Effect of Amino Acids and Effective Microorganisms on Meadow Silage Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Analysis
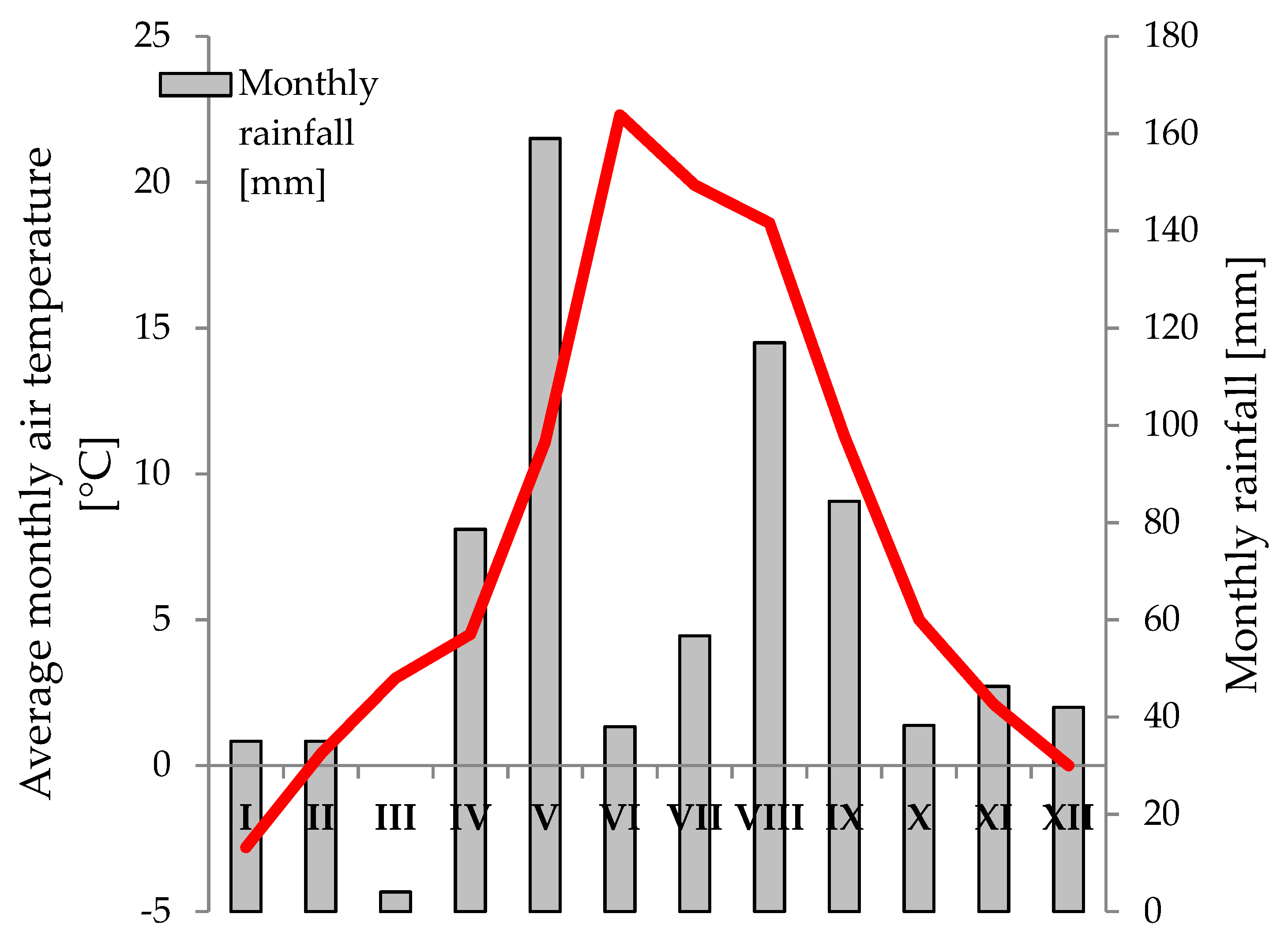
2.2. Weather Conditions
2.3. Materials and Experimental Designs
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Gugała, M.; Zarzecka, K.; Sikorska, A.; Mystkowska, I.; Dołęga, H. Wpływ herbicydów i biostymulatorów wzrostu na organiczenie zachwaszczenia i plonowanie ziemniaka jadalnego. Fragm. Agron. 2017, 34, 59–66. [Google Scholar]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A new trend towards solving an old problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawrońska, H.; Przybysz, A. Biostymulatory: Mechanizmy Działania i Przykłady Zastosowań; Mat. Konferencyjne, Targi sadownictwa i warzywnictwa: Warsaw, Poland, 2011; pp. 5–6. [Google Scholar]
- Chojnacka, K. Innovative bio-products for agriculture. Open Chem. 2015, 13, 932–937. [Google Scholar] [CrossRef]
- Lovatt, C.J. Use of a Natural Metabolite to Increase Crop Production. U.S. Patent 14/503,276, 21 May 2015. [Google Scholar]
- Wang, H.; Wu, L.; Tao, Q. Influence of partial replacement of nitrate by amino acids on nitrate accumulation of pakchoi (Brassica chinensis L.). China Environ. Sci. 2004, 24, 19–23. [Google Scholar]
- Persson, J.; Näshom, T. Regulation of amino acid uptake in conifers by exogenous and endogenous nitrogen. Planta 2002, 215, 639–644. [Google Scholar] [CrossRef]
- Persson, J.; Näshom, T. Regulation of amino acid uptake by carbon and nitrogen in Pinus sylvestris. Planta 2003, 217, 309–315. [Google Scholar] [CrossRef]
- Persson, J.; Hogberg, P.; Ekblad, A.; Hogberg, M.N.; Nordgren, A.; Nasholm, T. Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 2003, 137, 252–257. [Google Scholar] [CrossRef]
- Pessarakli, M. Handbook of Plant and Crop Physiology, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 385–394. [Google Scholar]
- Popova, O.V.; Dietz, K.J.; Golldack, D. Salt–dependent expression of a nitrate transporter and two amino acid transporter genes in Mesembryanthemum crystallinum. Plant Mol. Biol. 2003, 52, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Werdin-Pfisterer, N.R.; Kielland, K.; Boone, R.D. Soil amino acid composition across a boreal forest successional sequence. Soil Biol. Biochem. 2009, 41, 1210–1220. [Google Scholar] [CrossRef]
- Jones, D.L.; Shannon, D.; Junvee-Fortune, T.; Farrarc, J.F. Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol. Biochem. 2005, 37, 179–181. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassler, B.L. Cell-to-cell communication in bacteria: A chemical discourse. Harvey Lect. 2004, 100, 123–142. [Google Scholar] [PubMed]
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Method of Analysis, 18th ed.; Method 935.14 and 992.24; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Ahmed, Y.M.; Shalaby, E.A.; Shanan, N.T. The use of organic and inorganic cultures in improving vegetative growth, yield characters and antioxidant activity of roselle plants (Hibiscus sabdariffa L.). Afr. J. Biotechnol. 2011, 10, 1988–1996. [Google Scholar] [CrossRef]
- Sadak, M.S.H.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of amino acids on plant yield and physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.M.; Seadh, S.E.; AlTai, D.S.K. Role of humic acid and amino acids in limiting loss of nitrogen fertilizer and increasing productivity of some wheat cultivars grown under newly reclaimed sandy soil. Int. J. Adv. Res. Biol. Sci. 2016, 3, 123–136. [Google Scholar]
- Radkowski, A.; Radkowska, I.; Bocianowski, J. Effect of the fertilization of meadow sward with amino acids obtained from enzymatic hydrolysis on silage quality. J. Elem. 2020, 25, 259–277. [Google Scholar] [CrossRef]
- Saeed, M.R.; Kheir, A.M.; Al-Sayed, A.A. Supperssive effect of some amino acids against Meloidogyne incognita on soybeans. J. Agric. Sci. Mansoura Univ. 2005, 30, 1097–1103. [Google Scholar]
- El-Zohiri, S.S.M.; Asfour, Y.M. Effect of some organic compounds on growth and productivity of some potato cultivars. Ann. Agric. Sci. Moshtohor. J. 2009, 47, 403–415. [Google Scholar]
- Tarraf, S.A.; Talaat, I.M.; El-Sayed, A.E.B.; Balbaa, L.K. Influence of foliar application of algae extract and amino acids mixture on fenugreek plants in sandy and clay soils. Nusant. Biosci. 2015, 7, 33–37. [Google Scholar] [CrossRef]
- Gamal El-Din, K.M.; Abd El-Wahed, M.S.A. Effect of some amino acids on growth and essential oil content of chamomile plant. J. Agri. Biol. 2005, 7, 376–380. [Google Scholar]
- Zewail, R.M.Y. Effect of seaweed extract and amino acids on growth and productivity and some biocostituents of common bean (Phaseolus vulgaris L.) plants. J. Plant Prod. 2014, 5, 1441–1453. [Google Scholar] [CrossRef] [Green Version]
- Pooryousef, M.; Alizadeh, K. Effect of foliar application of free amino acids on alfalfa performance under rainfed conditions. Res. Crops 2014, 15, 254–258. [Google Scholar] [CrossRef]
- Goss, J.A. Amino acid synthesis and metabolism. In Physiology of Plants and Their Cells; Pergamon Press, Inc.: New York, NY, USA, 1973; p. 202. [Google Scholar] [CrossRef]
- Mulabagal, V.; Tsay, H.S. Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int. J. Appl. Sci. Eng. 2004, 2, 29–48. [Google Scholar]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, A.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Bettoni, M.M.; Mogor, Á.F.; Pauletti, V.; Goicoechea, N.; Aranjuelo, I.; Garmendia, I. Nutritional quality and yield of onion as affected by different application methods and doses of humic substances. J. Food Comp. Anal. 2016, 51, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Shahabivand, S.; Padash, A.; Aghaee, A.; Nasiri, Y.; Rezaei, P.F. Plant biostimulants (Funneliformis mosseae and humic substances) rather than chemical fertilizer improved biochemical responses in peppermint. Iran. J. Plant Physiol. 2018, 8, 2333–2344. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wojtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef] [Green Version]
- Hołubowicz-Kliza, G. Soybean Cultivation; IUNG Puławy: Puławy, Poland, 2007. [Google Scholar]
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arrigoni, G.; Masi, A. Quantitative proteomics of maize roots treated with a protein hydrolysate: A comparative study with transcriptomics highlights the molecular mechanisms responsive to Biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic Responses of Maize Shoots and Roots Elicited by Combinatorial Seed Treatments With Microbial and Non-microbial Biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Trivedi, K.; Anand, K.G.V.; Ghosh, A. Science behind biostimulant action of seaweed extract on growth and crop yield: Insights into transcriptional changes in roots of maize treated with Kappaphycus alvarezii seaweed extract under soil moisture stressed conditions. J. Appl. Phycol. 2020, 32, 599–613. [Google Scholar] [CrossRef]
- Brzóska, F. Roughage feeds from grassland and their use in feeding of livestock. Wieś Jutra 2008, 3, 28–33. [Google Scholar]
- Brzóska, F.; Śliwiński, B. Jakość pasz objętościowych w żywieniu przeżuwaczy i metody jej oceny. Cz. II. Metody analizy i oceny wartości pokarmowej pasz objętościowych. Wiad. Zoot. 2011, 4, 57–68. [Google Scholar]
- Podkówka, W.; Potkański, A. Wpływ czynników chemicznych i fizycznych na przydatność pasz do zakiszania. Post. Nauk Rol. 1993, 40, 29–42. [Google Scholar]
- Nowak, J.; Šařec, P. Wybrane czynniki decydujące o jakości kiszonek w belach cylindrycznych. Post. Nauk Rol. 2001, 5, 95–110. [Google Scholar]
- Tokeshi, H.; Aloes, M.C.; Sanches, A.B.; Harada, D.Y. Effective Microorganisms for controlling the phytopathogenic fungus Sclerotinia sclerotiorum in lettuce. In Proceedings of the Conference on Effective Microorganisms for a sustainable agriculture and environment. In Proceedings of the 4th International Conference on Kyusei Nature Farming, Bellingham, WA, USA, 19–21 June 1998; pp. 131–139. [Google Scholar]
- Kaczmarek, Z.; Jakubus, M.; Grzelak, M.; Mrugalska, L. Impact of the addition of various doses of Effective Microorganisms to arable-humus horizons of mineral soils on their physical and water properties. J. Res. Appl. Agric. Eng. 2008, 53, 118–121. [Google Scholar]
- Kaczmarek, Z.; Wolna-Maruwka, A.; Jakubus, M. Zmiany liczebności wybranych grup drobnoustrojów glebowych oraz aktywności enzymatycznej w glebie inokulowanej efektywnymi mikroorganizmami (EM). J. Res. Appl. Agric. Eng. 2008, 53, 122–128. [Google Scholar]
- Kucharski, J.; Jastrzębska, E. Rola mikroorganizmów efektywnych (EM) i glebowych w kształtowaniu właściwości mikrobiologicznych gleby. Zesz. Probl. Post. Nauk Rol. 2005, 507, 315–322. [Google Scholar]
- Badura, L. Czy znamy wszystkie uwarunkowania funkcji mikroorganizmów w ekosystemach lądowych? Kosm. Probl. Nauk Biol. 2004, 53, 373–379. [Google Scholar]
- Ollel, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotech. 2013, 88, 380–386. [Google Scholar] [CrossRef]
| Grass Species | 80% |
| Perennial ryegrass (Lolium perenne L.) | 25% |
| Meadow fescue (Festuca pratensis Huds.) | 15% |
| Timothy grass (Phleum pratense L.) | 14% |
| Kentucky bluegrass (Poa pratensis L.) | 12% |
| Meadow grass (Dactylis glomerata L.) | 6% |
| Red fescue (Festuca rubra L.) | 6% |
| False oat-grass (Arrhenatherum elatius L.) | 2% |
| Fabaceae | 7% |
| Red clover (Trifolium pratense L.) | 7% |
| Dicotyledonous plants | 13% |
| Broadleaf plantain (Plantago maior L.) | 1% |
| Buckhorn plantain (Plantago lanceolata L.) | 2% |
| Chickweed (Stellaria media (L.) Vill.) | 1% |
| Common dandelion (Taraxacum officinale F. H. Wigg.) | 2% |
| Common yarrow (Achillea millefolium L.) | 2% |
| Germander speedwell (Veronica chamaedrys L.) | 2% |
| Red dead-nettle (Lamium purpureum L.) | 2% |
| Shepherd’s purse (Capsella bursa pastoris) | 1% |
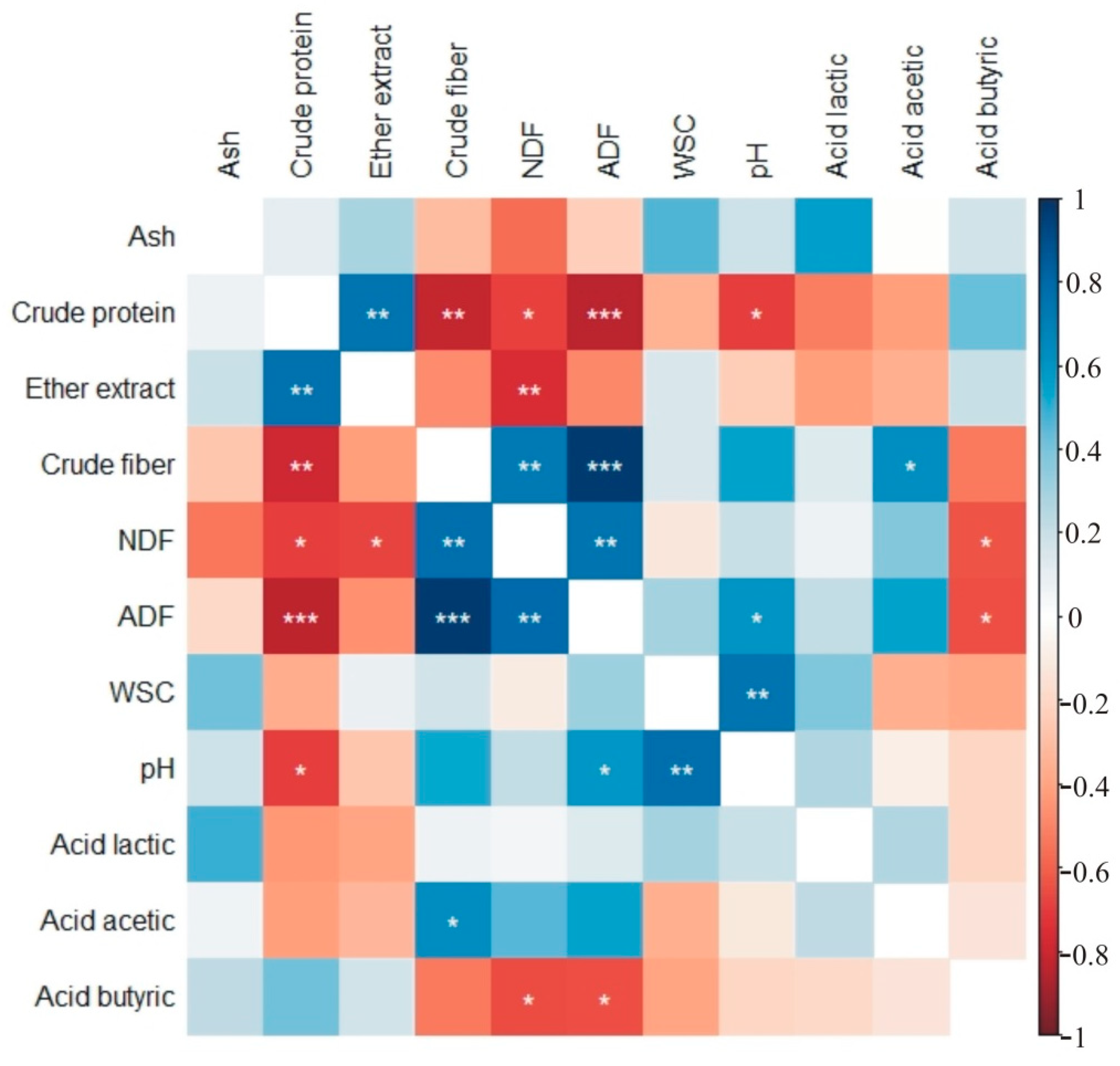
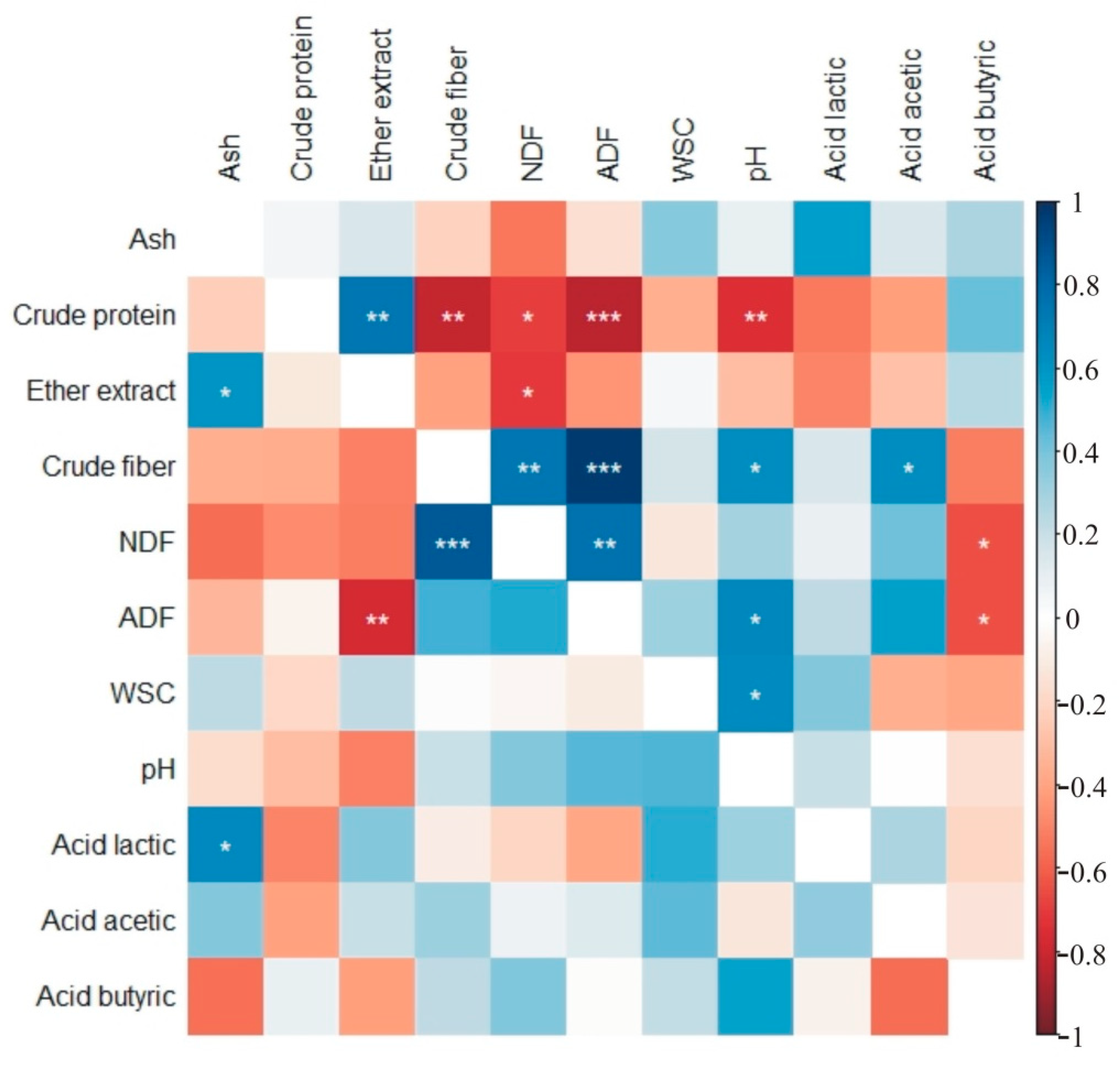
| Cut | Ash (g kg−1 DM) | Crude Protein (g kg−1 DM) | Ether Extract (g kg−1 DM) | Crude Fiber (g kg−1 DM) | NDF (g kg−1 DM) | ADF (g kg−1 DM) | Water-Soluble Carbohydrates (g kg−1 DM) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD 1 | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||||
| Control | I | 75.6 | abc 2 | 1.53 | 97.3 | b | 1.97 | 22.94 | d | 0.47 | 359.1 | a | 7.28 | 656.6 | a | 13.32 | 417.7 | ab | 8.47 | 84.9 | a | 1.72 |
| II | 65.01 | c | 1.69 | 128.8 | a | 2.89 | 28.2 | abc | 0.73 | 323.4 | abcd | 8.4 | 613.2 | a | 15.93 | 376.5 | bc | 9.78 | 64.91 | a | 1.69 | |
| III | 88.25 | a | 28.5 | 121.8 | a | 13.68 | 23.38 | cd | 5.88 | 275.1 | d | 64.7 | 599.2 | a | 113.5 | 344.6 | c | 64.8 | 75.88 | a | 40.97 | |
| Amino acids | I | 76.52 | abc | 1.55 | 98.3 | b | 1.83 | 22.97 | d | 0.51 | 362.7 | a | 6.83 | 664.3 | a | 13.26 | 423.1 | ab | 8.91 | 85.68 | a | 1.58 |
| II | 65.8 | c | 1.71 | 130.3 | a | 2.92 | 28.54 | ab | 0.74 | 327.4 | abc | 8.5 | 620.6 | a | 16.12 | 381 | bc | 9.9 | 65.7 | a | 1.71 | |
| III | 85.32 | a | 22 | 123.1 | a | 13.73 | 23.25 | d | 5.55 | 277.4 | cd | 64.9 | 601.5 | a | 107.5 | 348.2 | c | 65.05 | 75.05 | a | 38.03 | |
| Microorganisms | I | 78.16 | abc | 1.59 | 100.6 | b | 2.04 | 23.72 | bcd | 0.48 | 371.3 | a | 7.53 | 678.8 | a | 13.77 | 431.9 | a | 8.76 | 87.78 | a | 1.78 |
| II | 67.21 | bc | 1.75 | 133.1 | a | 2.99 | 29.15 | a | 0.76 | 333.9 | ab | 7.92 | 633.2 | a | 15.31 | 388.7 | abc | 9.89 | 67.11 | a | 1.74 | |
| III | 83.74 | ab | 17.5 | 125.9 | a | 14.15 | 23.67 | bcd | 5.47 | 283.9 | bcd | 66.3 | 617 | a | 113.6 | 355.7 | c | 66.46 | 77.94 | a | 41.38 | |
| Amino acids with microorganisms | I | 89.14 | a | 8.51 | 108.9 | b | 14.25 | 32.59 | a | 5.79 | 345.7 | a | 22.4 | 627.2 | a | 50.93 | 409.8 | ab | 21.17 | 87.49 | a | 2.02 |
| II | 75.85 | abc | 6.05 | 131.9 | a | 15.97 | 29.81 | a | 1 | 354.4 | a | 25.7 | 638.9 | a | 57.58 | 413.7 | ab | 21.99 | 67.06 | a | 1.73 | |
| III | 85.26 | a | 9.45 | 124.1 | a | 11.43 | 29.57 | a | 3.18 | 334.3 | ab | 19.4 | 619.6 | a | 22.8 | 426.5 | ab | 18.73 | 59.85 | a | 8.7 | |
| LSD0.05 | 17.65 | 14.35 | 4.94 | 50.5 | 87.88 | 50.1 | 29.09 | |||||||||||||||
| F pr. | 0.064 | <0.001 | <0.001 | 0.001 | 0.77 | 0.003 | 0.50 | |||||||||||||||
| Cut | pH | Acid Lactic (g kg−1 DM) | Acid Acetic (g kg−1 DM) | Acid Butyric (g kg−1 DM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD 1 | Mean | SD | Mean | SD | Mean | SD | ||||||
| Control | I | 6.418 | Abc 2 | 0.13 | 15.95 | ab | 2.85 | 1.176 | ab | 0.11 | 0.505 | abc | 0.08 |
| II | 5.974 | bcd | 0.16 | 12.07 | d | 0.31 | 0.284 | b | 0.04 | 0.663 | ab | 0.04 | |
| III | 5.812 | d | 0.74 | 14.82 | bc | 1.27 | 0.8 | ab | 1.4 | 0.875 | a | 0.82 | |
| Amino acids | I | 6.482 | ab | 0.12 | 15.89 | ab | 3.26 | 1.19 | ab | 0.11 | 0.512 | abc | 0.08 |
| II | 6.046 | bcd | 0.16 | 12.22 | d | 0.32 | 0.287 | b | 0.04 | 0.672 | ab | 0.04 | |
| III | 5.883 | cd | 0.75 | 14.87 | bc | 1.05 | 0.81 | ab | 1.42 | 0.886 | a | 0.83 | |
| Microorganisms | I | 6.635 | a | 0.13 | 16.49 | ab | 2.95 | 1.215 | ab | 0.12 | 0.523 | abc | 0.08 |
| II | 6.176 | abcd | 0.16 | 12.48 | cd | 0.32 | 0.293 | b | 0.04 | 0.686 | ab | 0.04 | |
| III | 5.958 | bcd | 0.74 | 15.29 | b | 1.25 | 0.827 | ab | 1.45 | 0.905 | a | 0.85 | |
| Amino acids with microorganisms | I | 6.361 | abcd | 0.29 | 18.21 | a | 1.44 | 1.475 | a | 0.33 | 0.108 | bc | 0.22 |
| II | 6.143 | abcd | 0.09 | 13.96 | bcd | 1.96 | 1.245 | ab | 0.73 | 0.16 | bc | 0.32 | |
| III | 6.237 | abcd | 0.19 | 14.23 | bcd | 1.07 | 0.905 | ab | 0.29 | 0 | c | 0 | |
| LSD0.05 | 0.57 | 2.59 | 1.08 | 0.62 | |||||||||
| F pr. | 0.024 | <0.001 | 0.035 | 0.049 | |||||||||
| Contrast | Control | Amino Acids | Microorganisms | Amino Acids with Microorganisms | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-II | I-III | II-III | I-II | I-III | II-III | I-II | I-III | II-III | I-II | I-III | II-III | |
| Ash | 11 | −13 | −23 * | 11 | −9 | −20 * | 11 | −6 | −17 | 13 | 4 | −9 |
| Crude protein | −31 *** | −24 ** | 7 | −32 *** | −25 ** | 7 | −33 *** | −25 ** | 7 | −23 ** | −15 * | 8 |
| Ether extract | −5 * | 0 | 5 | −6 * | 0 | 5 * | −5 * | 0 | 5 * | 3 | 3 | 0 |
| Crude fiber | 36 | 84 ** | 48 | 35 | 85 ** | 50 | 37 | 87 ** | 50 | −9 | 11 | 20 |
| NDF | 43 | 57 | 14 | 44 | 63 | 19 | 46 | 62 | 16 | −12 | 8 | 19 |
| ADF | 41 | 73 ** | 32 | 42 | 75 ** | 33 | 43 | 76 ** | 33 | −4 | −17 | −13 |
| Water-soluble carbohydrates | 20 | 9 | −11 | 20 | 11 | −9 | 21 | 10 | −11 | 20 | 28 | 7 |
| pH | 0.4 | 0.6 * | 0.2 | 0.4 | 0.6 * | 0.2 | 0.5 | 0.7 * | 0.2 | 0.2 | 0.1 | −0.1 |
| Lactic acid | 4 ** | 1 | −3 * | 4** | 1 | −3 * | 4 ** | 1 | −3 * | 4 ** | 4 ** | 0 |
| Acetic acid | 0.9 | 0.4 | −0.5 | 0.9 | 0.4 | −0.5 | 0.9 | 0.4 | −0.5 | 0.2 | 0.6 | 0.3 |
| Butyric acid | −0.2 | −0.4 | −0.2 | −0.2 | −0.4 | −0.2 | −0.2 | −0.4 | −0.2 | −0.1 | 0.1 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radkowski, A.; Radkowska, I.; Bocianowski, J.; Cyplik, A.; Wolski, K.; Bujak, H. Effect of Amino Acids and Effective Microorganisms on Meadow Silage Chemical Composition. Agronomy 2021, 11, 1198. https://doi.org/10.3390/agronomy11061198
Radkowski A, Radkowska I, Bocianowski J, Cyplik A, Wolski K, Bujak H. Effect of Amino Acids and Effective Microorganisms on Meadow Silage Chemical Composition. Agronomy. 2021; 11(6):1198. https://doi.org/10.3390/agronomy11061198
Chicago/Turabian StyleRadkowski, Adam, Iwona Radkowska, Jan Bocianowski, Adrian Cyplik, Karol Wolski, and Henryk Bujak. 2021. "Effect of Amino Acids and Effective Microorganisms on Meadow Silage Chemical Composition" Agronomy 11, no. 6: 1198. https://doi.org/10.3390/agronomy11061198






