62, 732–738. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. The Effect of Potassium and Sulfur Fertilization on Seed Quality of Faba Bean (Vicia faba L.). Agronomy 2019, 9, 209. [Google Scholar] [CrossRef] [Green Version]
- Vestreng, V.; Myhre, G.; Fagerli, H.; Reis, S.; Tarraśon, L. Twenty-five years of continuous sulphur dioxide emission reduction in Europe. Atmos. Chem. Phys. 2007, 7, 3663–3681. [Google Scholar] [CrossRef] [Green Version]
- Przygocka-Cyna, K.; Grzebisz, W. Sulphur balance in Poland—Regional analysis. Fragm. Agron. 2017, 34, 60–75. [Google Scholar]
- Zhou, L.I.; Cao, J.; Zhang, F.S.; Li, L. Rhizosphere acidification of faba bean, soybean and maize. Sci. Total Environ. 2009, 407, 4359–4362. [Google Scholar] [CrossRef]
- Yang, Y.; Haneklaus, S.; Singh, B.R.; Schnug, E. Effect of Repeated Applications of Elemental Sulfur on Microbial Population, Sulfate Concentration, and pH in Soils. Commun. Soil Sci. Plan. 2007, 39, 124–140. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Venieraki, A.; Chorianopoulou, S.N.; Katinakis, P. Impact of Elemental Sulfur on the Rhizospheric Bacteria of Durum Wheat Crop Cultivated on a Calcareous Soil. Plants 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- WRB. World reference base for soil resources 2014. In Word Soil Resources Reports, 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 19 March 2021).
- Kęsik, K.; Jadczyszyn, T.; Lipiński, W.; Jurga, B. Adaptation of the Mehlich 3 procedure for routine determination of phosphorus, potassium and magnesium in soil. Przemysł Chem. 2015, 94, 973–976. (In Polish) [Google Scholar] [CrossRef]
- Motowicka-Terelak, T.; Terelak, H. Siarka w glebach i roślinach Polski. Folia Univ. Agric. Stetin. 204 Agric. 2000, 81, 7–16. (In Polish) [Google Scholar]
- Rutkowska, A.; Fotyma, M. Calibration of Soil Test for Mineral Nitrogen in Poland. Commun. Soil Sci. Plant Anal. 2009, 40, 987–998. [Google Scholar] [CrossRef]
- Pszółkowski, P.; Sawicka, B. Phenotopic variability of the yield and structure of mid-early potato cultivars. Acta Sci. Pol. Agric. 2017, 16, 147–161. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph.; Meier, U., Ed.; Julius Kühn-Institut (JKI): Quedlinbur, Germany, 2018; Available online: https://www.julius-kuehn.de/media/Veroeffentlichungen/bbch%20epaper%20en/page.pdf (accessed on 27 February 2021).
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagents. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Reining, E. Assessment tool for biological nitrogen fixation of Vicia faba cultivated as spring main crop. Eur. J. Agron. 2005, 23, 392–400. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Data Science Textbook. 2020. Available online: https://docs.tibco.com/data-science/textbook (accessed on 7 February 2021).
- Podleśny, J. Effect of amount and distribution of precipitation during vegetation on growth, development and yielding of determinate and traditional faba bean varieties. Acta Agrophys. 2009, 14, 413–425. [Google Scholar]
- Najeeb, H.; Alharbi, A.B.; Adhikari, K.N. Factors of yield determination in faba bean (Vicia faba). Crop Pasture Sci. 2020, 71, 305–321. [Google Scholar] [CrossRef]
- Łabuda, H. Flowering and characteristics of useful traits of some faba bean (Vicia faba L. var.major Harz) cultivars and breeding lines. Acta Agrobot. 2012, 65, 139–148. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Ziegler, K.V.; Kriegner, S.; Kaul, H.-P. Limited winter survival and compensation mechanisms of yield components constrain winter faba bean production in Central Europe. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 496–505. [Google Scholar] [CrossRef]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 1999, 9, 273–293. [Google Scholar] [CrossRef]
- Pettigrew, W.W. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Barłóg, P.; Borowiak, K.; Wolna-Maruwka, A. The effect of sulphur and potassium fertilisation on the nitrogenase and microbial activity in soil under broad bean (Vicia faba L.) cultivation. Fresen. Environ. Bull. 2015, 24, 723–732. [Google Scholar]
- Abd-Alla, M.H.; Abdel Wahab, A.M. Response of nitrogen fixation, nodule activities, and growth to potassium supply in water-stressed broad bean. J. Plant Nutr. 1995, 18, 1391–1402. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Faba bean yield and growth dynamics in response to soil potassium availability and sulfur application. Field Crop Res. 2018, 219, 87–97. [Google Scholar] [CrossRef]
- Khan, T.A.; Mazid, M. Nutritional significance of sulphur in pulse cropping system. Biol. Med. 2011, 3, 114–133. [Google Scholar]
- Mendel, R.R.; Bittner, F. Cell biology of molybdenum. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 621–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, H.W. Impact of sulfur on N2 fixation of legumes. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 43–54. [Google Scholar] [CrossRef]
- Varin, S.; Cliquet, J.B.; Personeni, E.; Avice, J.C.; Lemauviel-Lavenant, S. How does sulphur availability modify N acquisition of white clover (Trifolium repens L.)? J. Exp. Bot. 2010, 61, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisany, W.; Raei, Y.; Allahverdipoor, K.H. Role of Some of Mineral Nutrients in Biological Nitrogen Fixation. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 77–84. [Google Scholar]
- Akhtar, A.; Hisamuddin, A.; Robab, M.I.; Abbasi, H.; Sharf, R. Plant growth promoting Rhizobacteria: An overview. J. Nat. Prod. Plant Resour. 2012, 2, 19–31. [Google Scholar]
- Elscheikh, E.A.E.; Elzidany, A.A. Effect of Rhizobium inoculation, organic and chemical fertilizers on proximate composition, in vitro protein digestibility, tannin and sulphur content of faba beans. Food Chem. 1997, 59, 41–45. [Google Scholar] [CrossRef]
- Ganeshamurthy, A.N.; Sammi Reddy, K. Effect of integrated used of farmyard Manure and Sulphur in a soyabean and wheat cropping systems on nodulation dry matter production and chlorophyll content of soyabean on swell-shrink soil in central India. J. Agron. Crop Sci. 2000, 185, 91–97. [Google Scholar] [CrossRef]
- Turpin, J.E.; Herridge, D.F.; Robertson, M.J. Nitrogen fixation and soil nitrate interactions in field-grown chickpea (Cicer arietinum) and faba bean (Vicia faba). Aust. J. Agric. Res. 2002, 53, 599–608. [Google Scholar] [CrossRef]
- Allito, B.B.; Ewusi-Mensah, N.; Alemneh, A.A. Rhizobia strain and host-legume interaction effects on nitrogen fixation and yield of grain legume: A review. Mol. Soil Biol. 2014, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tripolskaja, L.; Asakaviciute, R. Effects of fertilisers on pulse crop productivity and nitrogen assimilation on acid soil. Plant Soil Environ. 2019, 65, 536–540. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E.; Russelle, M.P. Understanding the variability in soybean nitrogen fixation across agroecosystems. Plant Soil 2010, 329, 379–397. [Google Scholar] [CrossRef]
- Guinet, M.; Nicolardot, B.; Revellin, C.; Durey, V.; Carlsson, G.; Voisin, A.-S. Comparative effect of inorganic N on plant growth and N2 fixation of ten legume crops: Towards a better understanding of the differential response among species. Plant Soil 2018, 432, 207–227. [Google Scholar] [CrossRef]
- Hossain, Z.; Wang, X.; Hamel, C.; Knight, J.D.; Morrison, M.J.; Gan, Y. Biological nitrogen fixation by pulse crops on semiarid Canadian prairies. Can. J. Plant Sci. 2016, 97, 119–131. [Google Scholar] [CrossRef]
- Denton, M.D.; Pearce, D.J.; Peoples, M.B. Nitrogen contributions from faba bean (Vicia faba L.) reliant on soil rhizobia or inoculation. Plant Soil 2013, 365, 363–374. [Google Scholar] [CrossRef]
- Fahmi, A.I.; Nagaty, H.H.; Eissa, R.A.; Hassan, M.M. Effects of Salt Stress on Some Nitrogen Fixation Parameters in Faba Bean. Pak. J. Biol. Sci. 2011, 14, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.K.; Naamala, J.; Dakora, F.D. Nature and mechanisms of aluminum toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol. Fert. Soils 2018, 54, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rochester, I.J.; Peoples, M.B.; Constable, G.A.; Gault, R.R. Faba beans and other legumes add nitrogen to irrigated cotton cropping systems. Aust. J. Exp. Agric. 1998, 38, 253–260. [Google Scholar] [CrossRef]






| Factor | Acronyms and Factor Levels |
|---|---|
| Potassium treatments | K1—control, low K concentration in soil, without any K dose in crop rotation |
| K2—medium K concentration in soil + 33.3 kg K ha−1 (= 25% of the full K dose) | |
| K3—medium K concentration in soil + 66.5 kg K ha−1 (= 50% of the full K dose) | |
| K4—high K concentration in soil + 133.0 kg K ha−1 (= 100% of the nutrient requirement for faba bean) | |
| Sulfur application | S0—control, without S application |
| S25—25.0 kg S ha−1 | |
| S50—50.0 kg S ha−1 |
| Treatment | pH 1 | P 2 | K 2 | Mg 2 | Ca 2 | SO4-S 3 | NH4-N 4 | NO3-N 4 |
|---|---|---|---|---|---|---|---|---|
| mg kg−1 | kg ha−1 | |||||||
| Soil depth: 0.0–0.3 m | ||||||||
| K1 | 6.34 | 157.3 H | 89.3 L | 60.4 M | 1184 L | 10.4 L | 11.7 | 29.0 |
| K2 | 6.49 | 165.9 H | 133.2 M | 60.0 M | 1089 L | 12.0 L | 15.0 | 30.4 |
| K3 | 6.41 | 167.8 H | 115.0 M | 57.4 M | 1132 L | 10.4 L | 11.4 | 34.6 |
| K4 | 6.52 | 163.6 H | 163.0 H | 62.4 M | 1021 L | 9.4 L | 10.2 | 33.8 |
| Soil depth: 0.3–0.6 m | ||||||||
| K1 | 5.93 | 98.8 | 81.9 | 69.8 | 1289 | 8.4 | 11.5 | 28.8 |
| K2 | 6.13 | 110.2 | 104.0 | 74.9 | 1371 | 11.5 | 9.8 | 28.0 |
| K3 | 5.97 | 108.0 | 98.7 | 84.3 | 1309 | 10.6 | 10.1 | 28.9 |
| K4 | 6.14 | 109.7 | 112.7 | 79.2 | 1286 | 9.3 | 9.9 | 32.8 |
| Soil depth: 0.6–0.9 m | ||||||||
| K1 | 6.25 | 39.2 | 87.0 | 90.50 | 1390 | 8.5 | 9.3 | 35.5 |
| K2 | 6.03 | 48.7 | 97.0 | 84.00 | 1401 | 11.1 | 9.7 | 33.4 |
| K3 | 6.09 | 33.2 | 95.4 | 105.50 | 1293 | 10.6 | 11.7 | 29.3 |
| K4 | 6.29 | 44.3 | 95.8 | 98.00 | 1328 | 11.5 | 10.0 | 29.7 |
| Factors | Nin (kg ha−1) | Nb (kg ha−1) | Ngain (kg ha−1) | NinT (kg ha−1) | NgainT (kg ha−1) | NinE (kg kg−1) | |
|---|---|---|---|---|---|---|---|
| K treatments (K) | |||||||
| K1 | 274.7 ± 6.9 b | 184.7 ± 6.0 b | 27.7 ± 6.9 | 302.4 ± 8.3 b | 102.2 ± 7.6 | 32.8 ± 1.3 | |
| K2 | 305.4 ± 9.7 a | 202.6 ± 6.5 a | 33.2 ± 8.5 | 338.6 ± 10.7 a | 124.8 ± 10.1 | 33.4 ± 1.0 | |
| K3 | 292.9 ± 8.3 ab | 189.9 ± 6.8 ab | 36.2 ± 6.8 | 329.1 ± 9.0 ab | 116.0 ± 7.2 | 34.9 ± 1.6 | |
| K4 | 303.5 ± 7.6 a | 205.3 ± 6.9 a | 26.7 ± 7.6 | 330.1 ± 8.1 ab | 121.8 ± 7.9 | 32.4 ± 1.3 | |
| S treatments (S) | |||||||
| S0 | 282.8 ± 8.6 b | 192.7 ± 6.1 | 23.1 ± 6.8 | 305.8 ± 9.0 | 105.4 ± 7.7 | 31.6 ± 1.0 b | |
| S25 | 294.5 ± 6.3 ab | 196.0 ± 5.5 | 35.2 ± 6.3 | 329.7 ± 7.2 | 120.9 ± 7.1 | 33.5 ± 1.2 ab | |
| S50 | 305.0 ± 6.4 a | 198.2 ± 5.7 | 34.5 ± 6.0 | 339.5 ± 7.1 | 122.4 ± 6.1 | 35.1 ± 1.2 a | |
| K × S interaction | |||||||
| K1 | S0 | 254.1 ± 13.1 | 178.6 ± 10.6 | 16.2 ± 8.5 | 270.3 ± 11.4 c | 84.6 ± 7.9 | 29.9 ± 1.7 |
| S25 | 276.5 ± 8.9 | 185.7 ± 10.4 | 25.0 ± 15.6 | 301.4 ± 14.1 abc | 100.4 ± 17.8 | 33.2 ± 2.4 | |
| S50 | 293.5 ± 11.4 | 190.0 ± 10.8 | 41.9 ± 10.4 | 335.4 ± 11.7 abc | 121.7 ± 10.7 | 35.4 ± 2.5 | |
| K2 | S0 | 302.1 ± 19.9 | 204.8 ± 13.4 | 29.8 ± 19.0 | 331.8 ± 22.5 abc | 123.6 ± 21.4 | 32.0 ± 1.4 |
| S25 | 301.4 ± 15.7 | 203.1 ± 10.4 | 44.2 ± 9.5 | 345.5 ± 15.5 ab | 136.3 ± 12.2 | 32.2 ± 1.9 | |
| S50 | 312.7 ± 15.7 | 199.9 ± 10.6 | 25.6 ± 14.8 | 338.3 ± 18.4 ab | 114.5 ± 18.6 | 36.0 ± 1.8 | |
| K3 | S0 | 263.4 ± 16.1 | 178.3 ± 11.6 | 20.3 ± 11.6 | 283.7 ± 12.1 bc | 88.5 ± 10.9 | 31.8 ± 2.8 |
| S25 | 297.4 ± 12.7 | 189.9 ± 11.8 | 41.1 ± 12.6 | 338.5 ± 14.2 ab | 120.8 ± 11.6 | 36.2 ± 2.8 | |
| S50 | 318.0 ± 9.8 | 201.6 ± 11.7 | 47.1 ± 10.4 | 365.1 ± 10.3 a | 138.6 ± 11.4 | 36.8 ± 2.6 | |
| K4 | S0 | 311.6 ± 14.7 | 209.1 ± 11.8 | 25.9 ± 15.0 | 337.5 ± 16.9 ab | 124.8 ± 15.9 | 32.8 ± 2.0 |
| S25 | 302.8 ± 12.1 | 205.3 ± 11.2 | 30.8 ± 13.2 | 333.6 ± 11.6 abc | 125.9 ± 13.9 | 32.2 ± 2.2 | |
| S50 | 296.0 ± 13.5 | 201.5 ± 13.4 | 23.3 ± 12.2 | 319.3 ± 13.5 abc | 114.7 ± 12.3 | 32.2 ± 2.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barłóg, P.; Łukowiak, R. Potassium and Elemental Sulfur as Factors Determining Nitrogen Management Indices of Soil and Faba Bean (Vicia faba L.). Agronomy 2021, 11, 1137. https://doi.org/10.3390/agronomy11061137
Barłóg P, Łukowiak R. Potassium and Elemental Sulfur as Factors Determining Nitrogen Management Indices of Soil and Faba Bean (Vicia faba L.). Agronomy. 2021; 11(6):1137. https://doi.org/10.3390/agronomy11061137
Chicago/Turabian StyleBarłóg, Przemysław, and Remigiusz Łukowiak. 2021. "Potassium and Elemental Sulfur as Factors Determining Nitrogen Management Indices of Soil and Faba Bean (Vicia faba L.)" Agronomy 11, no. 6: 1137. https://doi.org/10.3390/agronomy11061137
APA StyleBarłóg, P., & Łukowiak, R. (2021). Potassium and Elemental Sulfur as Factors Determining Nitrogen Management Indices of Soil and Faba Bean (Vicia faba L.). Agronomy, 11(6), 1137. https://doi.org/10.3390/agronomy11061137






