Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions, Experimental Design and Plant Material
2.2. Biostimulant Application
2.3. Sampling, Yield and Growth Assessment
2.4. Soil Plant Analysis Development (SPAD) Index, Chlorophyll Fluorescence and Photosynthetic Parameters
2.5. Leaf Mineral Content Analysis
2.6. Leaf Total Chlorophylls and Carotenoids
2.7. Total Phenols and Total Ascorbic Acid Analysis
2.8. Antioxidant Activity Analysis
2.9. Statistical Processing of the Data
3. Results
3.1. Growth and Yield Assessment
3.2. SPAD Index, Chlorophyll Fluorescence, Photosyntethic Parameters
3.3. Leaf Total Nitrogen, Nitrate and Mineral Content
3.4. Leaf Pigments and Qualitative Parameters
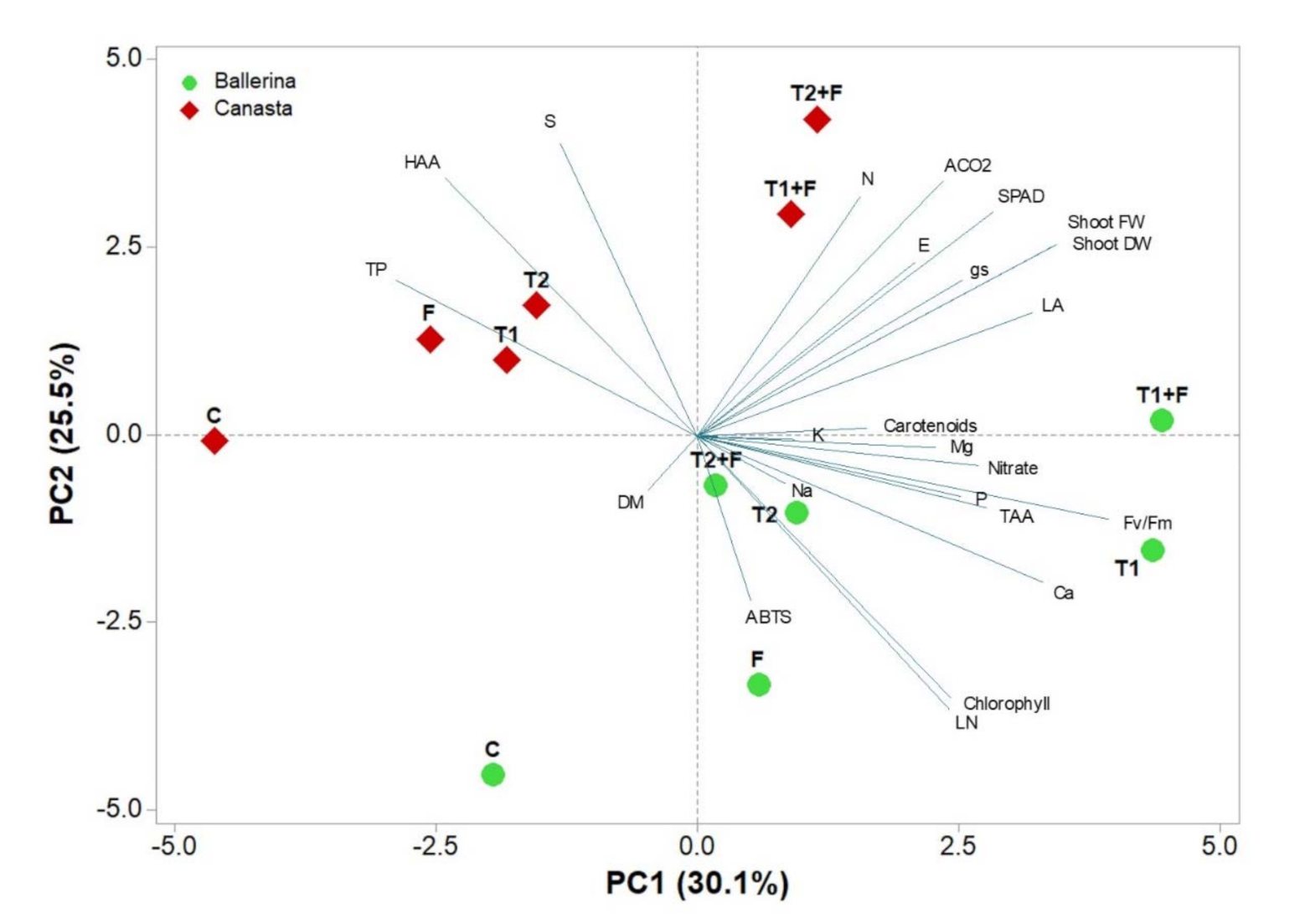
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agricultural Organization of the United Nations Statistical Data (FAOSTAT). Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 2 May 2021).
- Stima Delle Superfici E Produzioni Delle Coltivazioni Agrarie, Floricole E Delle Piante Intere Da Vaso. Available online: http://dati.istat.it/Index.aspx?QueryId=33703# (accessed on 2 May 2021).
- Van Duyn, M.A.S.; Pivonka, E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: Selected literature. J. Am. Diet. Assoc. 2000, 100, 1511–1521. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004, 23, 605–614. [Google Scholar] [CrossRef]
- Hernández, T.; Chocano, C.; Moreno, J.L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops-Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Gilardi, G.; Gullino, M.L.; Garibaldi, A. New diseases of wild and cultivated rocket in Italy. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 15 September 2013; Volume 1005, pp. 569–572. [Google Scholar]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustain 2016, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Nicola, S.; Hoeberechts, J.; Fontana, E. Comparison between traditional and soilless culture systems to Produce rocket (Eruca sativa) with Low Nitrate Content. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 31 December 2005; Volume 697, pp. 549–555. [Google Scholar]
- Barbosa, G.L.; Almeida Gadelha, F.D.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. Conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef] [Green Version]
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban agriculture in the developing world: A review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar] [CrossRef] [Green Version]
- El-Nakhel, C.; Petropoulos, S.A.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Colla, G.; Troise, A.D.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. The bioactive profile of lettuce produced in a closed soilless system as configured by combinatorial effects of genotype and macrocation supply composition. Food Chem. 2020, 309, 125713. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Paliament and of the Council of 5 June 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 2 May 2021).
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. In response to an Alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Mou, B. Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. Horttechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of vegetal- And seaweed extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel-grown baby lettuce under four regimes of nitrogen fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Vernizzi, P.; Borghesi, E.; Ferrante, A. Application of Biostimulants in Floating System for Improving Rocket Quality Plant responses to biostimulants application View project Bioactive Compounds Biosynthesis and Metabolism in Fruit and Vegetables View project. J. Food Agric. Environ. 2005, 3, 86. [Google Scholar]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Mou, B. Lettuce. In Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2008; pp. 75–116. ISBN 978-0-387-30443-4. [Google Scholar]
- Kim, D.E.; Shang, X.; Assefa, A.D.; Keum, Y.S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis. Part 2. CHemical and Microbiological Properties. Agronomy Monograph 9; Black, C.A., Evans, D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality Through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Burkart, S. Photosynthesis and high light stress. Wild 1999, 25, 3–16. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Variety-specific responses of lettuce grown in a gravel-film technique closed hydroponic system to N supply on yield, morphology, phytochemicals, mineral content and safety. J. Integr. Agric. 2018, 17, 2447–2457. [Google Scholar] [CrossRef] [Green Version]
- Manzocco, L.; Foschia, M.; Tomasi, N.; Maifreni, M.; Dalla Costa, L.; Marino, M.; Cortella, G.; Cesco, S. Influence of hydroponic and soil cultivation on quality and shelf life of ready-to-eat lamb’s lettuce (Valerianella locusta L. Laterr). J. Sci. Food Agric. 2011, 91, 1373–1380. [Google Scholar] [CrossRef]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Comparative Cultivation and Biochemical Analysis of Iceberg Lettuce Grown in Sand Soil and Hydroponics With or Without Microbubbles and Macrobubbles. J. Soil Sci. Plant Nutr. 2020, 21, 389–403. [Google Scholar] [CrossRef]
- de Souza, P.F.; Borghezan, M.; Zappelini, J.; de Carvalho, L.R.; Ree, J.; Barcelos-Oliveira, J.L.; Pescador, R. Physiological differences of ‘crocantela’ lettuce cultivated in conventional and hydroponic systems. Hortic. Bras. 2019, 37, 101–105. [Google Scholar] [CrossRef]
- Ouyang, Z.; Tian, J.; Yan, X.; Shen, H. Effects of different concentrations of dissolved oxygen or temperatures on the growth, photosynthesis, yield and quality of lettuce. Agric. Water Manag. 2020, 228, 105896. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Minhas, P.S.; Rane, J.; Pasala, R.K. Abiotic stresses in agriculture: An overview. In Abiotic Stress Management for Resilient Agriculture; Minhas, P.S., Rane, J., Pasala, R.K., Eds.; Springer: Singapore, 2017; pp. 3–8. ISBN 9789811057441. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, M. Soybean Peptide: Novel Plant Growth Promoting Peptide from Soybean. Soybean Nutr. 2011, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Carletti, P.; Arrigoni, G.; Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Masi, A. Quantitative proteomics of maize roots treated with a protein hydrolysate: A comparative study with transcriptomics highlights the molecular mechanisms responsive to biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Pecha, J.; Fürst, T.; Kolomazník, K.; Friebrová, V.; Svoboda, P. Protein biostimulant foliar uptake modeling: The impact of climatic conditions. AIChE J. 2012, 58, 2010–2019. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-alone and combinatorial effects of plant-based biostimulants on the production and leaf quality of perennial wall rocket. Plants 2020, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bonner, C.A.; Jensen, R.A. Recognition of specific patterns of amino acid inhibition of growth in higher plants, uncomplicated by glutamine-reversible “general amino acid inhibition”. Plant Sci. 1997, 130, 133–143. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Bernard, A.; Falchetto, L.; Marget, P.; Chauvel, B.; Steinberg, C.; Morris, C.E.; Gibot-Leclerc, S.; Boari, A.; Vurro, M.; et al. Investigation of amino acids as herbicides for control of orobanche minor parasitism in red clover. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhai, J.; Shao, L.; Lin, W.; Peng, C. Accumulation of Anthocyanins: An Adaptation Strategy of Mikania micrantha to Low Temperature in Winter. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Rothstein, S.J. ROS-Induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal. Behav. 2018, 13, e1451708. [Google Scholar] [CrossRef] [Green Version]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.; Dogliotti, E.; Di Domenico, A.; Fernández-cruz, M.L.; Fink-gremmels, J.; Fürst, P.; Galli, C.; et al. Nitrate in vegetables-Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2008, 6, 689. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
| Source of Variance | Leaf Number | Leaf Area | Shoot Fresh Yield | Dry Shoot Biomass | Leaf Dry Matter |
|---|---|---|---|---|---|
| (no. plant−1) | (cm2 plant−1) | (g plant−1) | (g plant−1) | (%) | |
| Cultivar (C) | |||||
| Ballerina | 22.39 ± 0.32 | 1603 ± 50 | 80.28 ± 3.6 | 4.42 ± 0.21 | 5.52 ± 0.08 |
| Canasta | 17.33 ± 0.57 | 1522 ± 35 | 79.86 ± 3.17 | 4.34 ± 0.16 | 5.43 ± 0.05 |
| t-test | *** | ns | ns | ns | ns |
| Biostimulant (B) | |||||
| Control | 20.50 ± 1.67 | 1264 ± 44 c | 57.70 ± 3.17 d | 3.30 ± 0.19 d | 5.81 ± 0.04 a |
| F | 19.50 ± 1.57 | 1515 ± 34 b | 74.68 ± 1.44 c | 3.81 ± 0.05 c | 5.10 ± 0.06 d |
| T1 | 20.00 ± 1.81 | 1523 ± 70 b | 81.01 ± 5.32 b | 4.39 ± 0.28 b | 5.39 ± 0.07 c |
| T2 | 20.17 ± 0.95 | 1675 ± 54 a | 83.45 ± 2.01 b | 4.59 ± 0.11 b | 5.47 ± 0.05 bc |
| T1 + F | 19.83 ± 1.14 | 1695 ± 13 a | 92.95 ± 2.01 a | 5.14 ± 0.16 a | 5.51 ± 0.13 bc |
| T2 + F | 19.17 ± 1.05 | 1702 ± 20 a | 90.65 ± 4.46 a | 5.06 ± 0.23 a | 5.58 ± 0.07 b |
| ns | *** | *** | *** | *** | |
| C × B | |||||
| Ballerina × Control | 23.67 ± 0.88 | 1193 ± 55 f | 50.79 ± 1.58 h | 2.88 ± 0.10 f | 5.86 ± 0.06 a |
| Ballerina × F | 22.67 ± 0.33 | 1557 ± 58 bc | 76.69 ± 2.30 de | 3.82 ± 0.10 e | 5.00 ± 0.06 f |
| Ballerina × T1 | 23.33 ± 0.67 | 1665 ± 33 ab | 92.81 ± 1.58 b | 5.02 ± 0.02 b | 5.36 ± 0.10 de |
| Ballerina × T2 | 21.67 ± 0.88 | 1783 ± 52 a | 87.19 ± 2.59 c | 4.82 ± 0.08 bc | 5.51 ± 0.09 bcd |
| Ballerina × T1 + F | 22.00 ± 0.58 | 1694 ± 28 a | 93.81 ± 4.03 b | 5.42 ± 0.23 a | 5.76 ± 0.14 ab |
| Ballerina × T2 + F | 21.00 ± 0.58 | 1729 ± 32 a | 80.86 ± 1.15 de | 4.58 ± 0.14 cd | 5.65 ± 0.12 abc |
| Canasta × Control | 17.33 ± 1.76 | 1335 ± 41 e | 64.60 ± 0.72 g | 3.72 ± 0.07 e | 5.76 ± 0.02 ab |
| Canasta × F | 16.33 ± 1.45 | 1473 ± 26 cd | 72.66 ± 0.58 ef | 3.80 ± 0.02 e | 5.19 ± 0.05 ef |
| Canasta × T1 | 16.67 ± 2.19 | 1382 ± 57 de | 69.21 ± 1.01 fg | 3.77 ± 0.10 e | 5.43 ± 0.10 cde |
| Canasta × T2 | 18.67 ± 1.20 | 1568 ± 24 bc | 79.71 ± 0.43 de | 4.35 ± 0.05 d | 5.42 ± 0.06 cde |
| Canasta × T1 + F | 17.67 ± 1.20 | 1696 ± 8 a | 92.09 ± 1.58 bc | 4.87 ± 0.03 bc | 5.27 ± 0.06 de |
| Canasta × T2 + F | 17.33 ± 1.33 | 1676 ± 19 ab | 100.43 ± 1.29 a | 5.54 ± 0.09 a | 5.51 ± 0.09 bcd |
| ns | *** | *** | *** | ** |
| Source of Variance | SPAD Index | Fluorescence | ACO2 | gs | E |
|---|---|---|---|---|---|
| Fv/Fm Ratio | (μmol CO2 m−2 s−1) | (mol H2O m−2 s−1) | (mmol H2O m−2 s−1) | ||
| Cultivar (C) | |||||
| Ballerina | 32.56 ± 0.25 | 0.837 ± 0.00 | 19.64 ± 0.48 | 0.64 ± 0.04 | 8.86 ± 0.29 |
| Canasta | 33.01 ± 0.29 | 0.830 ± 0.00 | 20.41 ± 0.39 | 0.67 ± 0.04 | 9.29 ± 0.24 |
| t-test | ns | *** | ns | ns | ns |
| Biostimulant (B) | |||||
| Control | 31.70 ± 0.40 d | 0.831 ± 0.00 bc | 18.27 ± 0.67 c | 0.50 ± 0.04 c | 8.41 ± 0.38 c |
| F | 32.13 ± 0.24 d | 0.830 ± 0.00 c | 18.33 ± 0.67 c | 0.64 ± 0.07 b | 8.00 ± 0.41 c |
| T1 | 33.03 ± 0.29 bc | 0.836 ± 0.00 a | 19.94 ± 0.50 b | 0.62 ± 0.03 b | 9.73 ± 0.15 ab |
| T2 | 32.35 ± 0.14 cd | 0.835 ± 0.00 ab | 20.31 ± 0.25 b | 0.67 ± 0.04 b | 9.10 ± 0.33 b |
| T1 + F | 33.98 ± 0.18 a | 0.836 ± 0.00 a | 21.54 ± 0.31 a | 0.85 ± 0.09 a | 10.00 ± 0.35 a |
| T2 + F | 33.52 ± 0.66 ab | 0.835 ± 0.00 ab | 21.76 ± 0.72 a | 0.66 ± 0.07 b | 9.22 ± 0.59 b |
| *** | * | *** | *** | *** | |
| C × B | |||||
| Ballerina × Control | 31.19 ± 0.52 d | 0.834 ± 0.00 bcd | 17.12 ± 0.81 f | 0.58 ± 0.04 cdef | 8.99 ± 0.28 cde |
| Ballerina × F | 32.30 ± 0.31 c | 0.834 ± 0.00 bcd | 17.21 ± 0.83 f | 0.52 ± 0.03 def | 7.39 ± 0.52 f |
| Ballerina × T1 | 33.62 ± 0.18 b | 0.842 ± 0.00 a | 20.93 ± 0.53 bcd | 0.68 ± 0.04 bcde | 9.65 ± 0.33 abc |
| Ballerina × T2 | 32.30 ± 0.20 c | 0.838 ± 0.00 ab | 20.64 ± 0.42 bcd | 0.58 ± 0.01 cdef | 8.41 ± 0.00 def |
| Ballerina × T1 + F | 33.88 ± 0.30 b | 0.842 ± 0.00 a | 21.78 ± 0.43 b | 1.00 ± 0.10 a | 10.65 ± 0.30 a |
| Ballerina × T2 + F | 32.08 ± 0.17 cd | 0.833 ± 0.00 bcd | 20.16 ± 0.20 cde | 0.50 ± 0.02 ef | 8.07 ± 0.61 ef |
| Canasta × Control | 32.21 ± 0.51 c | 0.828 ± 0.00 de | 19.42 ± 0.53 de | 0.41 ± 0.03 f | 7.82 ± 0.55 ef |
| Canasta × F | 31.96 ± 0.41 cd | 0.826 ± 0.00 e | 19.45 ± 0.52 de | 0.76 ± 0.11 bc | 8.61 ± 0.45 cde |
| Canasta × T1 | 32.45 ± 0.21 c | 0.831 ± 0.00 cde | 18.95 ± 0.12 e | 0.57 ± 0.01 def | 9.81 ± 0.06 abc |
| Canasta × T2 | 32.41 ± 0.23 c | 0.831 ± 0.00 cde | 19.98 ± 0.16 cde | 0.75 ± 0.04 bc | 9.78 ± 0.27 abc |
| Canasta × T1 + F | 34.08 ± 0.26 ab | 0.830 ± 0.00 cde | 21.31 ± 0.49 bc | 0.71 ± 0.08 bcd | 9.35 ± 0.32 bcd |
| Canasta × T2 + F | 34.97 ± 0.26 a | 0.836 ± 0.00 abc | 23.36 ± 0.02 a | 0.81 ± 0.05 b | 10.36 ± 0.27 ab |
| *** | * | *** | *** | *** |
| Source of Variance | Total N | Nitrate | P | K | Ca | Mg | S | Na |
|---|---|---|---|---|---|---|---|---|
| (%) | (mg kg−1 FW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | |
| Cultivar (C) | ||||||||
| Ballerina | 3.88 ± 0.04 | 1446 ± 67 | 4.23 ± 0.09 | 43.83 ± 0.64 | 16.58 ± 0.33 | 5.91 ± 0.11 | 1.04 ± 0.02 | 4.45 ± 0.22 |
| Canasta | 3.99 ± 0.06 | 1106 ± 83 | 4.02 ± 0.08 | 44.13 ± 0.58 | 14.86 ± 0.20 | 5.78 ± 0.08 | 1.20 ± 0.02 | 4.36 ± 0.18 |
| t-test | ns | ** | ns | ns | *** | ns | *** | ns |
| Biostimulant (B) | ||||||||
| Control | 3.77 ± 0.04 c | 1087 ± 113 c | 3.78 ± 0.11 b | 42.09 ± 0.81 b | 15.11 ± 0.56 bc | 5.37 ± 0.10 d | 1.07 ± 0.05 b | 4.45 ± 0.38 b |
| F | 3.83 ± 0.07 bc | 1090 ± 157 c | 4.29 ± 0.12 a | 45.49 ± 0.83 a | 16.00 ± 0.54 ab | 6.04 ± 0.03 ab | 1.13 ± 0.04 ab | 4.28 ± 0.29 bc |
| T1 | 4.00 ± 0.09 ab | 1346 ± 40 b | 4.40 ± 0.08 a | 45.79 ± 0.72 a | 16.21 ± 0.64 ab | 6.27 ± 0.23 a | 1.04 ± 0.03 b | 5.50 ± 0.17 a |
| T2 | 3.89 ± 0.09 bc | 919 ± 72 d | 4.18 ± 0.14 a | 44.48 ± 0.81 a | 14.58 ± 0.47 c | 5.93 ± 0.11 abc | 1.12 ± 0.04 ab | 3.56 ± 0.20 d |
| T1 + F | 4.09 ± 0.09 a | 1480 ± 101 b | 4.43 ± 0.10 a | 45.46 ± 0.61 a | 16.55 ± 0.61 a | 5.82 ± 0.09 bc | 1.17 ± 0.06 a | 4.76 ± 0.28 b |
| T2 + F | 4.03 ± 0.06 ab | 1733 ± 76 a | 3.67 ± 0.08 b | 40.56 ± 0.29 b | 15.88 ± 0.49 ab | 5.62 ± 0.10 cd | 1.20 ± 0.05 a | 3.89 ± 0.08 cd |
| ** | *** | *** | *** | * | *** | * | *** | |
| C × B | ||||||||
| Ballerina × Control | 3.76 ± 0.07 | 1319 ± 28 c | 3.84 ± 0.16 | 41.85 ± 1.11 | 16.14 ± 0.55 | 5.31 ± 0.15 | 1.00 ± 0.01 | 5.07 ± 0.57 ab |
| Ballerina × F | 3.83 ± 0.13 | 1410 ± 91 bc | 4.42 ± 0.21 | 45.87 ± 1.81 | 17.15 ± 0.33 | 6.00 ± 0.03 | 1.05 ± 0.00 | 3.75 ± 0.07 de |
| Ballerina × T1 | 3.93 ± 0.10 | 1338 ± 66 c | 4.52 ± 0.07 | 45.07 ± 1.15 | 17.54 ± 0.46 | 6.63 ± 0.32 | 0.97 ± 0.02 | 5.37 ± 0.24 ab |
| Ballerina × T2 | 3.76 ± 0.06 | 1069 ± 48 de | 4.41 ± 0.19 | 44.14 ± 1.61 | 14.34 ± 0.55 | 5.90 ± 0.13 | 1.08 ± 0.04 | 3.16 ± 0.12 e |
| Ballerina × T1 + F | 4.00 ± 0.05 | 1692 ± 70 a | 4.46 ± 0.06 | 45.59 ± 0.60 | 17.78 ± 0.31 | 5.87 ± 0.07 | 1.05 ± 0.02 | 5.30 ± 0.12 ab |
| Ballerina × T2 + F | 4.00 ± 0.07 | 1846 ± 123 a | 3.71 ± 0.10 | 40.48 ± 0.33 | 16.54 ± 0.76 | 5.73 ± 0.11 | 1.11 ± 0.05 | 4.03 ± 0.01 d |
| Canasta × Control | 3.77 ± 0.04 | 854 ± 96 ef | 3.73 ± 0.18 | 42.33 ± 1.41 | 14.08 ± 0.44 | 5.44 ± 0.14 | 1.15 ± 0.08 | 3.83 ± 0.03 de |
| Canasta × F | 3.83 ± 0.09 | 770 ± 116 f | 4.15 ± 0.07 | 45.11 ± 0.12 | 14.85 ± 0.14 | 6.08 ± 0.06 | 1.21 ± 0.03 | 4.81 ± 0.37 bc |
| Canasta × T1 | 4.07 ± 0.15 | 1354 ± 59 c | 4.28 ± 0.10 | 46.51 ± 0.88 | 14.89 ± 0.31 | 5.92 ± 0.19 | 1.12 ± 0.00 | 5.62 ± 0.28 a |
| Canasta × T2 | 4.02 ± 0.14 | 770 ± 32 f | 3.96 ± 0.11 | 44.83 ± 0.74 | 14.83 ± 0.85 | 5.96 ± 0.21 | 1.16 ± 0.07 | 3.97 ± 0.12 d |
| Canasta × T1 + F | 4.18 ± 0.17 | 1269 ± 34 cd | 4.40 ± 0.21 | 45.34 ± 1.21 | 15.33 ± 0.53 | 5.77 ± 0.19 | 1.29 ± 0.03 | 4.22 ± 0.28 cd |
| Canasta × T2 + F | 4.07 ± 0.12 | 1621 ± 36 ab | 3.63 ± 0.15 | 40.64 ± 0.55 | 15.21 ± 0.42 | 5.51 ± 0.16 | 1.28 ± 0.03 | 3.74 ± 0.09 de |
| ns | ** | ns | ns | ns | ns | ns | *** |
| Source of Variance | Total Chlorophylls | TAA | HAA | Carotenoids | ABTS | Total Phenols |
|---|---|---|---|---|---|---|
| (mg g−1 FW) | (mg AA 100 g−1 FW) | (mmol AA eq. 100 g−1 DW) | (mg g−1 FW) | (mmol Trolox eq. 100 g−1 DW) | (mg gallic acid eq. g−1 DW) | |
| Cultivar (C) | ||||||
| Ballerina | 1.49 ± 0.02 | 145.9 ± 7.26 | 3.77 ± 0.28 | 0.34 ± 0.01 | 27.30 ± 0.66 | 3.64 ± 0.14 |
| Canasta | 1.22 ± 0.03 | 84.45 ± 15.3 | 7.40 ± 0.26 | 0.34 ± 0.00 | 24.67 ± 0.78 | 4.98 ± 0.20 |
| t-test | *** | *** | *** | ns | ns | *** |
| Biostimulant (B) | ||||||
| Control | 1.40 ± 0.05 | 81.86 ± 24.6 d | 5.35 ± 0.73 c | 0.33 ± 0.00 b | 27.86 ± 1.31 a | 4.85 ± 0.68 |
| F | 1.33 ± 0.09 | 123.7 ± 37.1 c | 4.75 ± 0.71 c | 0.32 ± 0.01 b | 24.92 ± 2.47 b | 4.18 ± 0.24 |
| T1 | 1.38 ± 0.10 | 86.02 ± 16.8 d | 4.83 ± 0.98 c | 0.39 ± 0.03 a | 24.71 ± 1.20 b | 4.48 ± 0.27 |
| T2 | 1.32 ± 0.05 | 86.42 ± 17.8 d | 6.41 ± 1.18 b | 0.34 ± 0.00 b | 27.36 ± 0.91 a | 4.07 ± 0.50 |
| T1 + F | 1.34 ± 0.05 | 164.3 ± 12.2 a | 5.00 ± 0.88 c | 0.33 ± 0.00 b | 24.25 ± 0.34 b | 3.73 ± 0.28 |
| T2 + F | 1.33 ± 0.06 | 148.9 ± 6.39 b | 7.16 ± 0.55 a | 0.34 ± 0.00 b | 26.79 ± 0.31 a | 4.54 ± 0.27 |
| ns | *** | *** | *** | *** | ns | |
| C × B | ||||||
| Ballerina × Control | 1.48 ± 0.03 | 136.6 ± 4.69 bcd | 3.83 ± 0.57 c | 0.33 ± 0.00 b | 30.54 ± 1.12 a | 3.52 ± 0.48 |
| Ballerina × F | 1.52 ± 0.02 | 206.5 ± 3.11 a | 3.23 ± 0.47 cd | 0.30 ± 0.00 c | 30.29 ± 1.32 a | 4.00 ± 0.40 |
| Ballerina × T1 | 1.59 ± 0.00 | 121.8 ± 8.29 d | 2.68 ± 0.08 d | 0.44 ± 0.02 a | 27.19 ± 0.64 bc | 3.90 ± 0.12 |
| Ballerina × T2 | 1.44 ± 0.02 | 125.3 ± 8.10 cd | 3.80 ± 0.16 c | 0.34 ± 0.01 b | 25.54 ± 0.66 cde | 3.10 ± 0.23 |
| Ballerina × T1 + F | 1.45 ± 0.02 | 139.3 ± 8.60 bcd | 3.12 ± 0.34 cd | 0.32 ± 0.00 bc | 23.81 ± 0.52 ef | 3.25 ± 0.22 |
| Ballerina × T2 + F | 1.43 ± 0.03 | 146.2 ± 7.03 bc | 5.97 ± 0.22 b | 0.33 ± 0.00 b | 26.43 ± 0.52 cd | 4.04 ± 0.21 |
| Canasta × Control | 1.32 ± 0.08 | 27.09 ± 2.06 f | 6.88 ± 0.22 b | 0.34 ± 0.01 b | 25.19 ± 0.45 cde | 6.18 ± 0.55 |
| Canasta × F | 1.13 ± 0.07 | 41.02 ± 4.83 ef | 6.27 ± 0.15 b | 0.35 ± 0.01 b | 19.56 ± 0.18 g | 4.35 ± 0.32 |
| Canasta × T1 | 1.18 ± 0.04 | 50.22 ± 7.49 e | 6.97 ± 0.40 b | 0.34 ± 0.01 b | 22.24 ± 0.79 f | 5.06 ± 0.12 |
| Canasta × T2 | 1.20 ± 0.02 | 47.52 ± 1.32 ef | 9.02 ± 0.38 a | 0.34 ± 0.01 b | 29.18 ± 0.60 ab | 5.05 ± 0.53 |
| Canasta × T1 + F | 1.24 ± 0.06 | 189.3 ± 7.01 a | 6.88 ± 0.44 b | 0.34 ± 0.01 b | 24.70 ± 0.33 de | 4.20 ± 0.32 |
| Canasta× T2 + F | 1.24 ± 0.08 | 151.6 ± 12.2 b | 8.36 ± 0.23 a | 0.34 ± 0.00 b | 27.14 ± 0.28 bc | 5.03 ± 0.27 |
| ns | *** | ** | *** | *** | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristofano, F.; El-Nakhel, C.; Pannico, A.; Giordano, M.; Colla, G.; Rouphael, Y. Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System. Agronomy 2021, 11, 1194. https://doi.org/10.3390/agronomy11061194
Cristofano F, El-Nakhel C, Pannico A, Giordano M, Colla G, Rouphael Y. Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System. Agronomy. 2021; 11(6):1194. https://doi.org/10.3390/agronomy11061194
Chicago/Turabian StyleCristofano, Francesco, Christophe El-Nakhel, Antonio Pannico, Maria Giordano, Giuseppe Colla, and Youssef Rouphael. 2021. "Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System" Agronomy 11, no. 6: 1194. https://doi.org/10.3390/agronomy11061194
APA StyleCristofano, F., El-Nakhel, C., Pannico, A., Giordano, M., Colla, G., & Rouphael, Y. (2021). Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System. Agronomy, 11(6), 1194. https://doi.org/10.3390/agronomy11061194











