Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement
Abstract
:1. Introduction
2. An Overview of Plant-Associated Bacteria
2.1. The Rhizobacteria
2.2. The Phyllobacteria
2.3. The Bacterial Endophytes
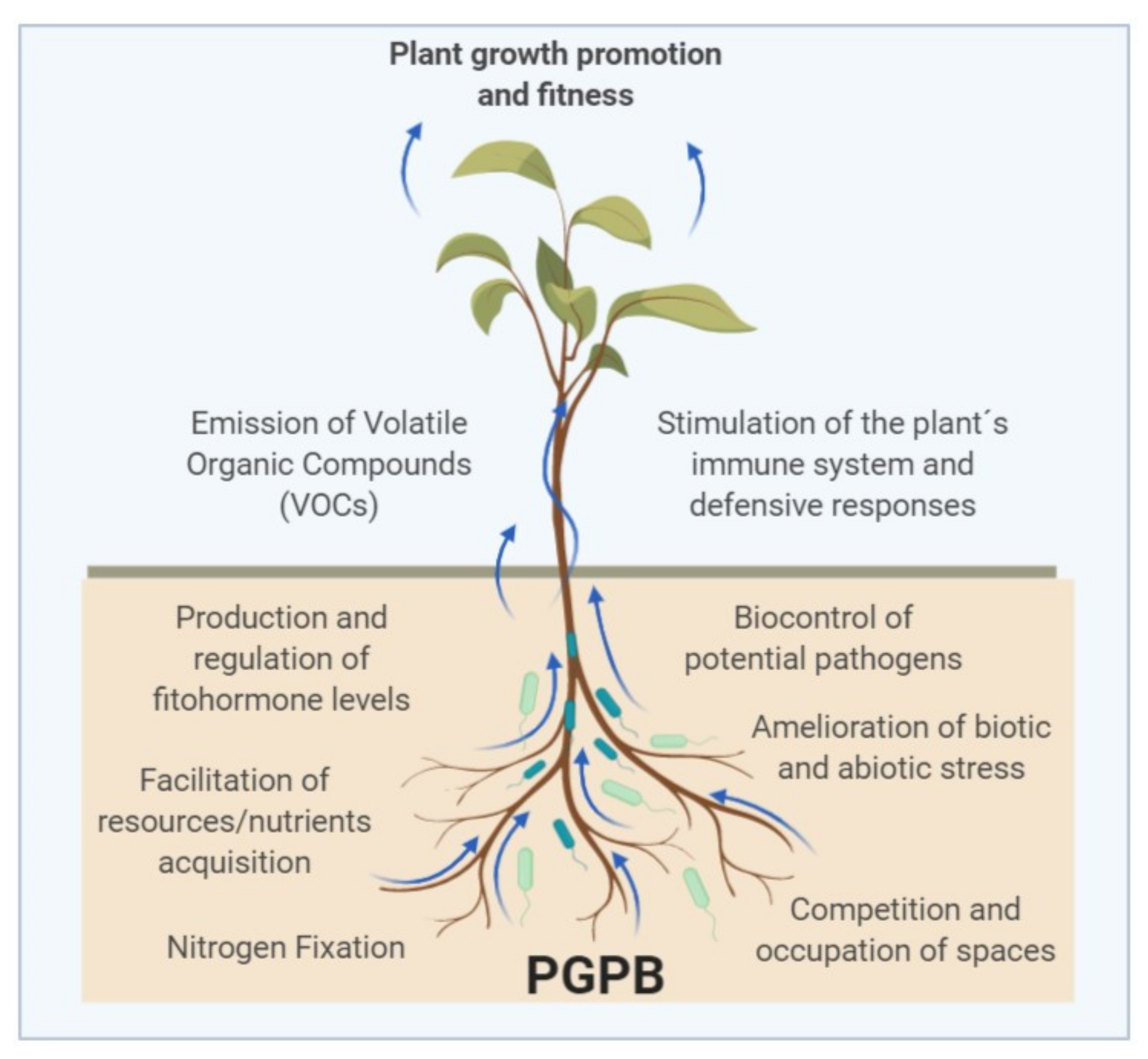
3. Beneficial Activities of PGPB
3.1. Direct Mechanisms
3.2. Indirect Mechanisms
| Bacterial Species and Strain | Mechanism and/or Benefit in the Host Plant | Plant Host Species | Type of Test or Applied Technique | Reference |
|---|---|---|---|---|
| Bacillus subtilis ES748, ES749 and a bacterial consortium | Synergistic interaction between species | Arabidopsis thaliana | Generation of mutants, colonization and maintenance assays in vitro | [41] |
| Agrobacterium rhizogenes K599 | Improvement in the acquisition of nitrogen and change in the secretion of organic compounds | Phaseolus vulgaris L. | In vitro colonization assays and microbial community analysis | [42] |
| Pseudomonas umsongensis, Arthrobacter defluvii, Streptomyces gardneri, Microbacterium yannicii, Variovorax ginsengisoli, Cupriavidus laharis, Bosea vestrisii, Bosea robiniae | Production of phytohormones, secretion of siderophores and stimulating effects | Zea mays L., Populus nigra and Arabidopsis thaliana | Detection of siderophores, phytohormones and colonization and permanence tests in vitro and in the field | [1,43] |
| Sinorhizobium meliloti 2011 | Improved nitrogen acquisition, secretion of signaling compounds | Medicago truncatula | Detection of nitrogen-fixing nodules and in vitro colonization assays | [44] |
| Bacillus cereus YL6 | Improved phosphorus acquisition and phytohormone biosynthesis | Glycine max, Triticum vulgare, Brassica rapa subsp. pekinensis | Phosphorus solubilization test, in vitro and field colonization test of plant species | [45] |
| Pseudomonas aeruginosa NXHG29 | Dual antagonism, quorum sensing, and biofilm formation | Nicotiana tabacum | In vitro colonization and antagonism assays | [46] |
| Pseudomonas stutzeri E25, Stenotrophomas maltophilia CR71 | Antagonism, secretion of volatile organic compounds and synergism between bacterial species | Physalis ixocarpa, Lycopersicon esculentum cv Saladette | In vitro antagonism, promotion, colonization and volatile compound detection assays by GC-MS | [47] |
| Bacillus cereus SA1 | Production of phytohormones, secretion of organic acids. Improved the biomass and chlorophyll content. | Glycine max | Detection of phytohormones, HPLC compound determination and assays in plants. | [48] |
| Pseudomonas fluorescens UM270 | Genes involved in signaling, antioxidant activities, secretion systems, and biofilm production | not applicable | Genomic comparison Pseudomonas strains | [49] |
| Bacillus megaterium, Enterobacter C7 | Improvement in the acquisition of Na, Ca, Mg, production of antioxidants, phytohormones and secretion of secondary metabolites | Solanum lycopersicum | Detection of phytohormones, evaluation in the change of metabolic profiles by GC-MS and in vitro colonization assay | [50] |
| Bacillus subtilis SWR01 | Genes involved in swarm signaling and motility | Solanum lycopersicum | Generation of mutants and in vitro colonization assay | [51] |
| Bacillus thuringiensis UM96, Pseudomonas fluorescens UM16, UM240, UM256, UM270 | Synergistic interaction between species and plant growth stimulation of plants | Physalis ixocarpa | In vitro colonization assay | [2,52] |
| Bacillus altitudinis KP-14 | Production of phytohormones, secretion of siderophores, Improvement in the acquisition of phosphorus, Antagonism, secretion of volatile organic compounds | Miscanthus × giganteus (Mxg), Brassica alba | Detection of phytohormones and siderophores, phosphorus solubilization test, in vitro antagonism assays, volatile compound detection assays and assays in plants | [53] |
| Bacillus amyloliquefaciens NJN-6 | Organic compound secretion and biofilm generation | Musa paradisiaca | Chemotaxis Assays, In Vitro Colonization Assay, and HPLC Compound Determination | [54] |
| Rhizobium etli G12, Pseudomonas trivialis, Pseudomonas jessenii, Serratia plymuthica, Bacillus subtilis Sb4-23, Mc5-Re2, Mc2-Re2 | Antibiosis, biofilm formation, chemotaxis, phytohormone production, secretion of toxic compounds to nematodes and induced systemic resistance | Solanum lycopersicum cv moneymaker | In vitro colonization and antagonism assays | [55] |
| Paenibacillus polymyxa CF05 | Production of phytohormones, secretion of antioxidants and phenolic compounds | Solanum lycopersicum cv Zheza 203 | Antioxidant detection assays, in vitro and greenhouse antagonism and colonization assays | [56] |
| Bacillus subtilis HJ5 | Antibiosis and biofilm production | Gossypium herbaceum | In vitro colonization and antibiosis assays | [57] |
| Pseudomonas sp. DSMZ 13134 | Improvement in the acquisition of phosphorus, secretion of siderophores, antimicrobial compounds and induction of systemic resistance | Hordeum vulgare | Phosphorus solubilization test, siderophore detection, antagonism and colonization test in vitro | [58] |
| Kliebsella pneumoniae NG14 | Improved nitrogen acquisition and biofilm production | Oryza sativa L. | Detection of genes associated with nitrogen metabolism and in vitro colonization assay | [59] |
| Azospirillum brasilense SP245, SK048, SK051, SK454 | Genes involved in motility | Triticum vulgare | Generation of mutants and in vitro colonization assay | [60] |
4. Formulation of Bioinoculants and Recommendations on Their Application
5. Challenges in the Application of Bioinoculants
6. Other Challenges of PGPB Application: The Case of Latin America
7. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [Green Version]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Lugtenberg, B. Use of Plant Growth-Promoting Rhizobacteria to Alleviate Salinity Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 1, pp. 73–96. [Google Scholar]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Sánchez-Yáñez, J.M.; Santos-Villalobos, S.D.L. Methods for Detecting Biocontrol and Plant Growth-Promoting Traits in Rhizobacteria. In Microbes and Signaling Biomolecules against Plant Stress; Springer: Singapore, 2019; pp. 133–149. [Google Scholar]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant Growth Promoting Rhizobacterial Mitigation of Drought Stress in Crop Plants: Implications for Sustainable Agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, B.R. Issues Regarding the Use of PGPB. In Beneficial Plant-Bacterial Interactions; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Hartmann, A.; Rothballer, M.; Schmid, M. Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 2007, 312, 7–14. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Castillo, J.A. Influence of Light on Plant–Phyllosphere Interaction. Front. Plant Sci. 2018, 9, 1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunster, L.; Fokkema, N.J.; Schippers, B. Effect of Surface-Active Pseudomonas spp. on Leaf Wettability. Appl. Environ. Microbiol. 1989, 55, 1340–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the Phyllosphere Microbiome to Plant Health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef]
- Rodriguez, R.; Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol Res. 2005, 109, 661–687. [Google Scholar] [CrossRef] [Green Version]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, S.S.; Talat, M.A.; Dar, M.H.; Hamid, A.; Ahmad, L. Soil phosphorus fixation chemistry and role of phosphate solubilizing bacteria in enhancing its efficiency for sustainable cropping—A review. J. Pure. Appl. Microbiol. 2012, 6, 1905–1911. [Google Scholar]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2016, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial siderophores: A mini review. J. Basic Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth promoting rhizobacteria. J. Plant Growth. Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.D.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Batista, M.B.; Dixon, R. Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 2019, 47, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.X.; Tavares, M.J.; Franck, J.; Ali, S.; Glick, B.R.; Rossi, M.J. ACC deaminase plays a major role in Pseudomonas fluorescens YsS6 ability to promote the nodulation of Alpha- and Betaproteobacteria rhizobial strains. Arch. Microbiol. 2019, 201, 817–822. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Zafar-ul-Hye, M.; Sajjad, S.; Naveed, M. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for coinoculation with Rhizobium leguminosarum to improve growth, nodulation, and yield of lentil. Biol. Fertil Soils. 2011, 47, 457–465. [Google Scholar] [CrossRef]
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on Microbes: Diverse Roles of Microbial Volatile Organic Compounds in Plant Health. Am. Phytopathol. Soc. 2013, 26, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Hernández-León, R.; Rojas-Solís, D.; Contreras, M.; Orozco-Mosqueda, M.D.C.; Macías-Rodríguez, L.I.; de la Cruz, H.R.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; García-Pineda, E.; Valencia-Cantero, E. Bacterial Compound N,N-Dimethylhexadecylamine Modulates Expression of Iron Deficiency and Defense Response Genes in Medicago truncatula Independently of the Jasmonic Acid Pathway. Plants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Martínez-Absalón, S.; Rojas-Solís, D.; Hernández-León, R.; Prieto-Barajas, C.; Orozco-Mosqueda, M.D.C.; Peña-Cabriales, J.J.; Sakuda, S.; Valencia-Cantero, E.; Santoyo, G. Potential use and mode of action of the new strain Bacillus thuringiensis UM96 for the biological control of the grey mould phytopathogenBotrytis cinerea. Biocontrol Sci. Technol. 2014, 24, 1349–1362. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Leclère, V.; Béchet, M.; Adam, A.; Guez, J.S.; Wathelet, B.; Ongena, M.; Jacques, P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 2005, 71, 4577–4584. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.W.; Ryu, C.M. Bacterial endophytes as elicitors of induced systemic resistance. In Microbial Root Endophytes; Soil Biology; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 9, pp. 33–52. [Google Scholar]
- Eckshtain-Levi, N.; Harris, S.L.; Roscios, R.Q.; Shank, E.A. Bacterial Community Members Increase Bacillus subtilis Maintenance on the Roots of Arabidopsis thaliana. Phytobiomes J. 2020, 4, 303–313. [Google Scholar] [CrossRef]
- Barraza, A.; Vizuet-De-Rueda, J.C.; Alvarez-Venegas, R. Highly diverse root endophyte bacterial community is driven by growth substrate and is plant genotype-independent in common bean (Phaseolus vulgaris L.). PeerJ 2020, 8, e9423. [Google Scholar] [CrossRef]
- Bertola, M.; Mattarozzi, M.; Sanangelantoni, A.M.; Careri, M.; Visioli, G. PGPB Colonizing Three-Year Biochar-Amended Soil: Towards Biochar-Mediated Biofertilization. J. Soil Sci. Plant Nutr. 2019, 19, 841–850. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Wang, Q.; Zhang, C.; Yu, Y.; Tian, J.; Kong, Z. The host actin cytoskeleton channels rhizobia release and facilitates symbiosome accommodation during nodulation in Medicago truncatula. New Phytol. 2018, 221, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Ku, Y.; Xu, G.; Tian, X.; Xie, H.; Yang, X.; Cao, C. Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PLoS ONE 2018, 13, e0200181. [Google Scholar]
- Ma, L.; Zhang, H.-Y.; Zhou, X.-K.; Yang, C.-G.; Zheng, S.-C.; Duo, J.-L.; Mo, M.-H. Biological control tobacco bacterial wilt and black shank and root colonization by bio-organic fertilizer containing bacterium Pseudomonas aeruginosa NXHG29. Appl. Soil Ecol. 2018, 129, 136–144. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.D.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Salmerón, J.E.; Hernández-León, R.; Orozco-Mosqueda, M.D.C.; Valencia-Cantero, E.; Moreno-Hagelsieb, G.; Santoyo, G. Draft Genome Sequence of the Biocontrol and Plant Growth-Promoting Rhizobacterium Pseudomonas fluorescens strain UM270. Stand. Genom. Sci. 2016, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibort, P.; Molina, S.; Núñez, R.; Zamarreño, Á.M.; García-Mina, J.M.; Ruiz-Lozano, J.M.; Orozco-Mosqueda, M.D.C.; Glick, B.R.; Aroca, R. Tomato ethylene sensitivity determines interaction with plant growth-promoting bacteria. Ann. Bot. 2017, 120, 101–122. [Google Scholar] [CrossRef]
- Gao, S.; Wu, H.; Yu, X.; Qian, L.; Gao, X. Swarming motility plays the major role in migration during tomato root colonization by Bacillus subtilis SWR01. Biol. Control 2016, 98, 11–17. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Hernández-Pacheco, C.E.; Santoyo, G. Evaluation of Bacillus and Pseudomonas to colonize the rhizosphere and their effect on growth promotion in tomato (Physalis ixocarpa Brot. ex Horm.). Rev. Chapingo Ser. Hortic. 2016, 22, 45–57. [Google Scholar] [CrossRef]
- Pranaw, K.; Pidlisnyuk, V.; Trögl, J.; Malinská, H. Bioprospecting of a Novel Plant Growth-Promoting Bacterium Bacillus altitudinis KP-14 for Enhancing Miscanthus × giganteus Growth in Metals Contaminated Soil. Biology 2020, 9, 305. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, B.; Lv, N.; Sun, Y.; Jiang, X.; Li, R.; Ruan, Y.; Shen, Q. Effect of the combination of bio-organic fertiliser with Bacillus amyloliquefaciens NJN-6 on the control of banana Fusarium wilt disease, crop production and banana rhizosphere culturable microflora. Biocontrol Sci. Technol. 2015, 25, 716–731. [Google Scholar] [CrossRef]
- Adam, M.; Heuer, H.; Hallmann, J. Bacterial Antagonists of Fungal Pathogens also Control Root-Knot Nematodes by Induced Systemic Resistance of Tomato Plants. PLoS ONE 2014, 9, e90402. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Liang, Y.; Zhang, L.; Wang, Y.; Guo, Y. Induced systemic resistance and growth promotion in tomato by an indole-3-acetic acid-producing strain ofPaenibacillus polymyxa. Ann. Appl. Biol. 2014, 165, 270–279. [Google Scholar] [CrossRef]
- Li, X.; Rui, J.; Mao, Y.; Yannarell, A.; Mackie, R. Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar. Soil Biol. Biochem. 2014, 68, 392–401. [Google Scholar] [CrossRef]
- Fröhlich, A.; Buddrus-Schiemann, K.; Durner, J.; Hartmann, A.; von Rad, U. Response of barley to root colonization by Pseudomonas sp. DSMZ 13134 under laboratory, greenhouse, and field conditions. J. Plant. Interact. 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Sun, X.; Yang, H.; Wang, Y.; Song, W. Study on Mechanisms of Colonization of Nitrogen-Fixing PGPB, Klebsiella pneumoniae NG14 on the Root Surface of Rice and the Formation of Biofilm. Curr. Microbiol. 2010, 62, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Shelud’Ko, A.V.; Shirokov, A.; Sokolova, M.K.; Sokolov, O.I.; Petrova, L.; Matora, L.Y.; Katsy, E.I. Wheat root colonization by Azospirillum brasilense strains with different motility. Microbiology 2010, 79, 688–695. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.; Griffith, G.; Withers, P. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Sreedhar, S.S.; Mohan, V. Effect of bio-inoculants on seed germination and disease control of commercially important fast growing native tree species in nursery. Kavaka 2014, 43, 41–45. [Google Scholar]
- Kaymak, H.C. Potential of PGPR in Agricultural Innovations. In Plant Growth and Health Promoting Bacteria; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; pp. 45–79. [Google Scholar]
- Wong, C.; Saidi, N.B.; Vadamalai, G.; Teh, C.Y.; Zulperi, D. Effect of bioformulations on the biocontrol efficacy, microbial viability and storage stability of a consortium of biocontrol agents against Fusarium wilt of banana. J. Appl. Microbiol. 2019, 127, 544–555. [Google Scholar] [CrossRef]
- Arora, N.K.; Khare, E.; Maheshwari, D.K. Plant Growth Promoting Rhizobacteria: Constraints in Bioformulation, Commercialization, and Future Strategies. In Plant Growth and Health Promoting Bacteria; Microbiology Monographs; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; pp. 97–116. [Google Scholar]
- Anriquez, A.L.; Silberman, J.E.; Nuñez, J.A.D.; Albanesi, A.S. Biofertilizers in Argentina. In Biofertilizers for Sustainable Agriculture and Environment; Soil Biology; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 55, pp. 225–244. [Google Scholar]
- Keswani, C.; Dilnashin, H.; Birla, H.; Singh, S.P. Regulatory barriers to Agricultural Research commercialization: A case study of biopesticides in India. Rhizosphere 2019, 11, 100155. [Google Scholar] [CrossRef]
- Saritha, M.; Tollamadugu, N.P. The Status of Research and Application of Biofertilizers and Biopesticides: Global Scenario. In Recent Developments in Applied Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 2019; pp. 195–207. [Google Scholar] [CrossRef]
- Khalil, C.A.; Conforti, P.; Ergin, I.; Gennari, P. Defining Small Scale Food Producers to Monitor Target 2.3 of the 2030 Agenda for Sustainable Development. In FAO Statistics Division Working Paper Series ESS/17-12; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017. [Google Scholar]
- Durand, J.; Massey, D.S.; Zenteno, R.M. Mexican immigration to the United States: Continuities and changes. Lat. Am. Res. Rev. 2001, 36, 107–127. [Google Scholar] [PubMed]
- Pierson, W.; Kandel, Y.; Allen, T.; Faske, T.; Tenuta, A.; Wise, K.; Mueller, D. Soybean Yield Response to In-furrow Fungicides, Fertilizers, and Their Combinations. Crop Forage Turfgrass Manag. 2018, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Navarro, C. Mexico Continues to Promote Avocado Production, but Environmental Concerns Persist; University of New Mexico UNM Digital Repository: Albuquerque, NM, USA, 2018. [Google Scholar]
- Wightwick, A.; Walters, R.; Allinson, G.; Reichman, S.; Menzies, S.R.A.N. Environmental Risks of Fungicides Used in Horticultural Production Systems. In Fungicides; InTech: Rijeka, Croatia, 2010; pp. 273–304. [Google Scholar] [CrossRef] [Green Version]
- Stein, S.J.; Stein, B.H. La Herencia Colonial de América Latina/Colonial Heritage of Latin America; Siglo: Mexico City, Mexico, 2002; Volume 21. [Google Scholar]
- Lancho-Barrantes, B.S.; Cantú-Ortiz, F.J. Science in Mexico: A bibliometric analysis. Scientometrics 2019, 118, 499–517. [Google Scholar] [CrossRef]
- Chávez-Díaz, I.F.; Molina, L.X.Z.; Cárdenas, C.I.C.; Anaya, E.R.; Ramírez, S.R.; Villalobos, S.D.L.S. Consideraciones sobre el uso de biofertilizantes como alternativa agro- biotecnológica sostenible para la seguridad alimentaria en México. Rev. Mex. Cienc. Agrícolas 2020, 11, 1423–1436. [Google Scholar] [CrossRef]
- López, K.; Carrillo, M.F.Z. Hacia la inclusión de los pequeños agricultores, población vulnerable, en programas de vigilancia toxicológica mediante la implementación de marcadores biológicos de fácil acceso en zonas rurales de Colombia. Rev. Int. Cienc. Soc. 2016, 5, 95–102. [Google Scholar]
- Peeters, R.; Trujillo Jiménez, H.G.; O’Connor, E.; Ogarrio Rojas, P.; González Galindo, M.; Morales Tenorio, D.M. Low-Trust Bureaucracy: An Exploration of the Mechanisms and Costs of Administrative Burdens in Mexico. 2018. Available online: www.libreriacide.com/librospdf/DTAP-305.pdf (accessed on 1 April 2021).
- López Reyes, L.; Tapia Hernández, A.; Jiménez Salgado, T.; Espinosa Victoria, D.; Carcaño Montiel, M.D. José de Jesús Caballero Mellado líder de la microbiología de suelos en México. Terra Latinoam. 2014, 32, 87–97. [Google Scholar]
- Galindo, E.; Serrano-Carreón, L.; Gutiérrez, C.R.; Balderas-Ruíz, K.A.; Muñoz-Celaya, A.L.; Mezo-Villalobos, M.; Arroyo-Colín, J. Desarrollo histórico y los retos tecnológicos y legales para comercializar Fungifree AB®, el primer biofungicida 100% mexicano. TIP. Rev. Espec. Cienc. Quím.Biol. 2015, 18, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Tawate, S.; Gupta, R.; Jain, K. Technology Commercialization in Bio-fertilizer Firm: An Indian Case. Int. J. Bus. Compet. 2018, 13, 65–74. [Google Scholar]
- Altieri, M.A. Agroecología: Principios y Estrategias para Diseñar Sistemas Agrarios Sustentables. In Agroecología: El Camino Hacia una Agricultura Sustentable; Sarandon, S.J., Ed.; Ediciones Científicas Americanas: Buenos Aires, Argentina, 2002; pp. 49–56. [Google Scholar]
- Ortiz-Galeana, M.A.; Hernández-Salmerón, J.E.; Valenzuela-Aragón, B.; E Lossantos-Villalobos, S.D.; Rocha-Granados, M.D.C.; Santoyo, G. Diversidad de bacterias endófitas cultivables asociadas a plantas de arándano (Vaccinium corymbosum L.) cv. Biloxi con actividades promotoras del crecimiento vegetal. Chil. J. Agric. Anim. Sci. 2018, 34, 140–151. [Google Scholar] [CrossRef]
- Flores, A.; Diaz-Zamora, J.T.; Orozco-Mosqueda, M.D.C.; Chávez, A.; Villalobos, S.D.L.S.; Valencia-Cantero, E.; Santoyo, G. Bridging genomics and field research: Draft genome sequence of Bacillus thuringiensis CR71, an endophytic bacterium that promotes plant growth and fruit yield in Cucumis sativus L. 3 Biotech 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Morrison, B.A.; Cozatl-Manzano, R. Initial Evidence for Use of Periphyton as an Agricultural Fertilizer by the Ancient Maya Associated with El Eden Wetland, Northern Quintana Roo, Mexico. In The Lowland Maya Area: Three Millennia at the Human-Wildland Interface; Fedick, S., Allen, M., Jimenez-Osornio, J., Gomez-Pompa, A., Eds.; CRC Press: New York, NY, USA, 2003; pp. 401–413. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. https://doi.org/10.3390/agronomy11061167
Orozco-Mosqueda MdC, Flores A, Rojas-Sánchez B, Urtis-Flores CA, Morales-Cedeño LR, Valencia-Marin MF, Chávez-Avila S, Rojas-Solis D, Santoyo G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy. 2021; 11(6):1167. https://doi.org/10.3390/agronomy11061167
Chicago/Turabian StyleOrozco-Mosqueda, Ma. del Carmen, Aurora Flores, Blanca Rojas-Sánchez, Carlos A. Urtis-Flores, Luzmaria R. Morales-Cedeño, María F. Valencia-Marin, Salvador Chávez-Avila, Daniel Rojas-Solis, and Gustavo Santoyo. 2021. "Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement" Agronomy 11, no. 6: 1167. https://doi.org/10.3390/agronomy11061167
APA StyleOrozco-Mosqueda, M. d. C., Flores, A., Rojas-Sánchez, B., Urtis-Flores, C. A., Morales-Cedeño, L. R., Valencia-Marin, M. F., Chávez-Avila, S., Rojas-Solis, D., & Santoyo, G. (2021). Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy, 11(6), 1167. https://doi.org/10.3390/agronomy11061167







