Crop Response to Leaf and Seed Applications of the Biostimulant ComCat® under Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites, Details, and Data Collection
2.1.1. Field Experiments with Maize and Mixed Maize–Mung Bean in Benin to Investigate the Effect of ComCat® under Nutrient Deficiency
2.1.2. Pot Experiments with Maize in Germany to Investigate the Effect of ComCat® under Drought Conditions
2.1.3. Pot Experiments with Maize in Germany to Investigate the Effect of ComCat® under Weed Competition
2.1.4. Pot Experiment with Winter Barley in Germany to Investigate the Effect of ComCat® under Herbicide Stress
2.2. Data Analysis
3. Results
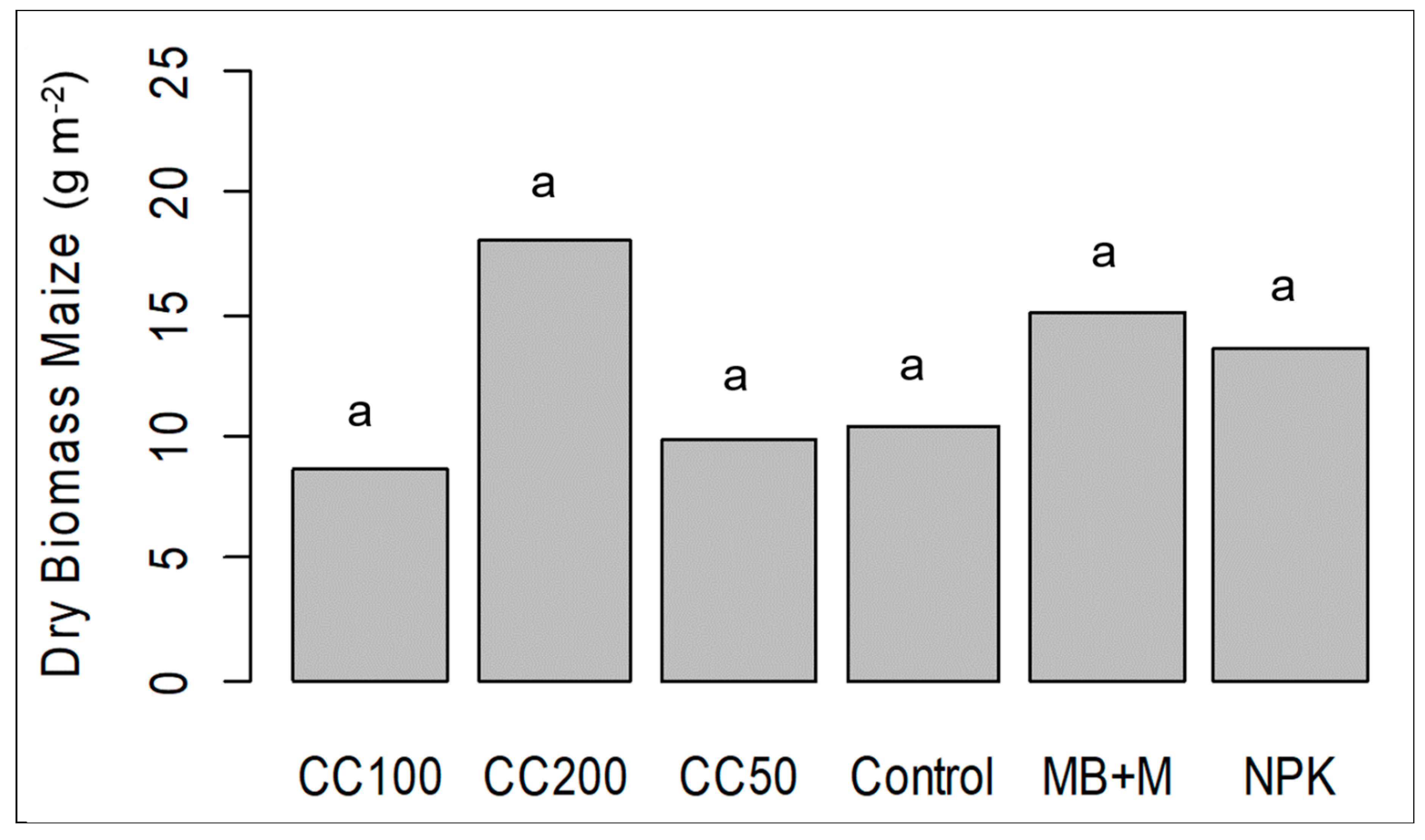
3.1. Field Experiments with Maize and Mixed Maize–Mung Bean in Benin to Investigate the Effect of ComCat® under Nutrient Deficiency
3.2. Pot Experiment with Maize in Germany to Investigate the Effect of ComCat® under Drought Conditions
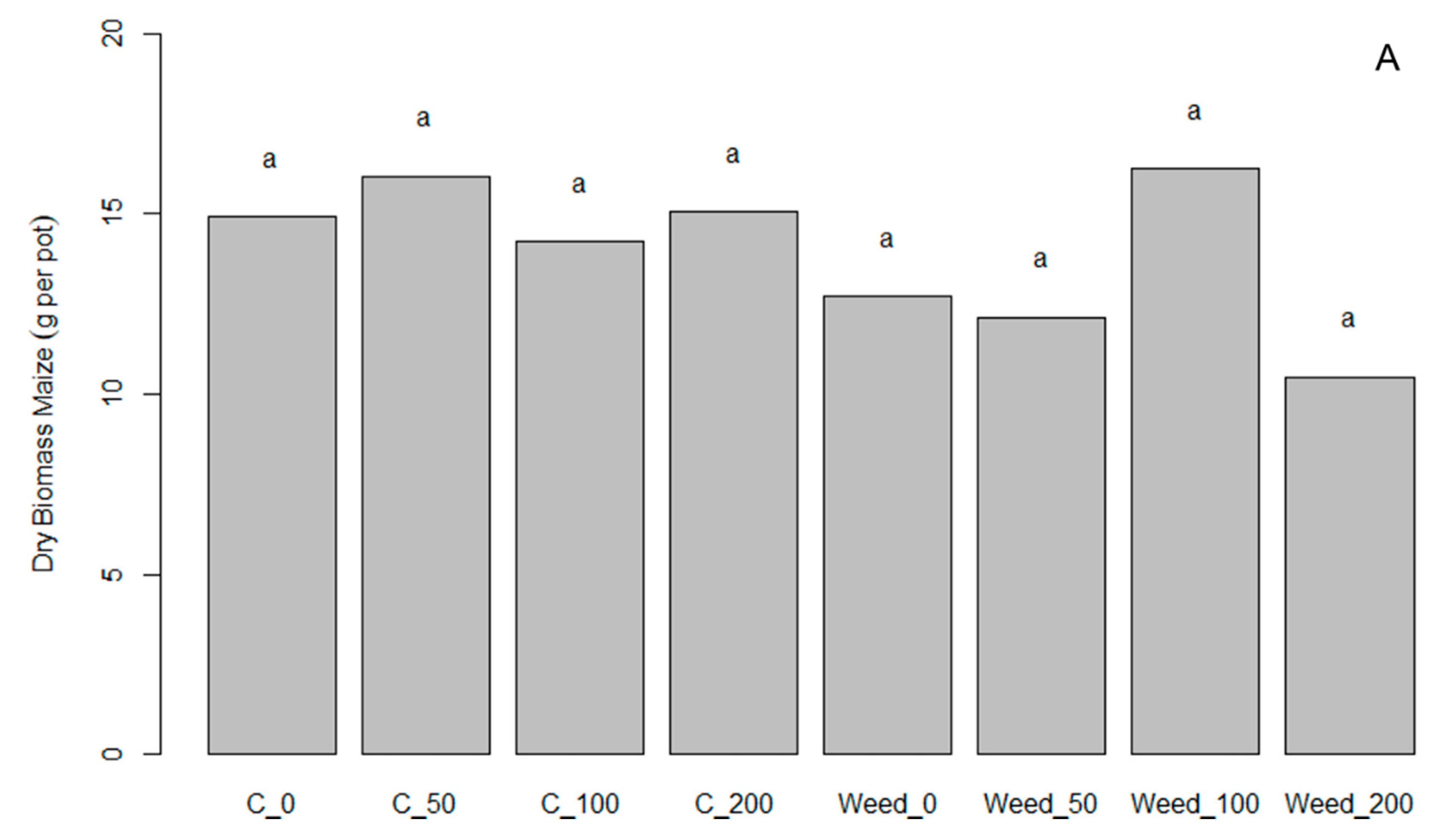
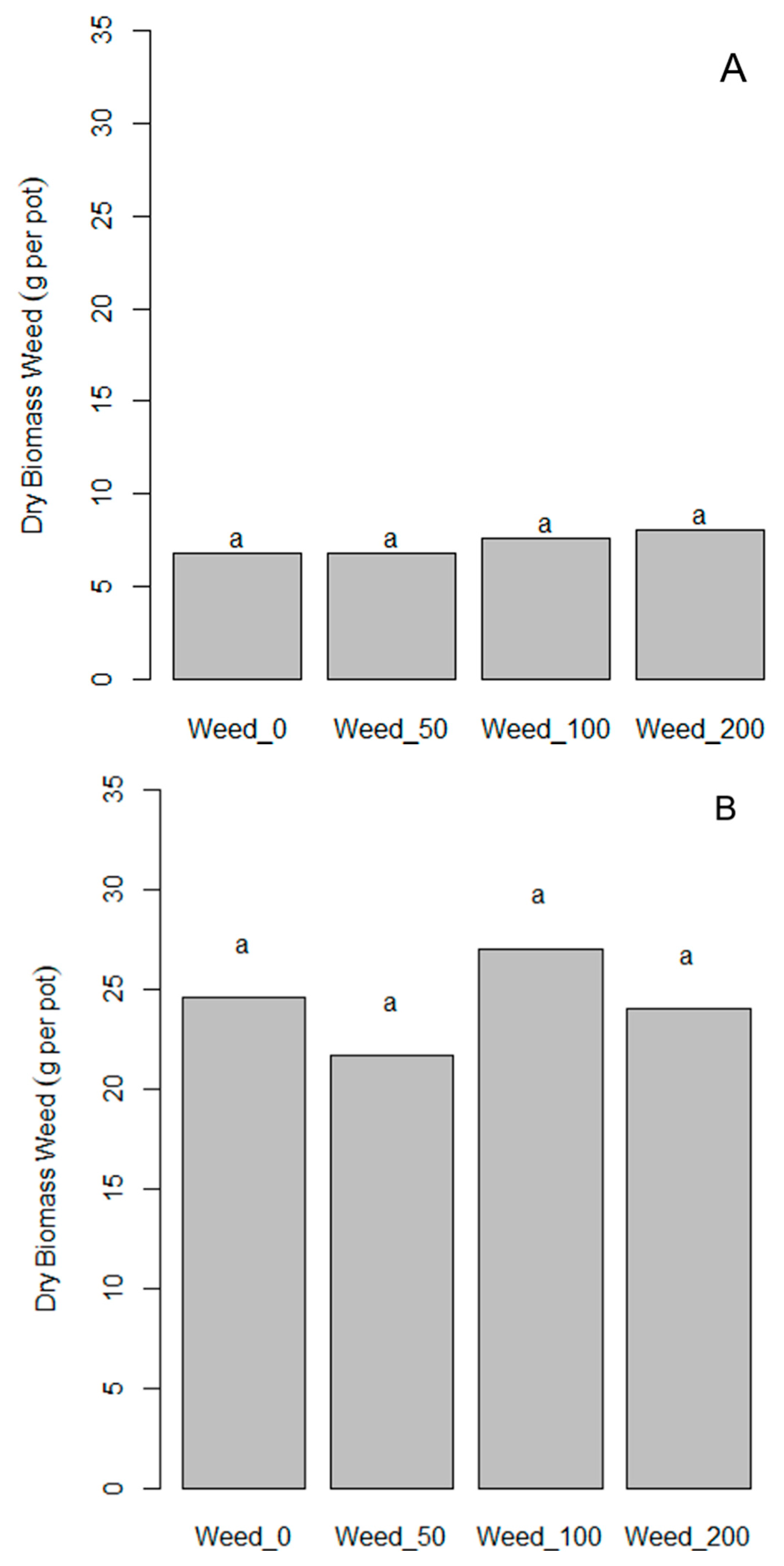
3.3. Pot Experiment with Maize in Germany to Investigate the Effect of ComCat® under Weed Competition
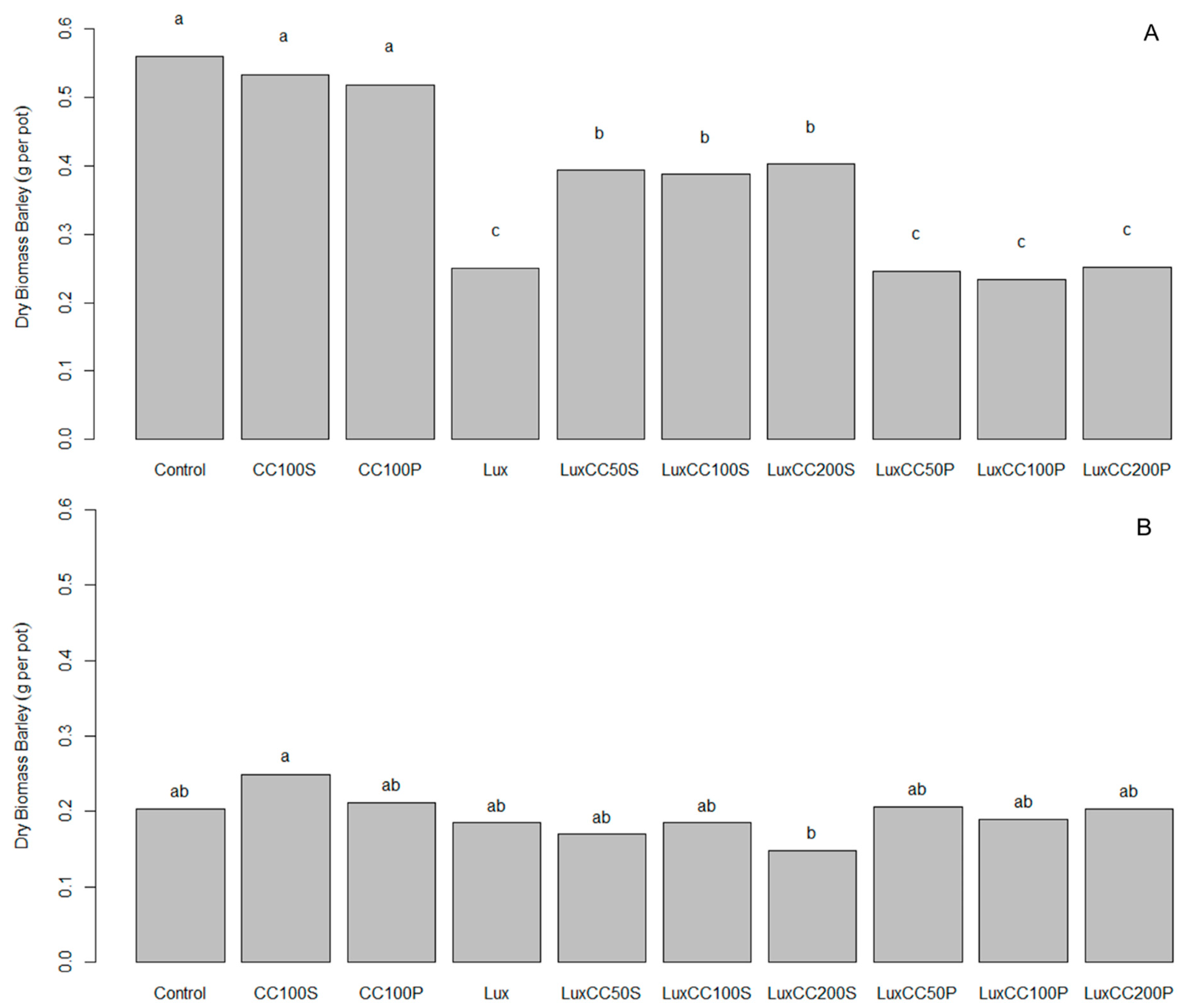
3.4. Pot Experiment with Winter Barley in Germany to Investigate the Effect of ComCat® under Herbicide Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Verma, A. Abiotic Stress and Crop Improvement: Current Scenario. Adv. Plants Agric. Res. 2016, 4, 345–346. [Google Scholar]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef] [Green Version]
- Le Mire, G.; Nguyen, M.L.; Fassotte, B.; du Jardin, P.; Verheggen, F.; Delaplace, P.; Jijakli, M.H. Review: Implementing Plant Biostimulants and Biocontrol Strategies in the Agroecological Management of Cultivated Ecosystems. Biotechnol. Agron. Soc. Environ. 2016, 20, 1–15. Available online: https://www.researchgate.net/publication/284168919 (accessed on 15 January 2021).
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Cropsunder Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Desfontaines, L.; Rotin, P.; Ozier-Lafontaine, H. Les Biostimulants: Qu’en savons-nous? Quelles alternatives pour l’agriculture Guyanaise? Innov. Agron. 2018, 64, 31–46. [Google Scholar]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Kruidhof, H.M.; van Dam, N.M.; Ritz, C.; Lotz, L.A.P.; Kropff, M.J.; Bastiaans, L. Mechanical wounding under field conditions: A potential tool to increase the allelopathic inhibitory effect of cover crops on weeds? Eur. J. Agron. 2014, 52, 229–236. [Google Scholar] [CrossRef]
- Friebe, A.; Volz, A.; Schmidt, J.; Voigt, B.; Adam, G.; Schnabl, H. 24-Epi-secasterone and 24-epi-castasterone from Lychnis viscaria seeds. Phytochemistry 1999, 52, 1607–1610. [Google Scholar] [CrossRef]
- Volz, A. Isolierung und Identifizierung aktiver Verbindungen aus Lychnis viscaria. Ph.D. Thesis, Rheinischen Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2000. [Google Scholar]
- Clouse, S.D.; Sasse, J.M. BRASSINOSTEROIDS: Essential Regulators of Plant Growth and Development. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AgraForUm. The Effect of Timed Applications of COMCAT on Vining Peas, Combining Peas and Spring Field Beans; Techn Report; PGRO Research Limited: Bomlitz, Germany, 2012; p. 5. [Google Scholar]
- Gajc-Wolska, J.; Spizewski, T.; Grabowska, A. The effect of seaweed extracts on the yield and quality parameters of Broccoli (Brassica oleracea var. cymosa L.) in open field production. Acta Hortic. 2013, 1009, 83–89. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2009; Volume 2, pp. 305–340. [Google Scholar]
- Heckman, J.R. Effect of an organic bio-stimulant on cabbage yield. J. Home Consum. Hortic. 1994, 1, 11–113. [Google Scholar]
- Petrozza, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Piaggesi, A. Evaluation of the effect of Radifarmw treatment on the morpho-physiological characteristics of root systems via image analysis. Acta Hortic. 2003, 1009, 149–153. [Google Scholar]
- Kaiser, Y.; Gerhards, R. Degradation and metabolism of fenoxaprop and mesosulfuron + iodosulfuron in multiple resistant blackgrass (Alopecurus myosuroides). Ges. Pflanzen 2015, 67, 109–117. [Google Scholar] [CrossRef]
- Coulibaly, K.; Gomgnimbou, A.P.K.; Traoré, M.; Nacro, H.B.; Sedogo, M.P. Effets des associations maïs-légumineuses sur le rendement du maïs (Zea mays L.) et la fertilité d’un sol ferrugineux tropical à l’Ouest du Burkina Faso. Afr. Sci. 2017, 13, 226–235. [Google Scholar]
- Diatta, A.A.; Abaye, O.; Thomason, W.E.; Lo Guèye, F.; Baldé, A.B.; Tine Vaughan, L.J.; Thompson, T.L. Effet de l’association du haricot mungo sur le rendement du mil dans le Bassin arachidier, Sénégal. Innov. Agron. INRAE 2019, 74, 69–81. [Google Scholar]
- Trail, P.; Abaye, O.; Thomason, W.E.; Thompson, T.L.; Gueye, F.; Diedhiou, I.; Diatta, M.B.; Faye, A. Evaluating Intercropping (Living Cover) and Mulching (Desiccated Cover) Practices for Increasing Millet Yields in Senegal. Agron. J. 2016, 108, 1742–1752. [Google Scholar] [CrossRef]
- Eilittä, M.; Sollenberger, L.E.; Littell, R.C.; Harrington, L.W. On-farm experiments with maize-mucuna systems in the Los Tuxtlas region of Veracruz, southern Mexico. II. Mucuna variety evaluation and subsequent maize grain yield. Exp. Agric. 2003, 39, 19. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.M.; Reddy, C.G.; Phogat, M.; Korav, S. Role of bio-fertilizers towards sustainable agricultural development: A review. J. Pharmacogn. Phytochem. 2018, 7, 1915–1921. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerhards, R.; Ouidoh, F.N.; Adjogboto, A.; Avohou, V.A.P.; Dossounon, B.L.S.; Adisso, A.K.D.; Heyn, A.; Messelhäuser, M.; Santel, H.-J.; Oebel, H. Crop Response to Leaf and Seed Applications of the Biostimulant ComCat® under Stress Conditions. Agronomy 2021, 11, 1161. https://doi.org/10.3390/agronomy11061161
Gerhards R, Ouidoh FN, Adjogboto A, Avohou VAP, Dossounon BLS, Adisso AKD, Heyn A, Messelhäuser M, Santel H-J, Oebel H. Crop Response to Leaf and Seed Applications of the Biostimulant ComCat® under Stress Conditions. Agronomy. 2021; 11(6):1161. https://doi.org/10.3390/agronomy11061161
Chicago/Turabian StyleGerhards, Roland, Fructueuse N. Ouidoh, André Adjogboto, Vodéa Armand Pascal Avohou, Berteulot Latus Sètondji Dossounon, Alexandra Koupamba Ditti Adisso, Alexandra Heyn, Miriam Messelhäuser, Hans-Joachim Santel, and Horst Oebel. 2021. "Crop Response to Leaf and Seed Applications of the Biostimulant ComCat® under Stress Conditions" Agronomy 11, no. 6: 1161. https://doi.org/10.3390/agronomy11061161





