Metabolic Responses of Two Contrasting Lentil Genotypes to PEG-Induced Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Osmotic Drought Stress Treatment and Experimental Design
2.3. Metabolite Extraction, Derivatization, and GC-MS Analysis
2.4. Statistical Analysis
3. Results
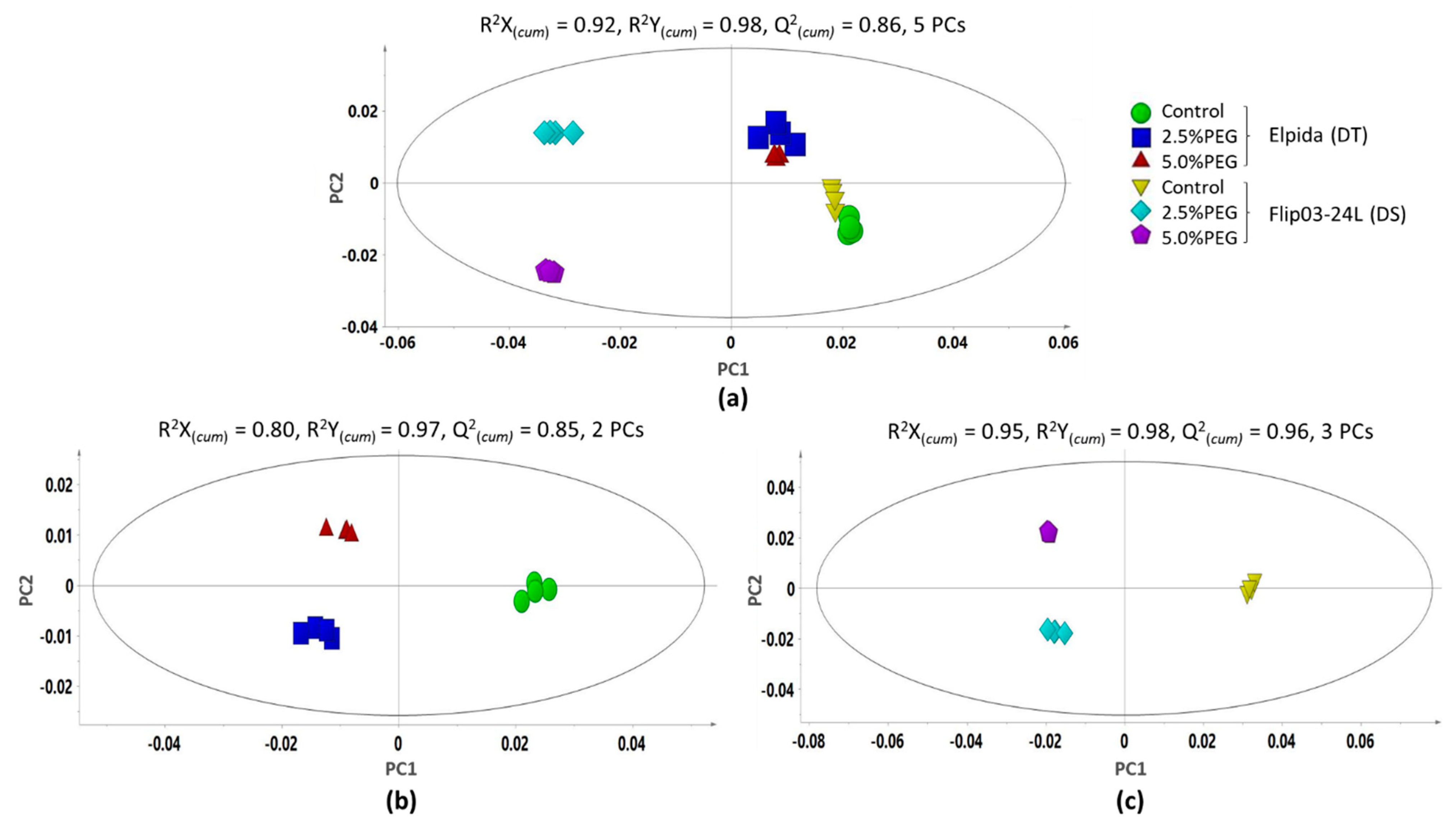
3.1. Comparative Metabolic Response of Drought-Tolerant and Drought-Sensitive Genotypes under Stress Conditions
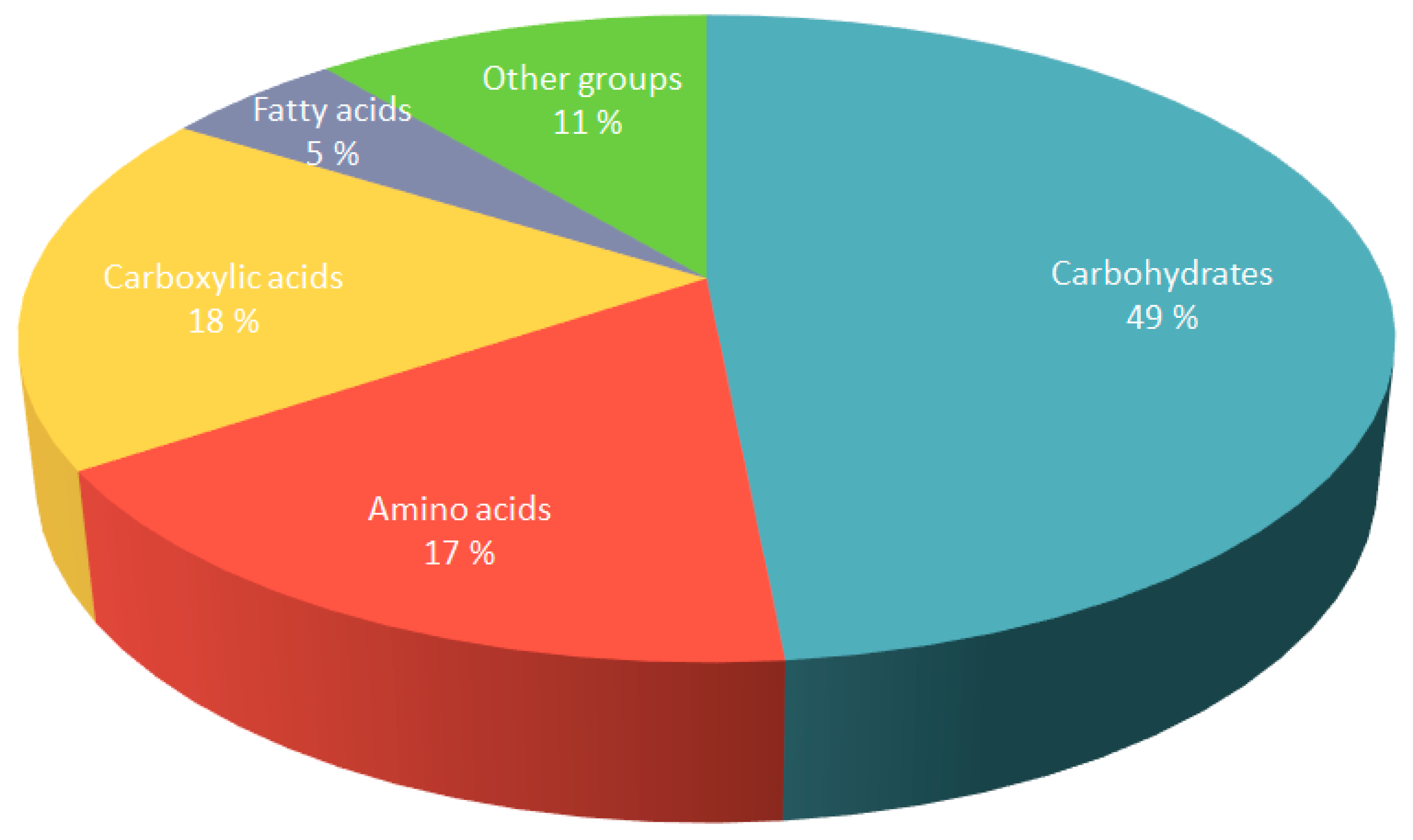
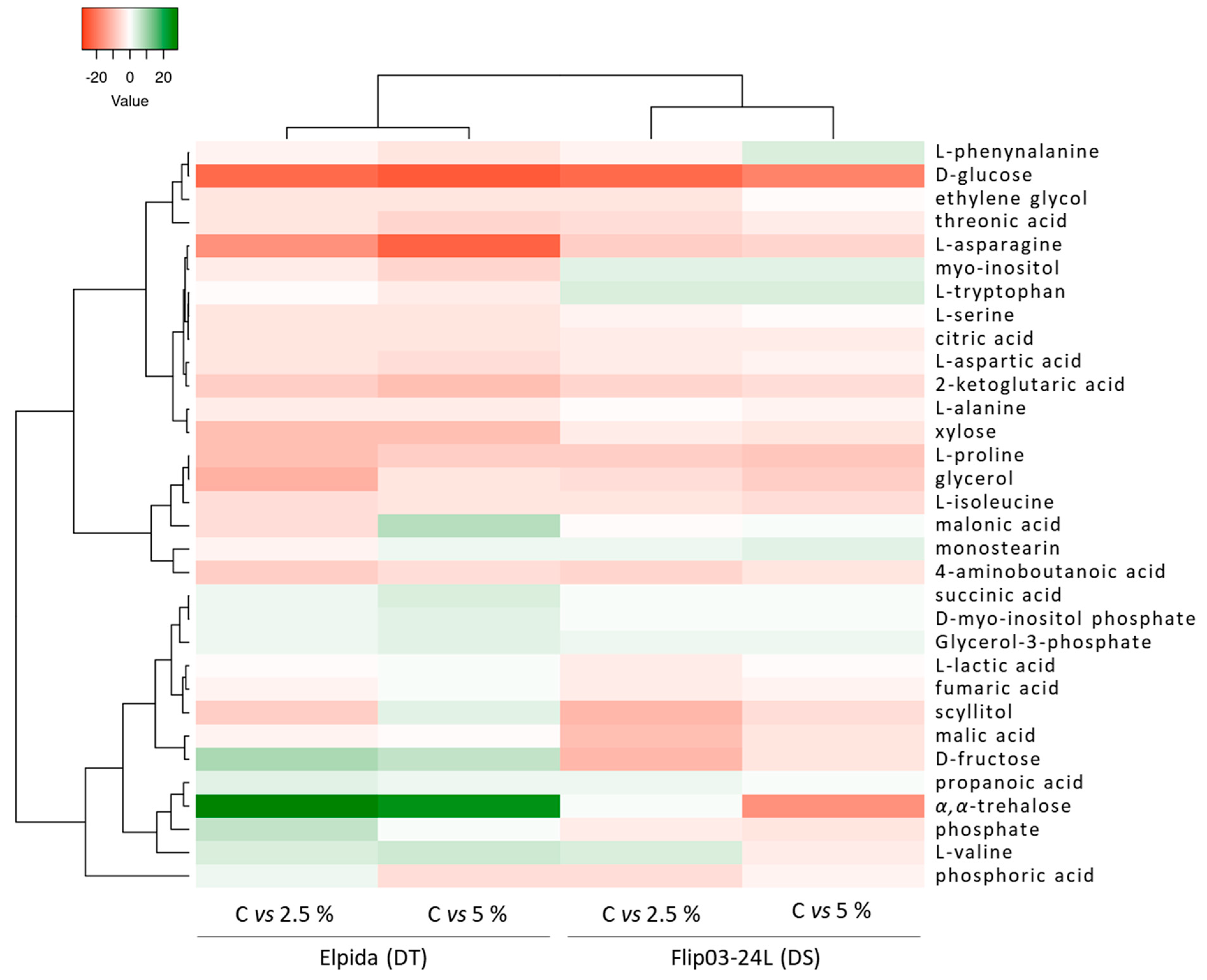
3.2. Overview of Fluctuations of Lentil Metabolome in Response to Osmotic Drought Stress
3.2.1. Carbohydrates
3.2.2. Carboxylic Acids
3.2.3. Amino Acids
3.2.4. Phosphoric Acids
3.2.5. Other Chemical Groups
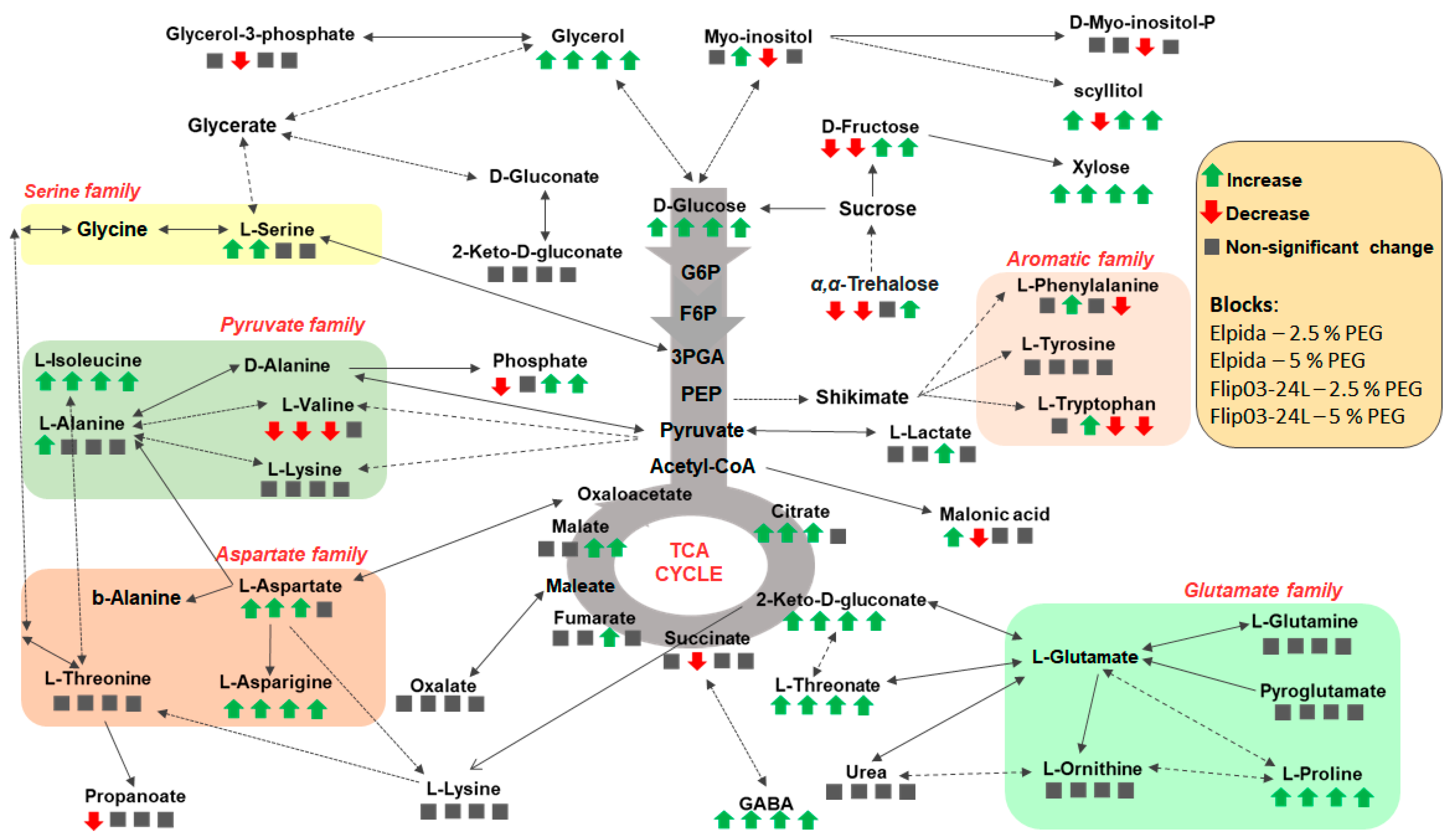
3.3. Regulatory Metabolic Networks in Osmotic Drought Stress Responses of Lentil
4. Discussion
4.1. Basic Aspects of Lentil Metabolism in Response to Osmotic Drought Stress
4.2. Fluctuations in the Content of Carbohydrates in Stressed Plants
4.3. Fluctuations in the Content of Carboxylic Acids in Stressed Plants
4.4. Fluctuations in the Content of Amino Acids in Stressed Plants
4.5. Genotypic-Dependent Metabolic Response to Osmotic Drought Stress: Opportunities for Biomarkers in Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaves, M.M.; Oliveira, M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought–From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Bray, E.A. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Mata, A.T.; Jorge, T.F.; Pires, M.V.; António, C. Drought stress tolerance in plants: Insights from metabolomics. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 2, pp. 187–216. [Google Scholar]
- Saxena, M.C. The challenge of developing biotic and abiotic stress resistance in cool-season food legumes. In Breeding for Stress Tolerance in Cool-Season Food Legumes; Singh, K.B., Saxena, M.C., Eds.; Wiley: Chichester, UK; Oxford, UK, 1993; pp. 3–14. [Google Scholar]
- Johansen, C.; Baldev, B.; Brouwer, J.B.; Erskine, W.; Jermyn, W.A.; Li-Juan, L.; Malik, B.A.; Miah, A.A.; Silim, S.N. Biotic and abiotic stresses constraining productivity of cool season food legumes in Asia, Africa and Oceania. In Expanding the Production and Use of Cool Season Food Legumes; Muehlbauer, F.J., Kaiser, W.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 175–194. [Google Scholar]
- Allahmoradi, P.; Mansourifar, C.; Saiedi, M.; Jalali Honarmand, S. Effect of different water deficiency levels on some antioxidants at different growth stages of lentil (Lens culinaris L.). Adv. Environ. Biol. 2013, 7, 535–543. [Google Scholar]
- Babayeva, S.; Akparov, Z.; Damania, A.; Izzatullayeva, V.; Aslanova, G.; Abbasov, M. Genetic diversity for drought tolerance in lentils from Central Asia and the Caucasus: CACLentil. Albanian J. Agric. Sci. 2014, 13, 1–8. [Google Scholar]
- Siddique, K.H.M.; Loss, S.P.; Regan, K.L.; Jettner, R.L. Adaptation and seed yield of cool season grain legumes in Mediterranean environments of south-western Australia. Austr. J. Agric. Res. 1999, 50, 375–387. [Google Scholar] [CrossRef]
- Bhandari, K.; Siddique, K.H.; Turner, N.C.; Kaur, J.; Singh, S.; Agrawal, S.K.; Nayyar, H. Heat stress at reproductive stage disrupts leaf carbohydrate metabolism, impairs reproductive function, and severely reduces seed yield in lentil. J. Crop. Improv. 2016, 30, 118–151. [Google Scholar] [CrossRef]
- Shrestha, R.; Turner, N.C.; Siddique, K.H.; Turner, D.W.; Speijers, J. A water deficit during pod development in lentils reduces flower and pod numbers but not seed size. Aust. J. Agric. Res. 2006, 57, 427–438. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Fiehn, O. Combining genomics, metabolome analysis and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Fiehn, O. Metabolomics–the link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Bino, R.J.; Hall, R.D.; Fiehn, O.; Kopka, J.; Saito, K.; Draper, J.; Nikolaus, B.J.; Mendes, P.; Roessner-Tunali, U.; Beale, M.H.; et al. Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 2004, 9, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Jorge, T.F.; António, C. Plant Metabolomics in a Changing world: Metabolite responses to abiotic stress combinations. In Plant, Abiotic Stress and Responses to Climate Change; Andjelkovic, V., Ed.; IntechOpen Limited: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Urano, K.; Kurihara, Y.; Seki, M.; Shinozaki, K. ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Curr. Opin. Plant Biol. 2010, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Sadia, B.; Raza, A.; Hameed, M.K.; Saleem, F. Metabolomics: A way forward for crop improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscolo, A.; Junker, A.; Klukas, C.; Weigelt-Fischer, K.; Riewe, D.; Altmann, T. Phenotypic and metabolic responses to drought and salinity of four contrasting lentil accessions. J. Exp. Bot. 2015, 18, 5467–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Singh, C.K.; Taunk, J.; Tomar, R.S.S.; Chaturvedi, A.K.; Gaikwad, K.; Pal, M. Transcriptome analysis of lentil (Lens culinaris Medikus) in response to seedling drought stress. BMC Genom. 2017, 18, 206. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; Pal, A.K.; Singh, A.K. Physiological, biochemical and molecular responses of lentil (Lens culinaris Medik.) genotypes under drought stress. Ind. J. Plant Physiol. 2018, 23, 772–784. [Google Scholar]
- Mia, M.W.; Yamauchi, A.; Kono, Y. Root system structure of six food legume species: Inter- and intraspecific variations. Jpn. J. Crop Sci. 1996, 65, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Basu, P.S.; Srivastava, E.; Chaturvedi, S.K.; Nadarajan, N.; Kumar, S. Phenotyping of traits imparting drought tolerance in lentil. Crop. Pasture Sci. 2012, 63, 547–554. [Google Scholar] [CrossRef]
- Idrissi, O.; Houasli, C.; Udupa, S.M.; De Keyser, E.; Van Damme, P.; De Riek, J. Genetic variability for root and shoot traits in a lentil (Lens culinaris Medik.) recombinant inbred line population and their association with drought tolerance. Euphytica 2015, 204, 693–709. [Google Scholar] [CrossRef]
- Foti, C.; Khah, E.M.; Pavli, O.I. Response of lentil genotypes under PEG-induced drought stress: Effect on germination and growth. Plant 2018, 6, 75–83. [Google Scholar]
- Steuter, A. Water potential of aqueous polyethylene glycol. Plant Physiol. 1981, 67, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, M.; Deshpande, U. In vitro screening of tomato genotypes for drought resistance using polyethylene glycol. Afr. J. Biotechnol. 2007, 6, 691–696. [Google Scholar]
- Govindaraj, M.; Shanmugasundaram, P.; Sumathi, P.; Muthiah, A. Simple, rapid and cost effective screening method for drought resistant breeding in pearl millet. Electr. J. Plant Breed. 2010, 1, 590–599. [Google Scholar]
- Kalampokis, I.F.; Kapetanakis, G.C.; Aliferis, K.A.; Diallinas, G. Multiple nucleobase transporters contribute to boscalid sensitivity in Aspergillus nidulans. Fung. Gen. Biol. 2018, 115, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, K.; Ntatsi, G.; Arapis, G.; Aliferis, K.A. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics. Chemosphere 2020, 239, 124582. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vandergheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Μethods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Aliferis, K.; Jabaji, S. 1H NMR and GC-MS metabolic fingerprinting of developmental stages of Rhizoctonia solani sclerotia. Metabolomics 2010, 6, 96–108. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chrysayi-Tokousbaliides, M. Metabolomics in pesticide research and development: Review and future perspectives. Metabolomics 2011, 7, 35–53. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Faubert, D.; Jabaji, S. A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS ONE 2014, 9, e111930. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis. Principles and Applications; Umetrics Academy: Umeå, Sweden, 2001. [Google Scholar]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef] [Green Version]
- Scossa, F.; Brotman, Y.; de Abreu, E.L.F.; Willmitzer, L.; Nikoloski, Z.; Tohge, T.; Fernie, A.R. Genomics-based strategies for the use of natural variation in the improvement of crop metabolism. Plant Sci. 2016, 242, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef] [Green Version]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, B.; Pantin, F.; Génard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [Green Version]
- Ramel, F.; Sulmon, C.; Bogard, M.; Couée, I.; Gouesbet, G. Differential dynamics of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Ramel, F.; Sulmon, C.; Gouesbet, G.; Couée, I. Natural variation reveals relationships between pre-stress carbohydrate nutritional status and subsequent responses to xenobiotic and oxidative stress in Arabidopsis thaliana. Ann. Bot. 2009, 104, 1323–1337. [Google Scholar] [CrossRef] [Green Version]
- Peshev, D.; Van den Ende, W. Sugars as antioxidants in plants. In Crop Improvement under Adverse Conditions; Tuteja, N., Gill, S.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 285–308. [Google Scholar]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y.H. Trehalose metabolism and signaling. Ann. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunn, J.E.; Delorge, Ι.; Figueroa, C.M.; Van Dijck, P.; Stitt, Μ. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Ilhan, S.; Ozdemir, F.; Bor, M. Contribution of trehalose biosynthetic pathway to drought stress tolerance of Capparis ovata Desf. Plant Biol. 2014, 17, 402–407. [Google Scholar] [CrossRef]
- Sadak, M.S.; El-Bassiouny, H.M.S.; Dawood, M.G. Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull. Natl. Res. Cent. 2019, 43, 5. [Google Scholar] [CrossRef]
- Ge, L.F.; Chao, D.Y.; Shi, M.; Zhu, M.Z.; Gao, J.P.; Lin, H.X. Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta 2008, 228, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Broyart, C.; Rabanal, F.A.; Jonak, C. The redox-sensitive chloroplast trehalose-6-phosphate phosphatase AtTPPD regulates salt stress tolerance. Antioxid. Redox Signal. 2014, 21, 1289–1304. [Google Scholar] [CrossRef] [Green Version]
- Mahabub, A.; Mirza, H.; Kamrun, N.; Masayuki, F. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2013, 7, 1053–1063. [Google Scholar]
- McKersie, B.D.; Leshema, Y.Y. Stress and Stress Coping in Cultivated Plants; Kluwer Academic Publisher: London, UK, 1994. [Google Scholar]
- Solomon, K.F.; Labuschagne, M.T. Differences in the level of D-glucose and sucrose among durum wheat (Triticum turgidum var durum L.) genotypes differing in their responses to drought stress. S. Afr. J. Plant Soil 2005, 22, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; De la Vega, O.M.; Guevara-García, A.; Herrera-Estrella, L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 2000, 18, 450–453. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Iqbal, N.; Ashraf, Y.; Ashraf, M. Modulation of endogenous levels of some key organic metabolites by exogenous application of glycine betaine in drought stressed plants of sunflower (Helianthus annuus L.). Plant Growth Regul. 2011, 63, 7–12. [Google Scholar] [CrossRef]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res. 2012, 125, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Marcin′ska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Bowne, J.B.; Erwin, Τ.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorová, Z.; Kováčik, J.; Klejdus, B.; Maglovski, M.; Kuna, R.; Hauptvogel, P.; Matušíková, Ι. Drought-induced responses of physiology, metabolites, and pr proteins in Triticum aestivum. J. Agric. Food Chem. 2015, 63, 8125–8133. [Google Scholar] [CrossRef] [PubMed]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, A.; Paterson, A.H.; Cakmak, I.; Saranga, Y. Metabolite and mineral analyses of cotton near-isogenic lines introgressed with QTLs for productivity and drought-related traits. Physiol. Plant 2011, 141, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Merewitz, E.B.; Du, H.; Yu, W.; Liu, Y.; Gianfagna, T.; Huang, B. Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. J. Exp. Bot. 2011, 63, 1315–1328. [Google Scholar] [CrossRef] [Green Version]
- El-Tohamy, W.A.; El-Abagy, H.M.; Badr, M.A.; Gruda, N. Drought tolerance and water status of bean plants (Phaseolus vulgaris L.) as affected by citric acid application. J. Appl. Bot. Food Qual. 2013, 86, 212–216. [Google Scholar]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosche, M.; Vinocur, B.; Alatalo, E.R.; Lamminmaki, A.; Teichmann, T.; Otow, E.A.; Djilianov, D.; Afif, D.; Bogeat-Triboulot, M.; Altman, A.; et al. Gene expression and metabolite profiling of Populus euphratica growing in the Negev desert. Genome Biol. 2005, 6, R101. [Google Scholar] [CrossRef] [Green Version]
- Zuther, E.; Koehl, K.; Kopka, J. Comparative metabolome analysis of the salt response in breeding cultivars of rice. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 285–315. [Google Scholar]
- Kempa, S.; Krasensky, J.; Dal Santo, S.; Kopka, J.; Jonak, C. A central role of abscisic acid in stress-regulated carbohydrate metabolism. PLoS ONE 2008, 3, e3935. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, D.H.; Lippold, F.; Redestig, H.; Hannah, A.M.; Erban, A.; Kramer, U.; Kopka, J.; Udvardi, K.M. Integrative functional genomics of salt acclimatization in the model legume Lotus japonicus. Plant 2008, 53, 973–987. [Google Scholar] [CrossRef]
- Usadel, B.; Blasing, O.E.; Gibon, Y.; Poree, F.; Hohne, M.; Gunter, M.; Trethewey, R.; Kamlage, B.; Poorter, H.; Stitt, M. Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non-freezing range. Plant Cell Environ. 2008, 31, 518–547. [Google Scholar] [CrossRef]
- Lugan, R.; Niogret, M.F.; Leport, L.; Guegan, J.P.; Larher, F.R.; Savoure, A.; Kopka, J.; Bouchereau, A. Metabolome and water homeostasis analysis of Thellungiella salsuginea suggests that dehydration tolerance is a key response to osmotic stress in this halophyte. Plant J. 2010, 64, 215–229. [Google Scholar] [CrossRef]
- Bayram, Ö.; Feussner, K.; Dumkow, M.; Herrfurth, C.; Feussner, I.; Braus, G.H. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet. Biol. 2016, 87, 30–53. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Akond, M.; Babar, M.A.; Beecher, C.; Erickson, J.; Thomason, K.; De Jong, F.A.; Manson, R.E. LC-HRMS based non-targeted metabolomic profiling of wheat (Triticum aestivum L.) under post-anthesis drought stress. Am. J. Plant Sci. 2017, 8, 3024–3061. [Google Scholar] [CrossRef] [Green Version]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS ONE 2012, 7, e38554. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and metabolomic studies disclose key metabolism pathways contributing to well-maintained photosynthesis under the drought and the consequent drought-tolerance in rice. Front. Plant Sci. 2016, 7, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassi, S.; Aydi, S.; Hessini, K.; Gonzalez, E.M.; Arrese-Igor, C. Long-term mannitol-induced osmotic stress leads to stomatal closure, carbohydrate accumulation and changes in leaf elasticity in Phaseolus vulgaris leaves. Afr. J. Biotechnol. 2010, 9, 6061–6069. [Google Scholar]
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2018, 42, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.M. Osmoregulation and water stress in higher plants. Ann. Rev. Plant Biol. 1984, 35, 299–319. [Google Scholar] [CrossRef]
- Yadav, S.K.; Jyothi Lakshmi, N.; Maheswari, M.; Vanaja, M.; Venkateswarlu, B. Influence of water deficit at vegetative, anthesis and grain filling stages on water relation and grain yield in sorghum. Indian J. Plant Physiol. 2005, 10, 20–24. [Google Scholar]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.B.; Bhal, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. J. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef] [Green Version]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.S.P. Asparagine: An amide of particular distinction in the regulation of symbiotic nitrogen fixation of legumes. Crit. Rev. Biotechnol. 2013, 33, 309–327. [Google Scholar] [CrossRef]
- King, G.A.; Woollard, D.C.; Irving, D.E.; Borst, W.M. Physiological changes in asparagus spear tips after harvest. Plant Physiol. 1990, 80, 393–400. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Ann. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotero-Martins, A.; da Silva Bon, E.P.; Carvajal, E. Asparaginase II-GFP fusion as a tool for studying the secretion of the enzyme under nitrogen starvation. Braz. J. Microb. 2003, 34, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Kusaka, M.; Ohta, M.; Fujimura, T. Contribution of inorganic components to osmotic adjustment and leaf folding for drought tolerance in pearl millet. Physiol. Planta. 2005, 125, 474–489. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 9, 479–509. [Google Scholar] [CrossRef]
- Bouché, Ν. Fromm, N. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not Just a Metabolite. Trends Plant Sci. 2016, 21, 10. [Google Scholar] [CrossRef]
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol. 2003, 131, 1748–1755. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–2018. [Google Scholar] [CrossRef]
- Fernie, A.R.; Schauer, N. Metabolomics-assisted breeding: A viable option for crop improvement? Trends Genet. 2009, 25, 39–48. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, Μ.; Ollas, C.D.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Stepien, P.; Dyson, B.C.; Rattray, N.J.W.; Ellis, D.I.; Goodacre, R.; Johnson, G.N. Biochemical analyses of sorghum varieties reveal differential responses to drought. PLoS ONE 2016, 11, e0154423. [Google Scholar] [CrossRef]
- Massacci, A.; Battistelli, A.; Loreto, F. Effect of drought stress on photosynthetic characteristics, growth and sugar accumulation of field-grown sweet sorghum. Funct. Plant Biol. 1996, 23, 331–340. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 1, 1–19. [Google Scholar] [CrossRef]
- Stevenson-Paulik, J.; Bastidas, R.J.; Chiou, S.T.; Frye, R.A.; York, J.D. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc. Natl. Acad. Sci. USA 2005, 102, 12612–12617. [Google Scholar] [CrossRef] [Green Version]
- Perera, I.Y.; Hung, C.Y.; Moore, C.; Stevenson-Paulik, J.; Bossa, W.F. Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase and altered abscisic acid signalling. Plant Cell 2008, 20, 2876–2893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, S.; Jinxi, H.; Lei, X.; Yanyan, A.; Shaozhen, H.; Quingchang, L. A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 2016, 14, 592–602. [Google Scholar] [CrossRef]
- Yildizli, A.; Çevik, S.; Ünyayar, S. Effects of exogenous myo-inositol on leaf water status and oxidative stress of Capsicum annuum under drought stress. Acta Physiolog. Plant 2018, 40, 122. [Google Scholar] [CrossRef]
- Sharma, N.; Chaudhary, C.; Khurana, P. Wheat Myo-inositol phosphate synthase influences plant growth and stress responses via ethylene mediated signaling. Sci. Rep. 2020, 10, 10766. [Google Scholar] [CrossRef] [PubMed]

| Chemical Group | Metabolite | Elpida (DT) | Flip03-24L (DS) | ||
|---|---|---|---|---|---|
| C vs. 2.5% | C vs. 5% | C vs. 2.5% | C vs. 5% | ||
| Carbohydrates | D-fructose | 8.3 * | 6.2 * | −11.1 * | −3.5 * |
| D-glucose | −22.2 * | −23.9 * | −22.1 * | −18.7 * | |
| α,α-trehalose | 28.4 * | 24.2 * | 0.7 | −17 * | |
| D-myo-inositol phosphate | 1.4 | 2.7 * | 0.6 | 0.8 | |
| glycerol | −11.4 * | −3.6 * | −5.0 * | −7.0 * | |
| glycerol-3-phosphate | 2 | 3.2 * | 1.9 | 1.3 | |
| myo-inositol | −2.3 | −6.7 * | 2.9 * | 2.3 | |
| scyllitol | −7.6 * | 3.4 * | −11.2 * | −5.5 * | |
| xylose | −9.9 * | −9.6 * | −2.5 * | −4.0 * | |
| Carboxylic acids | 2-ketoglutaric acid | −7.3 * | −9.6 * | −5.8 * | −4.8 * |
| citric acid | −3.8 * | −4.1 * | −2.6 * | −2.3 | |
| malonic acid | −4.6 * | 6.9 * | −0.6 | 0.3 | |
| propanoic acid | 2.5 * | 2.1 | 1.7 | 0.4 | |
| succinic acid | 2.2 | 3.9 * | 1 | 1.1 | |
| fumaric acid | −1.4 | 0.9 | −3.3 * | −1.9 | |
| malic acid | −1.2 | −1.1 | −9.7 * | −4.1 * | |
| L-lactic acid | −0.8 | 0.9 | −3.1 * | −1 | |
| Amino acids | 4-aminobutanoic acid | −7.9 * | −5.2 * | −6.3 * | −4.2 * |
| L-alanine | −2.6 * | −2.3 | −1 | −1.5 | |
| L-asparagine | −16.8 * | −22.9 * | −7.6 * | −6.0 * | |
| L-aspartic acid | −3.8 * | −4.6 * | −2.6 * | −1.7 | |
| L-isoleucine | −5.3 * | −4.3 * | −4.5 * | −5.0 * | |
| L-phenynalanine | −1.2 | −4.2 * | −1.9 | 3.5 * | |
| L-proline | −9.9 * | −7.0 * | −7.4 * | −8.1 * | |
| L-serine | −3.8 * | −4.2 * | −1.2 | −1.1 | |
| L-tryptophan | −1.1 | −2.6 * | 3.9 * | 4.2 * | |
| L-valine | 3.9 * | 4.6 * | 4.5 * | −2.3 | |
| Phosphoric acids | phosphate | 5.9 * | 0.8 | −2.8 * | −3.5 * |
| phosphoric acid | 1.6 | −4.8 * | −5.1 * | −2.2 | |
| Fatty acids | threonic acid | −4.0 * | −6.2 * | −5.5 * | −3.0 * |
| Alcohols | ethylene glycol | −4.1 * | −4.2 * | −3.7 * | 0 |
| Glycerolipids | monostearin | −1.7 | 1.9 | 2.2 | 2.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, C.; Kalampokis, I.F.; Aliferis, K.A.; Pavli, O.I. Metabolic Responses of Two Contrasting Lentil Genotypes to PEG-Induced Drought Stress. Agronomy 2021, 11, 1190. https://doi.org/10.3390/agronomy11061190
Foti C, Kalampokis IF, Aliferis KA, Pavli OI. Metabolic Responses of Two Contrasting Lentil Genotypes to PEG-Induced Drought Stress. Agronomy. 2021; 11(6):1190. https://doi.org/10.3390/agronomy11061190
Chicago/Turabian StyleFoti, Chrysanthi, Ioannis F. Kalampokis, Konstantinos A. Aliferis, and Ourania I. Pavli. 2021. "Metabolic Responses of Two Contrasting Lentil Genotypes to PEG-Induced Drought Stress" Agronomy 11, no. 6: 1190. https://doi.org/10.3390/agronomy11061190
APA StyleFoti, C., Kalampokis, I. F., Aliferis, K. A., & Pavli, O. I. (2021). Metabolic Responses of Two Contrasting Lentil Genotypes to PEG-Induced Drought Stress. Agronomy, 11(6), 1190. https://doi.org/10.3390/agronomy11061190






