Influence of High Tunnel Microclimate on Fruit Quality and Calcium Concentration in ‘Santina’ Sweet Cherries in a Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Study Site and Treatments
2.2. Environmental Monitoring, Fruit Development, and Fruit Quality
2.3. Fruit Mineral Analysis
2.4. Statystical Analysis
3. Results
3.1. Microclimate
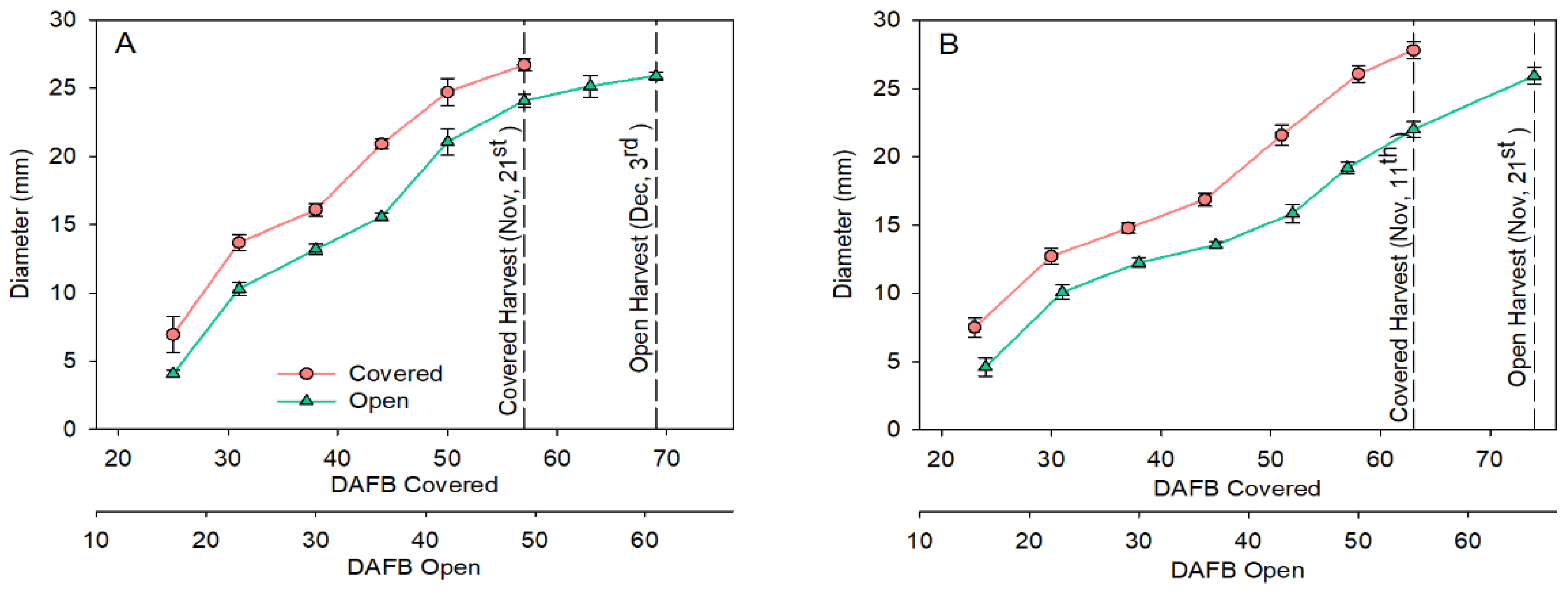
3.2. Fruit Development and Fruit Quality at Harvest
3.3. Cracking Incidence
3.4. Postharvest Evaluation
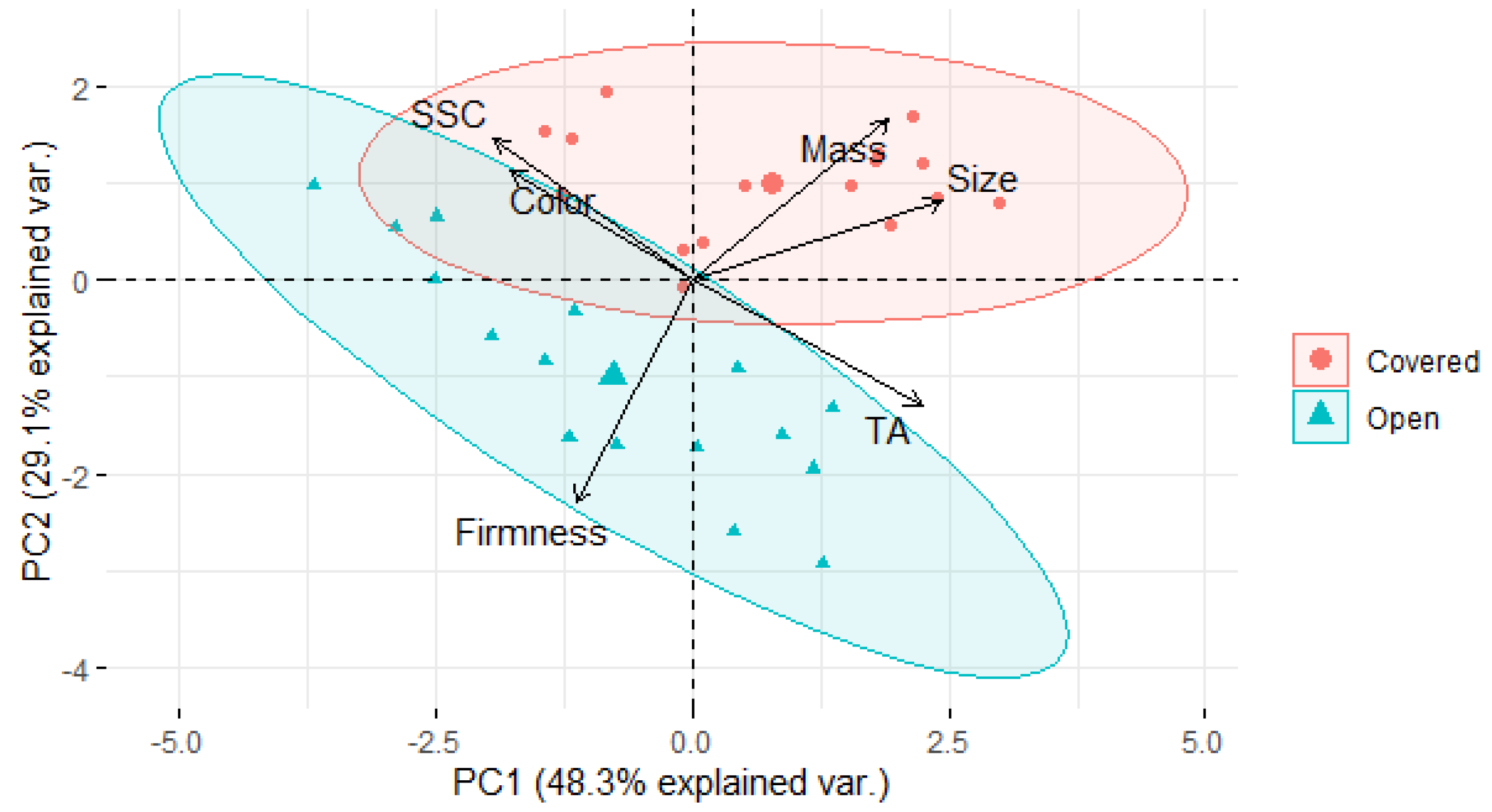
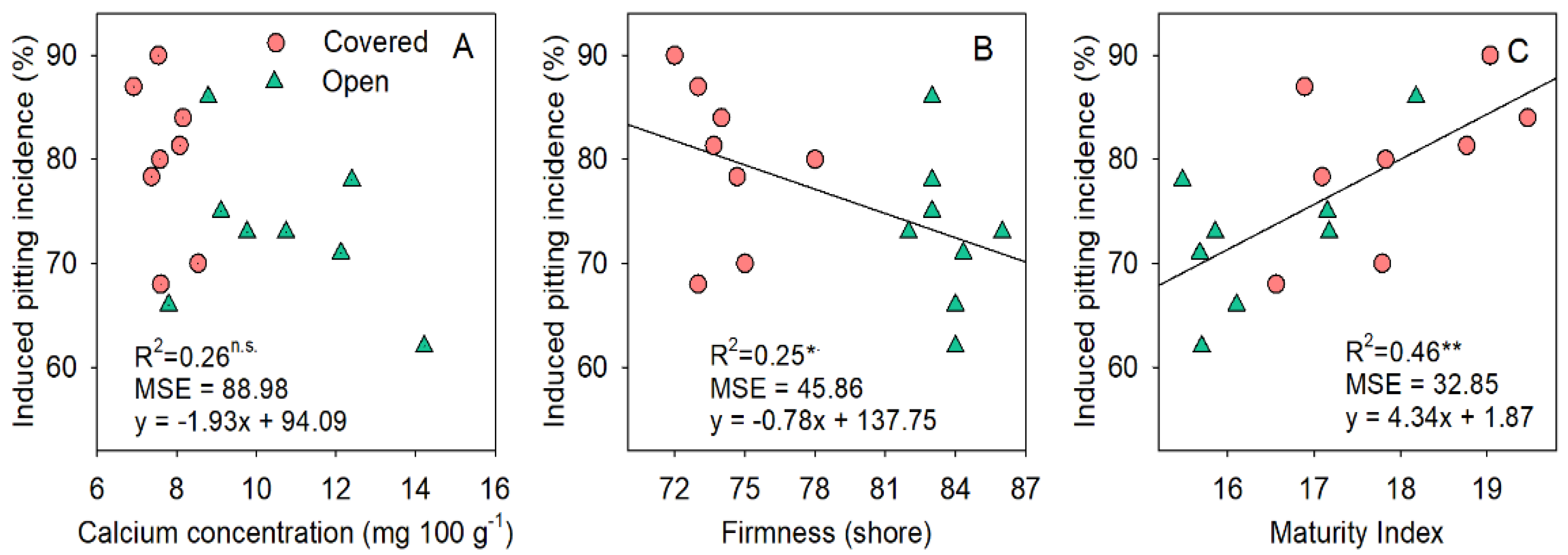
3.5. Fruit Mineral Concentration and Its Relationship with Fruit Firmness and Pitting Incidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FAOSTAT–Food and Agriculture Organization of the United Nations Statistics Division. Agricultural Production. Cherries. 2020. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 31 January 2021).
- Bujdosó, G.; Hrotkó, K. Chapter 1: Cherry production. In Cherries: Botany, Production and Uses, 1st ed.; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 1–13. [Google Scholar]
- Balbotin, C.; Ayala, H.; Bastías, R.M.; Tapia, G.; Ellena, M.; Torres, C.; Yuri, J.A.; Quero-García, J.; Ríos, J.C.; Silva, H. Cracking in sweet cherries: A comprehensive review from a physiological, molecular, and genomic perspective. Chil. J. Agric. Res. 2013, 73, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Brüggenwirth, M.; Knoche, M. Mechanical properties of skins of sweet cherry fruit of differing susceptibilities to cracking. J. Amer. Soc. Hort. Sci. 2016, 141, 162–168. [Google Scholar] [CrossRef]
- Blanke, M.M.; Lang, G.A.; Meland, M. Chapter 11: Orchard microclimate modification. In Cherries: Botany, Production and Uses, 1st ed.; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 244–268. [Google Scholar]
- Lang, G.A. High Tunnel Tree Fruit Production: The Final Frontier? HortTechnology 2009, 19, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Lamont, W.J. Overview of the Use of High Tunnels Worldwide. HortTechnology 2009, 19, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. Eco-physiological response, water productivity and fruit quality of sweet cherry trees under high tunnels. Sci. Hortic. 2021, 286, 110180. [Google Scholar] [CrossRef]
- Meland, M.; Frøynes, O.; Kaiser, C. High tunnel production systems improve yields and fruit size of sweet cherry. Acta Hortic. 2017, 1161, 117–124. [Google Scholar] [CrossRef]
- Lang, G.A. Growing sweet cherries under plastic covers and tunnels: Physiological aspects and practical considerations. Acta Hortic. 2014, 1020, 303–312. [Google Scholar] [CrossRef]
- Zoffoli, J.P.; Toivonen, P.; Wang, Y. Chapter 19: Postharvest biology and handling for fresh markets. In Cherries: Botany, Production and Uses, 1st ed.; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 460–484. [Google Scholar]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Mitchell, F.G.; Mayer, G.; Kader, A.A. Injuries cause deterioration of sweet cherries. Califotnia Agric. 1980, 34, 14–15. [Google Scholar]
- Mattheis, J.P.; Roberts, R.G. Fumigation of sweet cherry (Prunus avium ‘Bing’) fruit with low molecular weight aldehydes for postharvest decay control. Plant Dis. 1993, 77, 810–814. [Google Scholar] [CrossRef]
- Shao, Y.; Xuan, G.; Hu, Z.; Gao, Z.; Liu, L. Determination of the bruise degree for cherry using Vis-NIR reflection spectroscopy coupled with multivariate analysis. PLoS ONE 2019, 14, e0222633. [Google Scholar] [CrossRef]
- Param, N.; Zoffoli, J.P. Genotypic differences in sweet cherries are associated with the susceptibility to mechanical damage. Sci. Hortic. 2016, 211, 410–419. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Manganaris, G.A. Chapter 15.2: Stone fruits: Sweet cherries (Prunus avium L.). In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce. Part II: CA/MA Requirements and Recommendations for Fresh and Fresh-Cut Fruits, 1st ed.; Gil, M.I., Beaudry, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 323–329. [Google Scholar]
- Schlegel, H.J.; Grimm, E.; Winkler, A.; Knoche, M. Orange peel disorder in sweet cherry: Mechanism and triggers. Postharvest Biol. Technol. 2018, 137, 119–128. [Google Scholar] [CrossRef]
- Mitcham, E.J.; Crisosto, C.H. Postharvest handling systems: Stone fruits. III Sweet cherry. In Postharvest Technology of Horticultural Crops, 3rd ed.; Kader, A.A., Ed.; University of California, Division of Agricultural and Natural Resources: Richmond, CA, USA, 2002; pp. 353–356. [Google Scholar]
- Winkler, A.; Knoche, M. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar] [CrossRef]
- Measham, P.F.; Richardson, A.; Townsend, A. Calcium application and impacts on cherry fruit quality. Acta Hortic. 2017, 1161, 375–382. [Google Scholar] [CrossRef]
- Wang, Y.; Long, L.E. Physiological and biochemical changes relating to postharvest splitting of sweet cherries affected by calcium application in hydrocooling water. Food Chem. 2015, 181, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Candan, A.P.; Raffo, M.D.; Calvo, G.; Gomila, T. Study of the main points of impact during cherry handling and factors affecting pitting sensitivity. Acta Hortic. 2014, 1020, 137–141. [Google Scholar] [CrossRef]
- Clayton, M.; Biasi, W.V.; Agar, I.T.; Southwick, S.M.; Mitcham, E.J. Sensory quality of ‘bing’ sweet cherries following preharvest treatment with hydrogen cyanamide, calcium ammonium nitrate, or gibberellic acid. HortScience 2006, 41, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High tunnel cultivation of sweet cherry (Prunus avium L.): Physiological and production variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- Lang, G.A.; Sage, L.; Wilkinson, T. Ten years of studies on systems to modify sweet cherry production environments: Retractable roofs, high tunnels, and rain-shelters. Acta Hortic. 2016, 1130, 83–90. [Google Scholar] [CrossRef]
- Mika, A.; Buler, Z.; Wójcik, K.; Konopacka, D. Influence of the Plastic Cover on the Protection of Sweet Cherry Fruit Against Cracking, on the Microclimate under Cover and Fruit Quality. J. Hort. Res. 2019, 27, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Santibáñez, F.; Santibáñez, P.; Caroca, C.; González, P. Tomo III: Regiones de Valparaíso, Metropolitana, O’Higgins y Maule. In Atlas Agroclimático de Chile Estado Actual y Tendencias del Clima, 1st ed.; Universidad de Chile, Ed.; FIA: Santiago, Chile, 2017; pp. 157–163. [Google Scholar]
- Christensen, J.V. Cracking in cherries. III. Determination of cracking susceptibility. Acta Agric. Scand. 1972, 22, 128–136. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Kappel, F.; Stan, S.; McKenzie, D.-L.; Hocking, R. Firmness, Respiration, and Weight Loss of ‘Bing’, ‘Lapins’ and ‘Sweetheart’ Cherries in Relation to Fruit Maturity and Susceptibility to Surface Pitting. HortScience 2004, 39, 1066–1069. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual; International Center for Agricultural Research in the Dry Areas (ICARDA): Islamabad, Pakistan, 2001. [Google Scholar]
- Hedhly, A.; Hormaza, J.L.; Herrero, M. Warm temperatures at bloom reduce fruit set in sweet cherry. J. Appl. Bot. Food Qual. 2007, 81, 158–164. [Google Scholar]
- Zhang, L.; Ferguson, L.; Whiting, M.D. Temperature effects on pistil viability and fruit set in sweet cherry. Sci. Hortic. 2018, 241, 8–17. [Google Scholar] [CrossRef]
- Kwon, T.J.; Saeed, S. Effect of temperature on the foraging activity of Bombus terrestris L. (Hymenoptera: Apidae) on greenhouse hot pepper (Capsicum annuum L.). Appl. Entomol. Zool. 2003, 38, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Najla, S.; Vercambre, G.; Gérard, M. Effects of water deficit and variations of fruit microclimate on peach fruit growth and quality. Plant Stress 2011, 6, 33–38. [Google Scholar]
- Blanco, V.; Martínez-Hernández, G.B.; Artés-Hernández, F.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Water relations and quality changes throughout fruit development and shelf life of sweet cherry grown under regulated deficit irrigation. Agric. Water Manag. 2019, 217, 243–254. [Google Scholar] [CrossRef]
- Measham, P.F.; Wilson, S.J.; Gracie, A.J.; Bound, S.A. Tree water relations: Flow and fruit. Agric. Water Manag. 2014, 137, 59–67. [Google Scholar] [CrossRef]
- Blanco, V.; Domingo, R.; Pérez-Pastor, A.; Blaya-Ros, P.J.; Torres-Sánchez, R. Soil and plant water indicators for deficit irri-gation management of field-grown sweet cherry trees. Agric. Water Manag. 2018, 208, 83–94. [Google Scholar] [CrossRef]
- Kappel, F.; Lane, W.D.; MacDonald, R.; Lapins, K.; Schmidt, H. ‘Santina’, ‘Sumpaca Celeste’ and ‘Sumnue Cristalina’ Sweet Cherries. HortScience 1998, 33, 1087–1089. [Google Scholar] [CrossRef] [Green Version]
- Neilsen, G.H.; Neilsen, D.; Kappel, F.; Forge, T. Interaction of irrigation and soil management on sweet cherry productivity and fruit quality at different crop loads that simulate those occurring by environmental extremes. HortScience 2014, 49, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Bound, S.A.; Close, D.C.; Quentin, A.G.; Measham, P.F.; Whiting, M.D. Crop load and time of thinning interact to affect fruit quality in sweet cherry. J. Agric. Sci. 2013, 5, 216–230. [Google Scholar] [CrossRef] [Green Version]
- Blanco, V.; Torres-Sánchez, R.; Blaya-Ros, P.J.; Pérez-Pastor, A.; Domingo, R. Vegetative and reproductive response of ‘Prime Giant’ sweet cherry trees to regulated deficit irrigation. Sci. Hortic. 2019, 249, 478–489. [Google Scholar] [CrossRef]
- Mucha-Pelzer, T.; Müller, S.; Rohr, F.; Mewis, I. Cracking susceptibility of sweet cherries (Prunus avium L.) under different conditions. Commun. Agric. Appl. Biol. Sci. 2006, 71, 215–223. [Google Scholar]
- Lemus, G.; Valenzuela, J. Survey of the Chilean sweet cherry industry. Acta Hortic. 2006, 667, 379–387. [Google Scholar] [CrossRef]
- Wang, Y.; Long, L.E. Respiration and quality responses of sweet cherry to different atmospheres during cold storage and shipping. Postharvest Biol. Technol. 2014, 92, 62–69. [Google Scholar] [CrossRef]
- Cliff, M.A.; Stanich, K.; Toivonen, P.M.A. Evaluation of the Sensory, Physicochemical, and Visual Characteristics of a Sweet Cherry Cultivar Treated in a Commercial Orchard with a Cherry Cuticle Supplement when a Rainfall Event Does Not Occur. HortTechnology 2017, 27, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Neilsen, G.H.; Neilsen, D.; Forge, T. Chapter 9: Environmental limiting factors for Cherry production. In Cherries: Botany, Production and Uses, 1st ed.; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 189–222. [Google Scholar]
- Winkler, A.; Fiedler, B.; Knoche, M. Calcium physiology of sweet cherry fruits. Trees 2020, 34, 1157–1167. [Google Scholar] [CrossRef]
- Gomez, R.; Kalcsits, L. Physiological Factors Affecting Nutrient Uptake and Distribution and Fruit Quality in ‘Honeycrisp’ and ‘WA 38’ Apple (Malus × domestica Borkh.). HortScience 2020, 55, 1327–1336. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Beavers, W.B.; Sams, C.E.; Conway, W.S.; Brown, G.A. Calcium Source Affects Calcium Content, Firmness, and Degree of Injury of Apples during Storage. HortScience 1994, 29, 1520–1523. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.T.; Sajid, M. Influence of Calcium Sources and Concentrations on the Quality and Storage Performance of Peach. SJA 2017, 33, 532–539. [Google Scholar] [CrossRef]
- Hampson, C.R.; Stanich, K.; McKenzie, D.L.; Herbert, L.; Lu, R.; Li, J.; Cliff, M.A. Determining the optimum firmness for sweet cherries using Just-About-Right sensory methodology. Postharvest Biol. Technol. 2014, 91, 104–111. [Google Scholar] [CrossRef]
- Kappel, F.; Fisher-Fleming, B.; Hogue, E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 1996, 31, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Masoud, H.; Mousa, A.; Javad, N.M. Different K:Ca ratios affected fruit color and quality of strawberry ‘Selva’ in soilless system. J. Plant Nutr. 2018, 41, 243–252. [Google Scholar]
- Torkashvand, A.M.; Ahmadi, A.; Nikravesh, N.L. Prediction of kiwifruit firmness using fruit mineral nutrient concentration by artificial neural network (ANN) and multiple linear regressions (MLR). J. Integr. Agric. 2017, 16, 1634–1644. [Google Scholar] [CrossRef] [Green Version]
- Dilmaghani, M.R.; Malakouti, M.J.; Neilsen, G.H.; Fallahi, E. Interactive Effects of Potassium and Calcium on K/Ca Ratio and Its Consequences on Apple Fruit Quality in Calcareous Soils of Iran. J. Plant Nutr. 2005, 27, 1149–1162. [Google Scholar] [CrossRef]
- Kalcsits, L.; van der Heijden, G.; Reid, M.; Mullin, K. Calcium Absorption during Fruit Development in ‘Honeycrisp’ Apple Measured Using 44Ca as a Stable Isotope Tracer. HortScience 2017, 52, 1804–1809. [Google Scholar] [CrossRef] [Green Version]

| Covered | Open | |||||||
|---|---|---|---|---|---|---|---|---|
| Plot 1 | Plot 2 | Plot 1 | Plot 2 | |||||
| Developmental Stage | TMx (°C) | Tmin (°C) | TMx (°C) | Tmin (°C) | TMx (°C) | Tmin (°C) | TMx (°C) | Tmin (°C) |
| Full Bloom | 31.8 | 7.7 | 27.4 | 6.3 | 24.6 | 5.0 | 22.7 | 4.3 |
| Fruit Set | 28.6 | 9.6 | 28.1 | 5.4 | 23.5 | 7.9 | 24.2 | 4.9 |
| Stage I | 30.9 | 7.3 | 26.2 | 7.6 | 24.2 | 7.1 | 23.0 | 6.6 |
| Stage II | 32.8 | 8.2 | 28.3 | 8.0 | 29.0 | 8.0 | 26.3 | 7.5 |
| Stage III | 32.6 | 9.7 | 30.8 | 8.7 | 30.5 | 9.4 | 29.1 | 8.3 |
| Covered | Open | |||||||
| Plot 1 | Plot 2 | Plot 1 | Plot 2 | |||||
| Developmental Stage | RHMx (%) | RHmin (%) | RHMx (%) | RHmin (%) | RHMx (%) | RHmin (%) | RHMx (%) | RHmin (%) |
| Full Bloom | 95.7 | 33.6 | 94.8 | 41.5 | 85.1 | 23.4 | 90.3 | 38.2 |
| Fruit Set | 92.6 | 47.4 | 94.9 | 37.5 | 84.7 | 30.3 | 89.8 | 32.6 |
| Stage I | 94.0 | 35.6 | 95.8 | 43.6 | 90.8 | 24.4 | 89.4 | 28.3 |
| Stage II | 97.6 | 47.1 | 93.8 | 48.4 | 89.3 | 24.9 | 88.5 | 31.2 |
| Stage III | 94.8 | 33.1 | 95.2 | 36.2 | 89.4 | 29.1 | 89.9 | 27.9 |
| Mass (g) | Size (mm) | Firmness (Shore) | Color (1–5) | SSC (%) | TA (%) | MI | |||
|---|---|---|---|---|---|---|---|---|---|
| Plot 1 | Upper | Covered | 9.1 | 26.7 | 73.6 | 4.4 | 19.8 | 0.8 | 25.4 |
| Open | 9.2 | 24.9 | 82.4 | 4.4 | 20.8 | 0.7 | 27.9 | ||
| p-value | 0.717 | 0.013 | 0.021 | 0.722 | 0.153 | 0.013 | 0.079 | ||
| Lower | Covered | 9.7 | 27.4 | 78.9 | 4.2 | 19.2 | 0.9 | 21.2 | |
| Open | 8.9 | 26.0 | 84.3 | 4.4 | 17.3 | 0.8 | 22.3 | ||
| p-value | 0.042 | 0.088 | 0.015 | 0.194 | 0.026 | 0.145 | 0.312 | ||
| Plot 2 | Upper | Covered | 10.9 | 28.3 | 73.7 | 4.2 | 17.7 | 0.9 | 18.8 |
| Open | 8.6 | 26.4 | 83.0 | 4.2 | 17.4 | 1.0 | 17.2 | ||
| p-value | 0.008 | 0.041 | 0.001 | 0.671 | 0.763 | 0.055 | 0.108 | ||
| Lower | Covered | 11.0 | 28.5 | 74.7 | 4.2 | 16.9 | 1.0 | 17.1 | |
| Open | 9.2 | 27.3 | 84.3 | 4.1 | 16.7 | 1.1 | 15.7 | ||
| p-value | 0.039 | 0.048 | 0.007 | 0.592 | 0.652 | 0.037 | 0.023 |
| p-Value | Mass (g) | Size (mm) | Firm (Shore) | Color (1–5) | SSC (%) | TA (%) | MI | |
|---|---|---|---|---|---|---|---|---|
| Covering | A | <0.001 | <0.001 | <0.001 | 0.666 | 0.636 | 0.869 | 0.931 |
| Position | B | 0.494 | 0.222 | 0.239 | 0.202 | 0.075 | 0.254 | 0.059 |
| Plot | C | 0.071 | 0.005 | 0.670 | 0.029 | <0.001 | <0.001 | <0.001 |
| A × B | 0.546 | 0.223 | 0.191 | 0.670 | 0.035 | 0.644 | 0.508 | |
| A × C | 0.001 | 0.735 | 0.177 | 0.157 | 0.796 | <0.001 | 0.001 | |
| B × C | 0.571 | 0.303 | 0.379 | 0.607 | 0.052 | 0.490 | 0.001 | |
| A × B × C | 0.122 | 0.475 | 0.289 | 0.489 | 0.026 | 0.644 | 0.373 |
| Firmness (Shore) | Color (1–5) | SSC (%) | TA (%) | MI | ||
|---|---|---|---|---|---|---|
| 45 d | Covered | 72.9 | 4.1 | 18.8 | 0.7 | 24.3 |
| Open | 79.8 | 4.4 | 19.4 | 0.7 | 23.9 | |
| p-value | 0.040 | 0.277 | 0.582 | 0.322 | 0.736 | |
| 45 d + 3 d | Covered | 70.6 | 4.3 | 18.0 | 0.7 | 29.6 |
| Open | 76.3 | 4.5 | 17.4 | 0.6 | 29.2 | |
| p-value | 0.072 | 0.412 | 0.413 | 0.312 | 0.848 |
| Pitting (%) | Bruising (%) | Pebbling (%) | |
|---|---|---|---|
| Covered | 55.0 | 17.5 | 44.0 |
| Open | 71.3 | 15.0 | 30.0 |
| p-value | 0.093 | 0.689 | 0.356 |
| DM (%) | N (mg 100 g−1) | P (mg 100 g−1) | K (mg 100 g−1) | Mg (mg 100 g−1) | Ca (mg 100 g−1) | |
|---|---|---|---|---|---|---|
| Covered | 18.0 | 195.8 | 24.5 | 180.7 | 7.9 | 7.7 |
| Open | 17.4 | 177.0 | 22.5 | 179.1 | 8.6 | 10.6 |
| p-value | 0.181 | 0.453 | 0.209 | 0.859 | 0.292 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, V.; Zoffoli, J.P.; Ayala, M. Influence of High Tunnel Microclimate on Fruit Quality and Calcium Concentration in ‘Santina’ Sweet Cherries in a Mediterranean Climate. Agronomy 2021, 11, 1186. https://doi.org/10.3390/agronomy11061186
Blanco V, Zoffoli JP, Ayala M. Influence of High Tunnel Microclimate on Fruit Quality and Calcium Concentration in ‘Santina’ Sweet Cherries in a Mediterranean Climate. Agronomy. 2021; 11(6):1186. https://doi.org/10.3390/agronomy11061186
Chicago/Turabian StyleBlanco, Victor, Juan Pablo Zoffoli, and Marlene Ayala. 2021. "Influence of High Tunnel Microclimate on Fruit Quality and Calcium Concentration in ‘Santina’ Sweet Cherries in a Mediterranean Climate" Agronomy 11, no. 6: 1186. https://doi.org/10.3390/agronomy11061186
APA StyleBlanco, V., Zoffoli, J. P., & Ayala, M. (2021). Influence of High Tunnel Microclimate on Fruit Quality and Calcium Concentration in ‘Santina’ Sweet Cherries in a Mediterranean Climate. Agronomy, 11(6), 1186. https://doi.org/10.3390/agronomy11061186






