Tomato Graft Union Failure Is Associated with Alterations in Tissue Development and the Onset of Cell Wall Defense Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Grafting Method
2.3. Sample Collection
2.4. Fixation, Inclusion and Cuts
2.5. Histological Techniques
2.6. Amyloplast Frequency Estimation
2.7. Soluble Sugars Extraction
2.8. Sugar Determination
2.9. Statistical Analysis
3. Results and Discussion
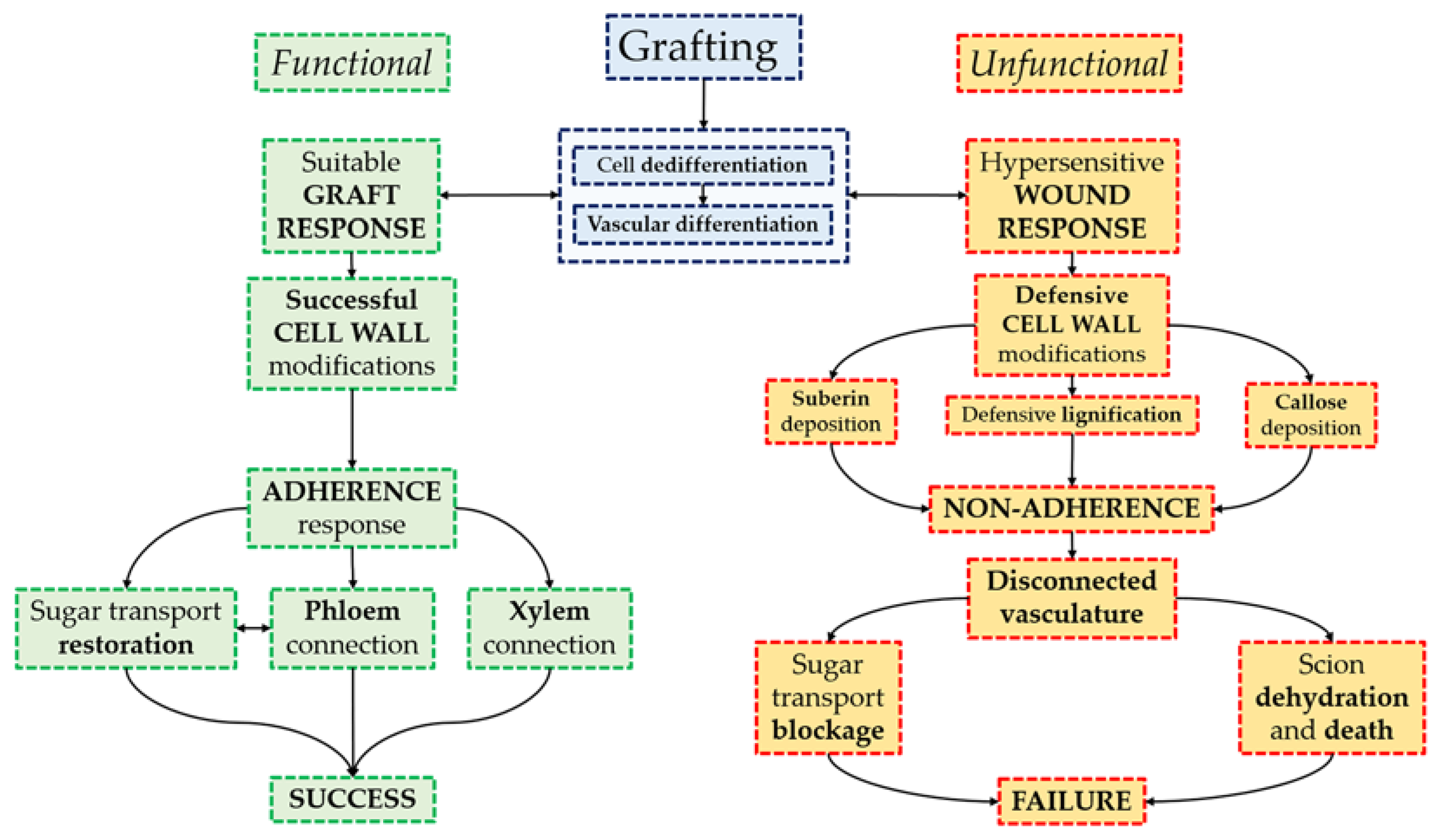
3.1. Tissue Development in Unfunctional Grafts
3.2. Asymmetry of Rootstock and Scion Responses in Unfunctional Grafts
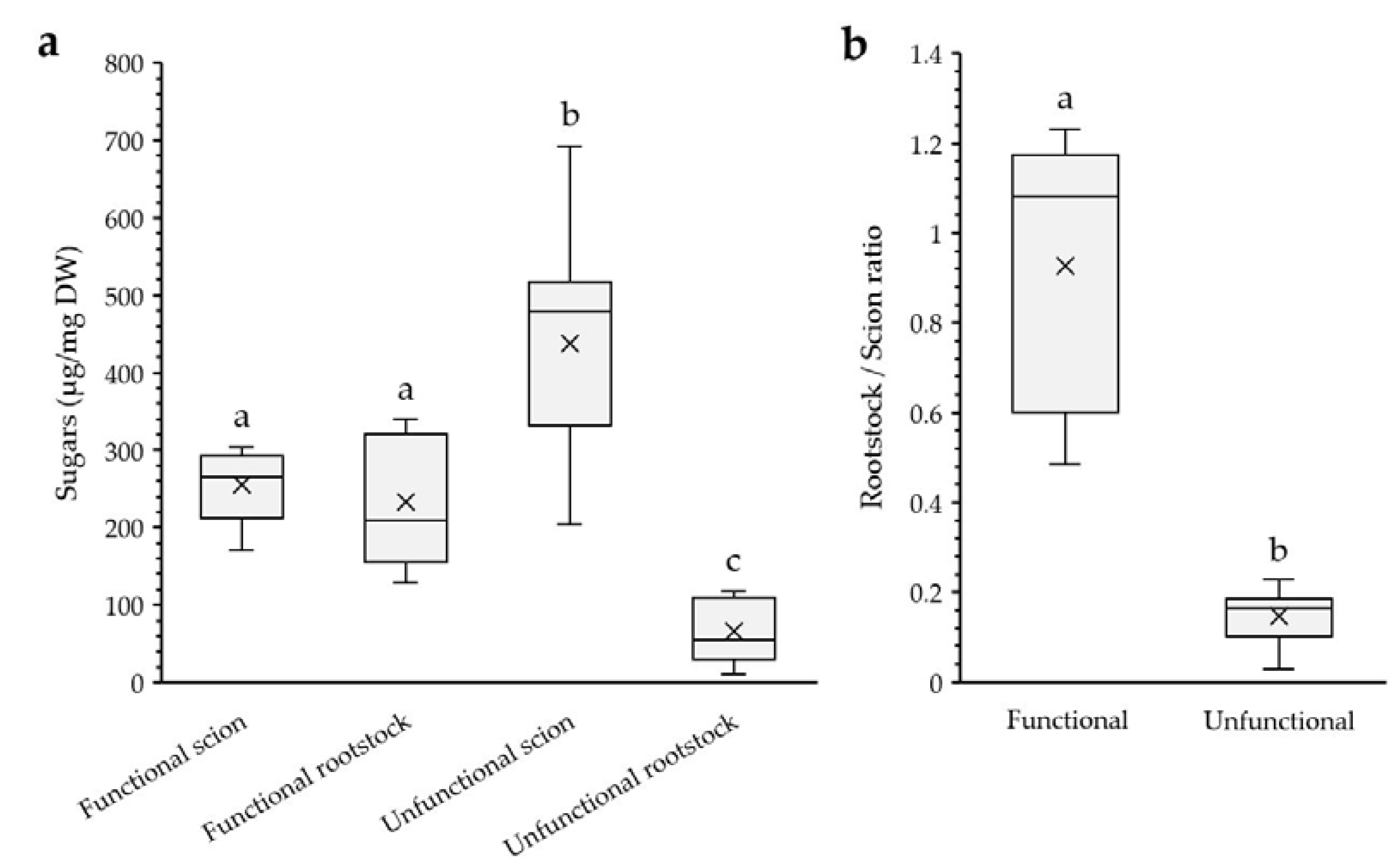
Uneven Distribution of Starch and Soluble Sugars Content
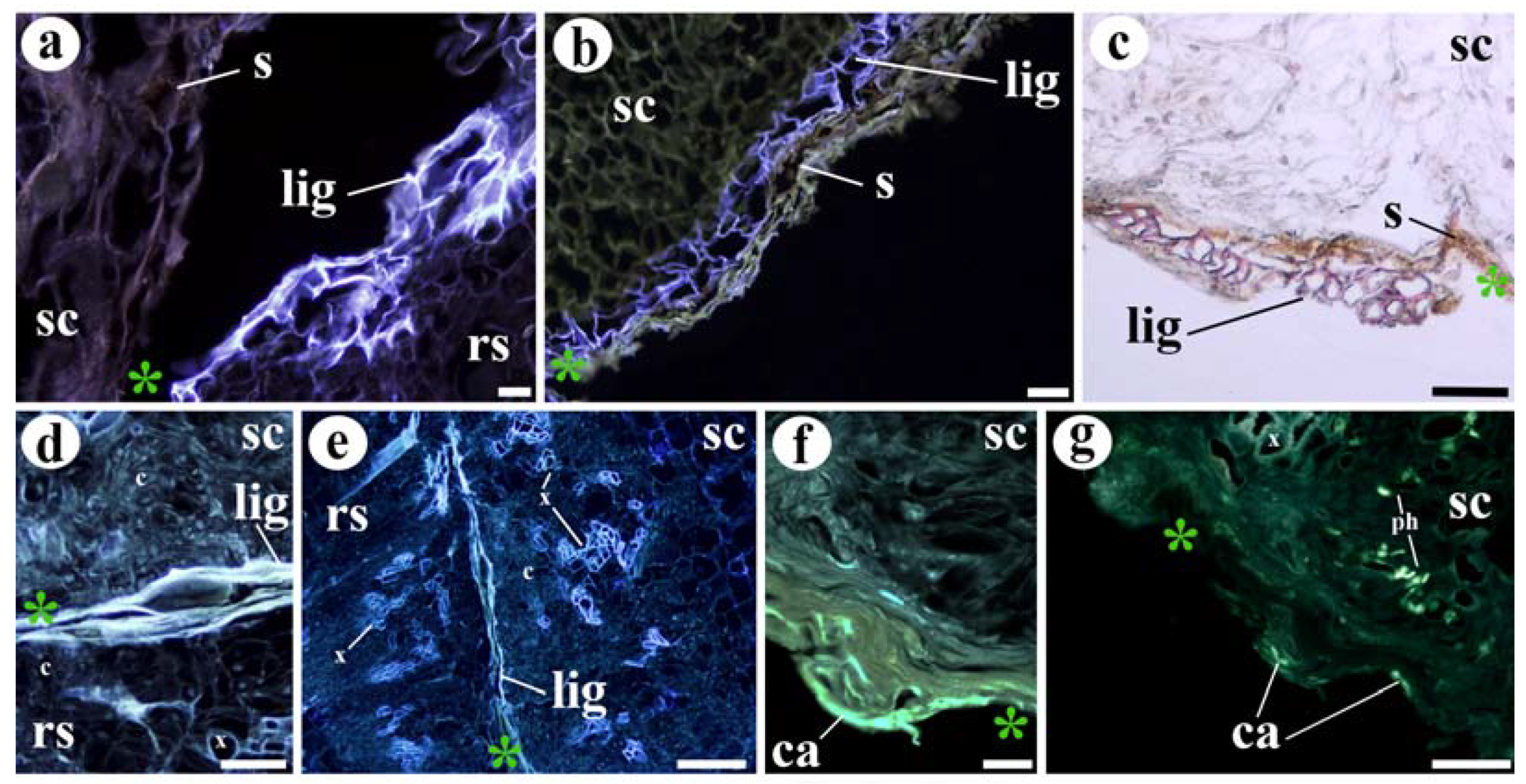
3.3. Cell Wall Responses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database (Crops). Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 December 2020).
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato grafting: A global perspective. HortScience 2017, 52, 1328–1336. [Google Scholar] [CrossRef]
- Lee, J.-M.; Oda, M. Grafting of herbaceous vegetable and ornamental crops. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Oxford, UK, 2010; Volume 28, pp. 61–124. [Google Scholar]
- Williams, B.; Ahsan, M.U.; Frank, M.H. Getting to the root of grafting-induced traits. Curr. Opin. Plant Biol. 2021, 59, 101988. [Google Scholar] [CrossRef]
- Loupit, G.; Cookson, S.J. Identifying molecular markers of successful graft union formation and compatibility. Front. Plant Sci. 2020, 11, 610352. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann & Kester’s Plant Propagation Principles and Practices, 9th ed.; Pearson: Harlow, UK, 2018; pp. 433–481. [Google Scholar]
- Lindsay, D.W.; Yeoman, M.M.; Brown, R. An analysis of the development of the graft union in Lycopersicon esculentum. Ann. Bot. 1974, 38, 639–646. [Google Scholar] [CrossRef]
- Moore, R.; Walker, D.B. Graft formation in Solanum pennellii (Solanaceae). Plant Cell Rep. 1984, 3, 172–175. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–Scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef]
- Frey, C.; Acebes, J.L.; Encina, A.; Álvarez, R. Histological changes associated with the graft union development in tomato. Plants 2020, 9, 1479. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E.M. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2017, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wulf, K.E.; Reid, J.B.; Foo, E. What drives interspecies graft union success? Exploring the role of phylogenetic relatedness and stem anatomy. Physiol. Plant. 2020, 170, 32–147. [Google Scholar] [CrossRef]
- Tsaballa, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Nianiou-Obeidat, I. Vegetable grafting from a molecular point of view: The involvement of epigenetics in rootstock-scion interaction. Front. Plant Sci. 2020, 11, 621999. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed]
- Moore, R. Studies of vegetative compatibility–Incompatibility in higher plants. V. A morphometric analysis of the development of a compatible and an incompatible graft. Can. J. Bot. 1982, 60, 2780–2787. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata incompatible and incompatible stem unions of Prunus spp. Sci. Hortic. 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Aloni, B.; Karni, L.; Deventurero, G.; Levin, Z.; Cohen, R.; Katzir, N.; Lotan-Pompan, M.; Edelstein, M.; Aktas, H.; Turhan, E.; et al. Physiological and biochemical changes at the rootstock–Scion interface in graft combinations between Cucurbita rootstocks and a melon scion. J. Hortic. Sci. Biotechnol. 2008, 83, 777–783. [Google Scholar] [CrossRef]
- Irisarri, P.; Binczycki, P.; Errea, P.; Juel, H.; Pina, A. Oxidative stress associated with rootstock–scion interactions in pear / quince combinations during early stages of graft development. J. Plant Physiol. 2015, 176, 25–35. [Google Scholar] [CrossRef]
- Baron, D.; Esteves Amaro, A.C.; Pina, A.; Ferreira, G. An overview of grafting re-establishment in woody fruit species. Sci. Hortic. 2019, 243, 84–91. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Jefree, C.E.; Yeoman, M.M. Development of intercellular connections between opposing cells in a graft union. New Phytol. 1983, 93, 491–509. [Google Scholar] [CrossRef]
- Fan, J.; Yang, R.; Li, X.; Zhao, W.; Zhao, F.; Wang, S. The processes of graft union formation in tomato. Hortic. Environ. Biotechnol. 2015, 56, 569–574. [Google Scholar] [CrossRef]
- Milewska-Hendel, A.; Polak, M.; Sala, K.; Zieleźnik-Rusinowska, P.; Gawecki, R.; Kurczyńska, E. Morpho-histological analysis of tomato (Solanum lycopersicum L.) plants after treatment with juglone. Acta Agrobot. 2017, 70, 1701. [Google Scholar] [CrossRef]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.; Zhang, Z.; Yan, X.; Chai, J.; Ren, Z.; Zheng, G.; Liu, H. Graft-union development: A delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. J. Exp. Bot. 2012, 63, 4219–4232. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Mitsuda, N.; Koyama, T.; Hiratsu, K.; Kojima, M.; Arai, T.; Inoue, Y.; Seki, M.; Sakakibara, H. Report the AP2 / ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 2011, 21, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Immanen, J.; Nieminen, K.; Smolander, O.-P.; Kojima, M.; Alonso Serra, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mähönen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Tiedemann, R. Graft union development and symplastic phloem contact in the heterograft Cucumis sativus on Cucurbita ficifolia. J. Plant Physiol. 1989, 134, 427–440. [Google Scholar] [CrossRef]
- Aloni, R. Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 1980, 150, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Donner, T.J.; Sherr, I.; Scarpella, E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 2009, 136, 3235–3246. [Google Scholar] [CrossRef]
- Sachs, T. The role of the root in the induction of xylem differentiation in peas. Ann. Bot. 1968, 32, 391–399. [Google Scholar] [CrossRef]
- Sachs, T. The control of the patterned differentiation of vascular tissues. Adv. Bot. Res. 1981, 9, 151–262. [Google Scholar]
- Biedroń, M.; Banasiak, A. Auxin-mediated regulation of vascular patterning in Arabidopsis thaliana leaves. Plant Cell Rep. 2018, 37, 1215–1229. [Google Scholar] [CrossRef]
- van de Pol, P.A.; Joosten, M.H.A.J.; Keizer, H. Stenting of roses, starch depletion and accumulation during the early development. Acta Hortic. 1986, 189, 51–60. [Google Scholar] [CrossRef]
- Asahina, M.; Azuma, K.; Pitaksaringkarn, W.; Yamazaki, T.; Mitsuda, N.; Ohme-Takagi, M.; Yamaguchi, S.; Kamiya, Y.; Okada, K.; Nishimura, T.; et al. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16128–16132. [Google Scholar] [CrossRef]
- Marsch-Martínez, N.; Franken, J.; Gonzalez-Aguilera, K.L.; de Folter, S.; Angenent, G.; Alvarez-Buylla, E.R. An efficient flat-surface collar-free grafting method for Arabidopsis thaliana seedlings. Plant Methods 2013, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, R.H.; Rier, J.P. Experimental induction of vascular tissues in callus of angiosperms. Am. J. Bot. 1963, 50, 418. [Google Scholar] [CrossRef]
- Herrero, J. Studies on compatible and incompatible graft combinations with special reference to hardy fruit trees. J. Hortic. Sci. 1951, 26, 186–237. [Google Scholar] [CrossRef]
- Kollmann, R.; Glockmann, C. Studies on graft unions III. On the mechanism of secondary formation of plasmodesmata at the graft interface. Protoplasma 1991, 165, 71–85. [Google Scholar] [CrossRef]
- Moore, R.; Walker, D.B. Studies of vegetative compatibility-incompatibility in higher plants. I. A structural study of a compatible autograft in Sedum telephoides (Crassulaceae). Am. J. Bot. 1981, 68, 820–830. [Google Scholar] [CrossRef]
- Moore, R.; Walker, D.B. Studies of vegetative compatibility-incompatibility in higher plants. II. A structural study of an incompatible heterograft between Sedum telephoides (Crassulaceae) and Solanum pennellii (Solanaceae). Am. J. Bot. 1981, 68, 831–842. [Google Scholar] [CrossRef]
- Asahina, M.; Iwai, H.; Kikuchi, A.; Yamaguchi, S.; Kamiya, Y.; Kamada, H.; Satoh, S. Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiol. 2002, 129, 201–210. [Google Scholar] [CrossRef]
- Sala, K.; Karcz, J.; Rypień, A.; Kurczyńska, E.U. Unmethyl-esterified homogalacturonan and extensins seal Arabidopsis graft union. BMC Plant Biol. 2019, 19, 151. [Google Scholar] [CrossRef]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M. XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. 2014, 80, 604–614. [Google Scholar] [CrossRef]
- Notaguchi, M.; Kurotani, K.I.; Sato, Y.; Tabata, R.; Kawakatsu, Y.; Okayasu, K.; Sawai, Y.; Okada, R.; Asahina, M.; Ichihashi, Y.; et al. Cell–cell adhesion in plant grafting is facilitated by β-1,4-glucanases. Science 2020, 369, 698–702. [Google Scholar] [CrossRef]
- Yeoman, M. Cellular reconigtion systems in grafting. In Cellular Interactions; Linskens, H.F., Heslop-Harrison, J., Eds.; Springer: Berlin, Germany, 1984; pp. 453–472. [Google Scholar]
- Nühse, T.S. Cell wall integrity signaling and innate immunity in plants. Front. Plant Sci. 2012, 3, 280. [Google Scholar] [CrossRef] [PubMed]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.; Boudet, A. Wound-induced lignin and suberin deposition in a woody angiosperm (Eucalyptus gunnii Hook.): Histochemistry of early changes in young plants. Protoplasma 1996, 191, 96–104. [Google Scholar] [CrossRef]
- Schneider, H. Deposition of wound gum, callose, and suberin as responses to diseases and wounding of citrus. Bull. Socete Bot. Fr. Actual. Bot. 1980, 127, 143–150. [Google Scholar] [CrossRef]
- Biggs, A.R.; Miles, N.W. Suberin deposition as a measure of wound response in peach bark. HortScience 1985, 20, 903–905. [Google Scholar]
- Rittinger, P.A.; Biggs, A.R.; Peirson, D.R. Histochemistry of lignin and suberin deposition in boundary layers formed after wounding in various plant species and organs. Can. J. Bot. 1987, 65, 1886–1892. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, G.T.N. Deposition of lignin, suberin and callose in relation to the restriction of infection by Botrytis cinerea in ghost spots of tomato fruits. J. Phytopathol. 1985, 112, 143–152. [Google Scholar] [CrossRef]
- Norday, T.; Shem, E.; Huat, J. Impacts of temperature and rootstocks on tomato grafting success rates. HortScience 2020, 55, 136–140. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, C.J.; Shang, Q. Healing responses of tube grafted tomato plug seedlings under different night temperatures. Acta Bot. Boreali-Occident. Sin. 2015, 35, 493–499. [Google Scholar]




| Staining | Mounting | Microscope | Target |
|---|---|---|---|
| Safranin–fast Green | Entellan | Bright-field | General staining |
| Hematoxylin–eosin | Entellan | Bright-field | General staining |
| Lugol | No | Bright-field | Starch |
| Sirofluor | No | Fluorescence | Callose |
| Phloroglucinol (Wiesner) | No | Bright-field | Lignin |
| Calcofluor | No | Fluorescence | Cellulose |
| Sudan III | No | Stereoscope | Suberin and hydrophobic compounds |
| - | Entellan | Fluorescence | Autofluorescence |
| Union Callus | Callus Cell Clusters | Vascular Differentiation | Adhesion | Vascular Reconnection | Amyloplast Frequency | Soluble Sugars | Cell Wall Defensive Deposits | |
|---|---|---|---|---|---|---|---|---|
| Functional graft | +++ | +++ | +++ | +++ | +++ | + | + | − |
| Unfunctional scion | − | ++ | ++ | −/± | − | +++ | +++ | +++ |
| Unfunctional rootstock | − | + | + | −/± | − | ± | ± | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frey, C.; Álvarez, R.; Encina, A.; Acebes, J.L. Tomato Graft Union Failure Is Associated with Alterations in Tissue Development and the Onset of Cell Wall Defense Responses. Agronomy 2021, 11, 1197. https://doi.org/10.3390/agronomy11061197
Frey C, Álvarez R, Encina A, Acebes JL. Tomato Graft Union Failure Is Associated with Alterations in Tissue Development and the Onset of Cell Wall Defense Responses. Agronomy. 2021; 11(6):1197. https://doi.org/10.3390/agronomy11061197
Chicago/Turabian StyleFrey, Carlos, Rafael Álvarez, Antonio Encina, and José Luis Acebes. 2021. "Tomato Graft Union Failure Is Associated with Alterations in Tissue Development and the Onset of Cell Wall Defense Responses" Agronomy 11, no. 6: 1197. https://doi.org/10.3390/agronomy11061197
APA StyleFrey, C., Álvarez, R., Encina, A., & Acebes, J. L. (2021). Tomato Graft Union Failure Is Associated with Alterations in Tissue Development and the Onset of Cell Wall Defense Responses. Agronomy, 11(6), 1197. https://doi.org/10.3390/agronomy11061197






