Abstract
The floating system (FL) is a common soilless method for baby-leaf production, whereas the ebb and flow system (EF) has been proposed as an alternative. Both of them allow managing plant saline stress while preventing reduction in plant growth and yield and increasing product quality. The oak-leaf lettuce response to the growing conditions (hydroponics, salinity) in interaction with climate and genotype has been little studied. Two experiments were carried out with two oak-leaf cultivars (green- and red-colored type) grown in FL and EF systems at two levels of nutrient solution (NS) electrical conductivity (EC) (EC = 2.5 and 3.5 dS m−1; EC2.5, EC3.5, respectively) under autumn and late-spring conditions. The EF system caused an increase in salinity in the substrate where roots mainly develop, so it overcomes the effect of the EC3.5 treatment. In the autumn cycle, irrespective of the EC, EF-grown plants had improved leaf thickness (specific leaf area), color, and antioxidative (total phenol and carotenoid contents) properties; however, a reduction in yield was observed in the most productive cultivar (green type). In late spring, higher yield and product quality (processability, nitrate content) were obtained at the expense of color, with the FL showing the best productivity in the green type cultivar. The red type cultivar had higher dry mass, phenol, and carotenoid concentrations.
1. Introduction
The baby-leaf category is important among the leafy vegetables. It is harvested at an early-vegetative phase (8–12 cm in length), hence the name, and it includes many species, with Lactuca sativa L. among the most popular [1]. Along with iceberg, other types of lettuce such as oak-leaf with attractive colors and shapes are produced as baby-leaf to combine the best quality characteristics from all varieties [2]. They are mainly used as minimally processed vegetable products [3] and consumed in increasing amounts as they provide an important source of health-promoting compounds such as carotenoids, vitamin C, and polyphenols [4,5,6].
Soilless cultivation systems (SCS) are rather common for baby-leaf production; in particular, the hydroponic floating system (FL) is most widely used for the production of high-quality, minimally processed vegetables as it is an easy, resource-saving, and profitable growing technique [7,8]. However, some concerns have arisen about this system as it is static (with no recirculation of NS), and a lack of oxygen frequently occurs in the NS, especially at high temperatures [3], so farmers are forced to provide continuous oxygen enrichment of the NS. To cope with this potential limit, the ebb and flow system (EF) can be used as an alternative for growing baby-leaf vegetables as it allows better root oxygenation deriving from the periodical NS supply to the root through sub-irrigation. Nevertheless, the information available on the effect of EF is mainly focused on vegetables other than lettuce [9,10,11,12], while the only published study on this species involves the romaine morphotype [13].
Hydroponics provides efficient tools to manage saline stress on plants, preventing salinity levels higher than the tolerance threshold of crops, which negatively affect plant growth and yield. Concurrently, controlled saline stress can be applied in order to increase the production of secondary metabolites (phytochemicals/antioxidants) and sensorial traits (color, firmness) [11,14] and to reduce anti-nutritional factors such as nitrate [13], improving the “whole” quality of vegetable products [15]. However, as a tool for imposing saline stress, the EF system has only been tested for wild rocket [11], romaine lettuce, and endive/escarole [13].
The “whole” quality of leafy vegetables also depends on other key factors such as climatic factors and genotype [15]. In lettuce, the seasonal climate variability is reported to affect plant growth, yield, and product quality in interaction with the growing conditions and genotypes [16,17,18,19].
In order to identify the best soilless approach for the scarcely studied oak-leaf lettuce to produce high-quality baby-leaf lettuce, this work aims to assess the effect of (a) ebb and flow and floating soilless cultivation systems; (b) nutrient solution salinity; and (c) the growing cycle on the growth and yield as well as the bio-morphological and nutritional traits of two genotypes.
2. Materials and Methods
2.1. Crop and Trial Set-Up
Two experiments were carried out in the years 2013–2014 using the species Lactuca sativa var. crispa L. (oak-leaf morphotype) grown either in a floating (FL) or ebb-and-flow (EF) soilless cultivation system (SCS) in an unheated greenhouse.
In both SCSs, the set-up consisted of aluminum benches where the trays containing the plantlets were laid. Nutrient solution (NS) was always maintained on the bench in FL, whereas it was periodically delivered in EF. Details on trials set-up and crops management are reported in Conversa et al. [13].
The climatic conditions recorded during the trial periods in the greenhouse are reported in Figure 1.
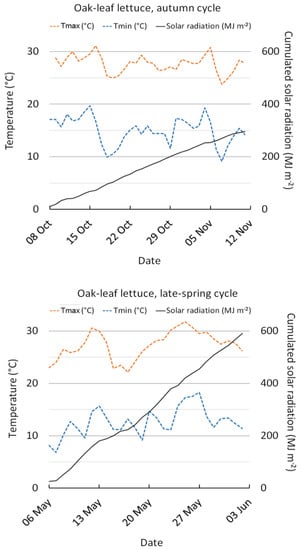
Figure 1.
Internal greenhouse minimum and maximum air temperature, and cumulated solar radiation during the autumn and late-spring periods.
2.2. Exp. 1
Two cultivars of oak-leaf lettuce namely, ‘Panisse’ (green type, Gautier Semenees—Eyragues, France) and ‘Oakly red’ (red type, Cora Seeds—FC, Italia), were sown on the 8 October 2013, autumn cycle). The harvest at the baby-leaf stage took place on 4 November 2013 (26 days after sowing). The following were compared: (a) floating (FL) and ebb and flow (EF) soilless cultivation systems; (b) two levels of electrical conductivity (EC) of the nutrient solution, 2.5 dS m−1 (EC2.5) and 3.5 dS m−1 (EC3.5); (c) two genotypes.
Treatments were arranged according to a split–split plot experimental design with three replications and with the SCS as the main plot, the EC of the NS (one bench with 16 trays) as sub-plots, the genotype as sub-sub-plots (8 trays per genotype on each bench) (the experimental unit).
The different salinity levels were obtained by adding 1.7 and 12 mmol L−1 of NaCl to the basal NS (EC 2.41 dS m−1) for the EC2.5 and EC3.5 treatment, respectively.
Over the whole crop cycle, the measured EC values of the NS (averaged over SCSs) were 2.9 ± 0.6 and 4.3 ± 0.3 dS m−1 (EC2.5 and EC3.5, respectively) and those of NS dissolved oxygen concentration (averaged over EC treatments) were 7.8 ± 0.5 and 8.9 ± 0.4 mg L−1 (FL and EF, respectively).
The productive (fresh weight (FW), dry weight (DW), leaf number, leaf height, leaf area), biophysiological (dry matter concentration (DM), specific leaf area (SLA), color indices (L*, h° and C*), concentration of chlorophylls, relative water content (RWC, %), electrolytic leakage (EL,%), and nutritional parameters (concentration of inorganic anions, phenols, and carotenoids) were determined for both genotypes.
The specific leaf area (SLA) is expressed as DW/leaf area (mg cm−2). The total chlorophylls (CHLtot) − (CHLa + CHLb), inorganic anions, carotenoids, and phenols (as gallic acid equivalents—GAE) are expressed on a FW basis. These latter analyses were performed on previously frozen samples (−80 °C).
More details about the NS composition and management, sampling, measurements, chemicals, and standards are reported in our previous paper [13].
2.3. Exp. 2
The above descripted trial was repeated in a late-spring (LS) cycle with the aim to assess the effect of the growing season. They used the same genotypes and soilless systems (FL and EF) but only at the 3.5 dS m−1 EC (EC3.5). The NS saline treatment was obtained as reported in Exp. 1. Treatments were arranged according to a split-plot experimental design with three replications and the SCS as main plots and the two genotypes as sub-plots.
In the late-spring cycle, lettuce was sown on 4 May 2014 and was harvested on 1 June 2014 (27 days after sowing). The productive and bio-physiological parameters reported for Exp. 1 were measured. Over the whole crop cycle, the averaged value of NS electrical conductivity was 4.8 ± 0.4 dS m−1, and the dissolved oxygen concentration was 8.5 ± 0.4 and 10.6 ± 0.2 mg L−1 in FL and EF, respectively.
2.4. Statistical Analysis
All data were statistically analyzed by ANOVA by using the GLM (General Linear Model) procedure—SAS/STAT® Statistical Analysis Software (SAS, Cary, NC, USA). In Experiment 2, a combined analysis of variance was performed using the season as a fixed variable. The least significant difference (LSD) test (α = 0.05) was used to establish differences between means.
For the visual analysis of the data, the principal component analysis (PCA) (PAST3 software [20] was performed on the main productive and qualitative parameters. The data matrix submitted to PCA was compiled considering all the treatments (2 SCSs, 2 ECs, 2 genotypes—autumn trial, and 2 SCSs, 2 genotypes—late spring trial) with 3 replications, so the PCA was made up of 36 records. Data were standardized ((x-mean)/standard deviation) before the analysis.
3. Results and Discussion
3.1. Effects of Soilless Cultivation System, Nutrient Solution Salinity, and Genotype
3.1.1. Growth, Yield, Leaf Bio-Physiological Traits
The plant dry weight (DW) (growth), fresh weight (yield) and dry mass concentration (DM) showed significant soilless cultivation system (SCS) × genotype interactions (Table 1).

Table 1.
Effect of soilless cultivation system (SCS), salinity level of nutrient solution (EC), and genotype (G) on yield and bio-morphological traits of oak-leaf lettuce leaves (autumn cycle). In brackets, the standard error of the mean.
Specifically, a reduction in growth and yield was observed for the cv. Panisse (green oak-leaf lettuce) grown in ebb and flow (EF) compared with the floating (FL) system (Figure 2A,B), whereas the ‘Oakly red’ (red oak-leaf lettuce) only showed a rise in leaf DM in EF (Figure S1). However, this latter cultivar was less productive than ‘Panisse’ (Figure 2).
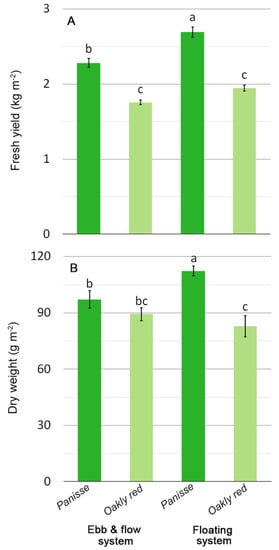
Figure 2.
Effect of soilless cultivation system and genotype on yield (A) and dry weight (B) of oak-leaf lettuce grown in a greenhouse during the autumn. Vertical bars indicate ±SE of mean (n = 6) of the observed values, with different letters significantly different according to the LSD test (α = 0.05).
Irrespective of genotype, the EF plants had a lower number, area, and height of leaves, but with a higher specific leaf area (SLA) (Table 1). The salinity of nutrient solution (NS) did not affect plant growth, whereas the NS at EC 3.5 dS m−2 provoked a slight yield reduction compared with the EC2.5 treatment associable to the decrease in leaf height, but with no further effects on the other plant morphological traits (Table 1).
The above-described plant responses were expected, as the salt stress is reported to result in a general decrease in fresh and/or dry weight, especially in the aerial part (lower leaf number and area). It is associable to the osmotic stress deriving from a lower water potential in the growing medium (soil/substrate or NS) [21,22,23]. The rise in SLA is known for plants under salinity stress [22], and it has been confirmed in soilless-grown romaine lettuce at 3.8/4.8 dS m−1 [14] or at 3.5 dS m−1 [13], which showed firmer leaves than at 2.8 or 2.5 dS m−1, respectively. Similar results have also been found for different leafy vegetables, such as wild rocket [11].
In this study, the evidence suggests that in the EF system, a salt stress higher than that imposed by the EC3.5 treatment may have occurred, as marked changes both in terms of plant growth and morphology were evident. In EF, the partial drying between the intermittent wettings (3 min wetting flux at the base of the trays every 100 min) may have exacerbated salt accumulation in the substrate, where roots mainly developed. On the contrary, the root apparatus in the FL system was always immersed in the NS with a more stable level of electrical conductivity maintained during the cycle. However, at the salinity load that occurred in EF, only the cv. Panisse exhibited detrimental effects on growth and productivity. On the contrary, the red oak-leaf type seems more tolerant to the EF-salinity load with the detected increase in leaf DM associable to plant osmotic adjustment to saline stress [22,24]. The lower productive potential exhibited by the cv. ‘Oakly red’, characterized by smaller plants (lower leaf number, height and area) (Table 1), seems linkable to its greater tolerance to salinity observed in this study, confirming previous studies comparing green vs. red lettuce cultivars [25]. However, in general, genotype differences in salt tolerance within butterhead, crisphead, romaine, and leaf lettuce have also been reported in other studies [13,21].
To the best of our knowledge, scarce information about oak-leaf lettuce grown in soilless systems under NaCl saline stress is available. Sgherri et al. [26] have reported a slight yield reduction (−9.8%) both in the green batavia and red oak-leaf lettuce grown with 200 mM NaCl-enriched NS for a short period (4 days). Luna et al. [17] compared this lettuce typology (red-colored) with the green butterhead at increasing EC levels of NS (1.4, 1.9, and 2.4 dS m−1). These authors only found a reduction in yield in the butterhead typology as an effect of the EC increase; nevertheless, the EC levels were obtained by raising the nitrate, ammonium, calcium, and potassium concentrations in the NS and maintaining Na and Cl unchanged. An increase in nutrient concentrations compared to a basic NS (EC < 1.3 dS m−1) was also applied to obtain saline treatment (10 dS m−1) with cultivar-specific yield response of oak-leaf, loose-leaf, and butterhead lettuce [23].
In agreement with results reported for other lettuce morphotypes, our research highlighted that the NaCl salt stress imposed by EC3.5 or EF can be considered from mild to moderate for oak-leaf lettuce.
A higher dry weight decrease (19–50%) than that observed in this study for ‘Panisse’ (13%) was observed in crisphead, butterhead, and romaine lettuce genotypes hydroponically grown at 6.3 dS m−1 EC of NS (with NaCl and CaCl added in a 2:1 ratio) [27]. A substantial reduction in dry weight has also been reported in romaine lettuce when irrigated for 15 days with very high saline water (>100 mM NaCl) [28] or grown in a soilless system with high saline nutrient solution (>100 mM NaCl) [29]. A drop in fresh yield has also been highlighted in butterhead lettuce at the rosette stage grown in soil irrigated with 7.2 dS m−1 EC water [30] and in many lettuce genotypes submitted to salt stress (6.3 dS m−1) [27]. In agreement with our results, these latter authors suggest an acceptable yield performance for lettuce at EC not higher than 3.6 dS m−1 [31].
It is noteworthy that the general enhancement of DM and SLA obtained in EF can be positively considered for baby-leaf vegetables, as it improves their suitability to be processed as fresh-cut produce [32,33]. The physiological status of leaves, tested through membrane electrolyte leakage (EL) and relative water content (RWC), highlights more stressed tissues for EF plants, showing a rise in membrane permeability and lower tissue hydration (lower RWC) compared to FL leaves (Table 2). This evidence confirms that higher salt-stressing conditions occurred in the EF system.

Table 2.
Effect of soilless cultivation system (SCS), salinity level (EC) of nutrient solution, and genotype (G) on the bio-physiological traits of oak-leaf lettuce (autumn cycle).
A lower RWC under saline stress has also been reported by other authors for lettuce and sage [34,35]. Additionally, in EF leaves, reductions in the levels of chlorophyll a (CHLa), b (CHLb), and total (CHLtot) were detected (Table 3). This detrimental effect could be related to the salinity increase that occurred in the EF system, with the lowest CHLtot value in ‘Panisse’ and the highest in FL for ‘Oakly red’ (Figure 3A). Chlorophyll level can be considered a biochemical marker of salt tolerance/sensitiveness in plants. A decrease in CHLs could occur in the salt-sensitive species/cultivars [25,36], resulting in appreciable growth reduction [22]. The decrease in growth showed by EF for ‘Panisse’ (Figure 2B) is probably due to the chlorophyll impairment caused by the salt stress. These differences in chlorophyll were not appreciable from the instrumental measurements of the color parameters, which were substantially affected by the genotype, with the red type ‘Oakly red’ showing a lower brightness (lower lightness index, L*) and colorfulness (C*) (Table 3), irrespective of the SCSs.

Table 3.
Effect of soilless cultivation system (SCS), salinity level (EC) of nutrient solution, and genotype (G) on inorganic anions and antioxidant compound concentrations of oak-leaf lettuce (autumn cycle).
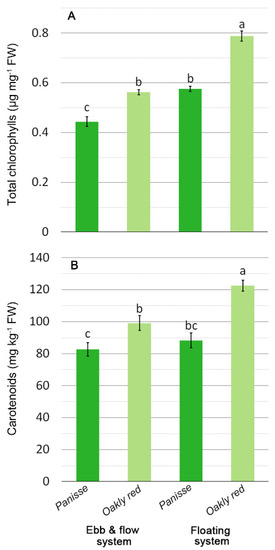
Figure 3.
Effect of soilless cultivation system and genotype on total chlorophylls (A) and carotenoids (B) of oak-leaf lettuce grown in a greenhouse during the autumn. Vertical bars indicate the ±SE of mean (n = 6) of the observed values, with different letters significantly different according to the LSD test (α = 0.05).
3.1.2. Leaf Inorganic Anion and Antioxidant Compound Contents
SCS affected the nitrate concentration in oak-leaf lettuce by increasing the level of this anion in EF plants, whereas the NS salinity had no effect (Table 3). Compared with romaine lettuce grown under the same conditions as the present work [13], oak-leaf seems more prone to accumulate nitrate, which is in agreement with findings reported by Di Gioia et al. [37] and Kappell et al. [23], whereas it showed values very similar to soilless-grown multi-leaf lettuce in the autumn–winter cycle [38]. However, the nitrate level was always below the limit imposed for autumn–winter lettuce by European Community Regulation 1258/2011.
Chloride concentration increased both with EC3.5 and EF compared with EC2.5 and FL, respectively, confirming the accumulation of Cl in plants exposed to saline (NaCl) stress [39] (Table 3).
It is noteworthy that a reduction in nitrate accumulation was expected as a consequence of Cl rise in EC3.5 and EF, as it is well known that salinity can contrast nitrate accumulation in leafy vegetables because of the antagonism between nitrate and chloride for the same root anion channel [40]. A decrease in nitrate concentration has been reported in baby-leaf romaine lettuce grown with NS at 4.8 dS m−1 [14] or 3.5 dS m−1 [13], which is in agreement with other experiments on wild rocket [11] and leafy vegetables [24]. Nevertheless, the nitrate–chloride antagonism cannot be confirmed for oak-leaf morphotype, as in EF-grown plants, nitrate accumulation even increased concurrently with chloride (Table 3).
This latter anion may exert negative effects if its level exceeds species/cultivar-specific critical toxicity values [41], as Cl accumulation may reduce the photosynthetic capacity and quantum yield due to chlorophyll degradation [42]. In this study, the salt-sensitiveness observed in the cv. Panisse in EF is not associable with the leaf chloride accumulation as, irrespective of SCS, it seems to accumulate a low level of Cl (Table 3). An unbalanced macronutrients uptake in the EF-grown ‘Panisse’ can be supposed, as it showed a reduction in phosphate uptake (Figure S2) that was likely involved in the observed reduction of dry biomass accumulation (Figure 2B) and negatively affecting chlorophylls (Figure 3A). Further research is required, also considering cation uptakes, to better understand cultivar-specific sensitiveness to NaCl load in the root medium.
No changes in carotenoids were detected among EC levels and SCSs, except for their improvement observed in ‘Oakly red’ plants grown in FL (Figure 3B), concordantly with chlorophylls (Figure 3A), whereas total phenols were higher in EF plants (Table 3). These compounds act as antioxidants to contrast reactive oxygen species (ROS) [43] produced by plants under biotic and abiotic stress, such as salinity [26]. Their increase is reported for lettuce and other green vegetables [28,29,44], which is linkable to a rise in the EC level in the cultivation medium.
In the present work, phenolic synthesis appears to be promoted only in EF-grown plants, confirming that these latter experienced a higher salinity load. This result would appear to be in disagreement with Sgherri et al. [26], who found that the content of phenols in oak-leaf lettuce was not affected by salinity; however, in their study, the salt stress lasted for a very short time (4 days).
No differences in inorganic anions emerged between cultivars, except for a higher concentration in Cl in ‘Oakly red’, which also showed higher total phenol content in agreement with other authors [17,19,25,26] reporting the red-type having a greater phenol content than the green-type lettuce.
3.2. Effects of the Growing Season, Soilless Cultivation System, and Genotype
Lettuce is considered a cool-season crop that thrives in areas where the mean temperatures range between 15 and 18 °C [45].
However, as repeated crop cycles can be carried out during the whole growing season (autumn–spring), plants may experience varying temperature regimes and radiation levels. It is well known that both temperature and solar radiation affect plant growth, yield, and product quality interacting with the growing conditions and genotypes [16,19]. Hence, this trial was performed to compare two crop cycles for oak-leaf lettuce cultivars both grown in EF and FL at EC3.5.
Growth, Yield, Leaf Bio-Physiological, Nutritional, and Antioxidative Traits
The significance of the F test for the crop cycle (CC), SCS, genotypes and their interactions are reported in Table 4. As the main effects of SCS, genotypes, and their interactions have been considered above, in this section, we focus on the main effect of the crop cycle and its interaction with both SCS and genotypes.

Table 4.
The significance of F test of ANOVA for the effects of the crop cycle (CC), soilless cultivation system (SCS), and genotype (G), and their interactions, on yield, bio-morpho-physiological traits, and the main inorganic and antioxidant compound concentrations in oak-leaf lettuce.
Neither CC × Genotype nor CC × SCS × Genotype interaction was statistically significant, whereas the CC affected both productive and qualitative parameters, frequently in interaction with the SCSs (Table 4). The dry weight showed an increasing trend passing from the autumn to late-spring (LS) cycle, with the greatest rise in growth observed for the FL-grown plants (Table 5).

Table 5.
Effect of the crop cycle and soilless cultivation system (SCS) on yield, bio-morpho-physiological traits, and the main inorganic anion and antioxidant compound concentrations of oak-leaf lettuce leaves. In brackets, the standard error of mean.
Compared with the autumn cycle, in the LS, a higher yield was obtained with plants exhibiting more expanded (Table 6) and taller leaves, especially in EF (higher height, Table 5). However, leaves were thicker due to the enhancement in DM and SLA (Table 5) and to a lower water content (RWC) (Table 6). Specifically, the FL-grown plants presented the largest increase in DM and SLA compared with the autumn product (Table 5). In general, the baby-leaf product obtained in the late-spring period may be considered to have better post-harvest processability [32,33] compared to that obtained under an autumn climate which, particularly in FL, seemed to have worse post-harvest handling resistance (thinner leaves).

Table 6.
Effect of crop cycle on yield, bio-morpho-physiological traits, and the main inorganic anion and antioxidant compound concentrations in oak-leaf lettuce.
During the late-spring cycle, despite temperatures in the greenhouse being similar to the autumn cycle, the solar radiation was higher (Figure 1). The longer photoperiod in the LS crop probably prompted plant growth and especially involved some morphological changes and physiological adjustments leading to the improvement of yield and product quality in terms of leaf firmness. Similar results have been also reported by other authors who found higher growth [16,19] and DM [19] in loose-leaf lettuce produced under higher solar radiation as well as in romaine lettuce leaves [13], endive/escarole [13], and baby-leaf wild rocket [11].
Additionally, the LS-grown leaves exhibited a reduction in phenol (Table 5), and carotenoid contents (Table 6), suggesting that they experienced less stressful conditions than under the autumn climate, as it has also been observed for C. endivia in the same crop cycle [13]. In particular, the higher radiation occurring in the LS cycle appears to alleviate the salt stress imposed by the EF, as EF plants exhibited the most substantial reduction in TP, in membrane permeability (EL), and showed an enhanced trend in chlorophyll concentration (Table 5).
As concerns inorganic anion content (Table 6), it is noteworthy that as expected, the late-spring conditions resulted in a decrease in nitrate concentration due to higher radiation availability, which prompts nitrogen assimilation through nitrate-reductase enzyme activation [40]. Accordingly, a rise in chloride concentration was observed confirming that Cl may replace NO3 in vacuoles as osmoticum when nitrate is required for sustaining higher plant growth [40]. The phosphate content decreased, unlike that of sulfate.
In terms of visual quality, oak-leaf lettuce leaves seemed to change in appearance in the two crop cycles, as LS leaves showed a reduction in their brightness (lower L* and Chroma), but also in greenness (lower h°) (Table 6).
3.3. Principal Component Analysis
The results of the principal component analysis (PCA) of all the selected data showed that the first two PCs explained 80.6% of the total variability, attributing 56.5% to PC1 and 24.1% to PC2 (Figure 4). PC1 allows a clear separation of the product obtained in late spring (on the right) from that obtained in the autumn (on the left) cycle.
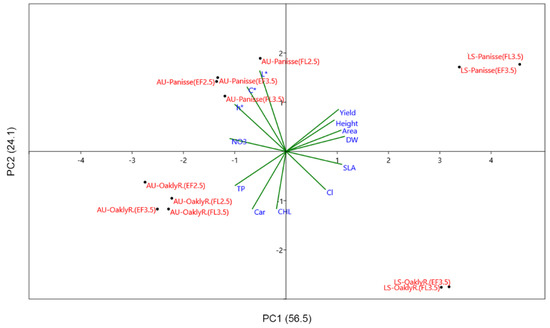
Figure 4.
Principal component analysis bi-plot (PC1 vs. PC2) showing the spatial distribution of the main productive and qualitative traits of oak-leaf lettuce as affected by growing season, soilless cultivation system, and genotype. The considered productive traits were yield (Yield), leaf area (Area), and height (H), dry weight (DW), specific leaf area (SLA). The considered qualitative traits were color indices (L*, h°, C*), concentration of total phenols (TP), carotenoids (CAR), chlorophylls, nitrate, and chloride.
The yield and its components (leaf area and height), plant growth (DW), SLA, and Cl were positively correlated to PC1 (loadings 0.31, 0.35, 0.33, and 0.24, respectively), whereas the color parameters (hue angle and chroma) (loadings −0.30, −0.23, respectively), and nutritional traits (nitrate, phenols, and carotenoids) (loadings −0.34, −0.31 and −0.20, respectively) were negatively correlated to PC1. PC2 separates the green type (‘Panisse’) (upper PC2 axis) from the red type (Oakly-red) cultivar (below the PC2 axis) for the color parameters, yield, and nutritional characteristics with the ‘Oakly-red’ being less productive, but with greater levels of chlorophylls, carotenoids, phenols, and chloride (loadings −0.36, −0.36 −0.24, and 0.23, respectively). Overall, the PC analysis highlights that in the late-spring cycle (right-hand side of PC1), the best productive results were obtained for ‘Panisse’ grown in FL (upper side of PC2), while in the autumn cycle (left-hand side of PC1), this occurred only when ‘Panisse’ was not exposed to saline stress (FL-EC2.5). However, this latter product had a poorer visual quality (higher L*) compared to ‘Panisse’ grown in EF/EC3.5, which clustered for greenness (higher hue angle). On the contrary, autumn-grown ‘Oakly-red’ (below the PC2 axis) clustered for the lowest productivity and an improved nutritional value (higher phenols and carotenoids, lower nitrate content) with negligible differences associable with SCS or NS EC.
4. Conclusions
Under Mediterranean greenhouse conditions, the climate changes during the growing season and genotype may be considered the driving force in affecting the response of oak-leaf lettuce to the soilless cultivation system. The best results in terms of crop productivity and product quality traits (processability, lower nitrate content) at the expense of color traits are expected in late spring when high temperatures are typically combined with high solar radiation, with FL advisable for highly productive cultivars such as ‘Panisse’. Conversely, under higher temperatures and lower solar radiation, the EF system should be preferred, as it produces baby-leaf lettuce with improved antioxidative properties, especially in the Oakly-red cultivar, and greenness in ‘Panisse’. In this highly productive cultivar, the reduction in yield with the EF is liable as a trade-off against the improved leaf quality. The described behaviors take place regardless of nutrient solution salinity, as their effects are substantially overcome if plants are grown in EF.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11061220/s1, Figure S1: Effect of soilless cultivation system and genotype on dry mass concentration of oak-leaf lettuce grown in a greenhouse during the autumn. Vertical bars indicate ±SE of mean (n = 6) of the observed values, with different letters significantly different according to the LSD test (α = 0.05). Figure S2: Effect of soilless cultivation system and genotype on phosphate concentration of oak-leaf lettuce grown in a greenhouse during the autumn. Vertical bars indicate ±SE of mean (n = 6) of the observed values, with different letters significantly different according to the LSD test (α = 0.05).
Author Contributions
Conceptualization, G.C., A.E.; methodology, G.C., A.B.; validation, G.C., A.E.; formal analysis, G.C.; investigation, G.C., A.B., C.L.; data curation, A.B., C.L.; resources, A.E.; writing—original draft preparation, G.C.; writing—review and editing, G.C., A.E.; visualization, G.C., A.E.; supervision, A.E.; funding acquisition, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 289719 (Project QUAFETY).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors are grateful to Paolo La Rotonda for technical support at Department of the Science of Agriculture, Food, Natural resources and Engineering (DAFNE) (University of Foggia).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicola, S.; Fontana, E. Chapter 9—Fresh-Cut Produce Quality: Implications for a Systems Approach. In Postharvest Handling, 3rd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 217–273. ISBN 978-0-12-408137-6. [Google Scholar]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-Leaf and Multi-Leaf of Green and Red Lettuces Are Suitable Raw Materials for the Fresh-Cut Industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Conesa, E.; Fernández, J.A.; Niñirola, D.; Egea-Gilabert, C. Nutrient Solution Aeration and Growing Cycles Affect Quality and Yield of Fresh-Cut Baby Leaf Red Lettuce. Agric. Food Sci. 2015, 24, 313–322. [Google Scholar] [CrossRef]
- DuPont, M.S.; Mondin, Z.; Williamson, G.; Price, K.R. Effect of Variety, Processing, and Storage on the Flavonoid Glycoside Content and Composition of Lettuce and Endive. J. Agric. Food Chem. 2000, 48, 3957–3964. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient Deprivation Eustress Elicits Differential Secondary Metabolites in Red and Green-Pigmented Butterhead Lettuce Grown in a Closed Soilless System. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef] [PubMed]
- Lanza Volpe, M.; Soto Vargas, V.C.; Morón, A.; González, R.E. Bioactive Compounds, Antioxidant Activity and Growth Behavior in Lettuce Cultivars Grown under Field and Greenhouse Conditions. Proceedings 2021, 70, 52. [Google Scholar] [CrossRef]
- Tomasi, N.; Pinton, R.; Dalla Costa, L.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New ‘Solutions’ for Floating Cultivation System of Ready-to-Eat Salad: A Review. Trends Food Sci. Technol. 2015, 46, 267–276. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Radiation and Water Use Efficiencies of Greenhouse Zucchini Squash in Relation to Different Climate Parameters. Eur. J. Agron. 2005, 23, 183–194. [Google Scholar] [CrossRef]
- Nicola, S.; Hoeberechts, J.; Fontana, E. Comparison between Traditional and Soilless Culture Systems to Produce Rocket (Eruca sativa) with Low Nitrate Content. Acta Hortic. 2005, 697, 549–555. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, Biophysical and Physiological Characteristics of Wild Rocket Genotypes As Affected by Soilless Cultivation System, Salinity Level of Nutrient Solution and Growing Period. Front. Plant Sci. 2017, 8, 300. [Google Scholar] [CrossRef]
- Hamilton, J.M.; Fonseca, J.M. Effect of Saline Irrigation Water on Antioxidants in Three Hydroponically Grown Leafy Vegetables: Diplotaxis tenuifolia, Eruca sativa, and Lepidium sativum. HortScience 2010, 45, 546–552. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; La Rotonda, P.; Elia, A. Reduction of Nitrate Content in Baby-Leaf Lettuce and Cichorium endivia through the Soilless Cultivation System, Electrical Conductivity and Management of Nutrient Solution. Front. Plant Sci. 2021, 12, 645671. [Google Scholar] [CrossRef]
- Scuderi, D.; Restuccia, C.; Chisari, M.; Barbagallo, R.N.; Caggia, C.; Giuffrida, F. Salinity of Nutrient Solution Influences the Shelf-Life of Fresh-Cut Lettuce Grown in Floating System. Postharvest Biol. Technol. 2011, 59, 132–137. [Google Scholar] [CrossRef]
- Ahuja, L.R.; Ma, L.; Green, T.R. Effective Soil Properties of Heterogeneous Areas for Modeling Infiltration and Redistribution. Soil Sci. Soc. Am. J. 2010, 74, 1469–1482. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Battistelli, A.; Colla, G. Yield and Quality of Leafy Lettuce in Response to Nutrient Solution Composition and Growing Season. J. Food Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- Luna, M.C.; Tudela, J.A.; Martínez-Sánchez, A.; Allende, A.; Marín, A.; Gil, M.I. Long-Term Deficit and Excess of Irrigation Influences Quality and Browning Related Enzymes and Phenolic Metabolism of Fresh-Cut Iceberg Lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2012, 73, 37–45. [Google Scholar] [CrossRef]
- Selma, M.V.; Luna, M.C.; Martínez-Sánchez, A.; Tudela, J.A.; Beltrán, D.; Baixauli, C.; Gil, M.I. Sensory Quality, Bioactive Constituents and Microbiological Quality of Green and Red Fresh-Cut Lettuces (Lactuca sativa L.) Are Influenced by Soil and Soilless Agricultural Production Systems. Postharvest Biol. Technol. 2012, 63, 16–24. [Google Scholar] [CrossRef]
- Sublett, W.L.; Barickman, T.C.; Sams, C.E. The Effect of Environment and Nutrients on Hydroponic Lettuce Yield, Quality, and Phytonutrients. Horticulturae 2018, 4, 48. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Xu, C.; Mou, B. Evaluation of Lettuce Genotypes for Salinity Tolerance. HortScience 2015, 50, 1441–1446. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Kappel, N.; Boros, I.F.; Ravelombola, F.S.; Sipos, L. EC Sensitivity of Hydroponically-Grown Lettuce (Lactuca sativa L.) Types in Terms of Nitrate Accumulation. Agriculture 2021, 11, 315. [Google Scholar] [CrossRef]
- Barbieri, G.; Bottino, A.; Di Stasio, E.; Vallone, S.; Maggio, A. Proline and Light as Quality Enhancers of Rocket (Eruca sativa Miller) Grown under Saline Conditions. Sci. Hortic. 2011, 128, 393–400. [Google Scholar] [CrossRef]
- Carillo, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Kyriacou, M.C.; Sifola, M.I.; Rouphael, Y. Physiological and Nutraceutical Quality of Green and Red Pigmented Lettuce in Response to NaCl Concentration in Two Successive Harvests. Agronomy 2020, 10, 1358. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and Salinity Are Responsible for Phenolics-Enrichment in Two Differently Pigmented Lettuces. Plant Physiol. Biochem. 2017, 115, 269–278. [Google Scholar] [CrossRef]
- Adhikari, N.D.; Simko, I.; Mou, B. Phenomic and Physiological Analysis of Salinity Effects on Lettuce. Sensors 2019, 19, 4814. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in Irrigation Water Affects the Nutritional and Visual Properties of Romaine Lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H.; Huang, J.; Gruber, M.Y.; Kaddour, R.; Lachaâl, M.; Ouerghi, Z.; Hannoufa, A. The Impact of Genotype and Salinity on Physiological Function, Secondary Metabolite Accumulation, and Antioxidative Responses in Lettuce. J. Agric. Food Chem. 2010, 58, 5122–5130. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Rouphael, Y.; Colla, G.; Fagnano, M.; Paradiso, R.; Mori, M. Morphophysiological Traits and Nitrate Content of Greenhouse Lettuce as Affected by Irrigation with Saline Water. HortScience 2017, 52, 1716–1721. [Google Scholar] [CrossRef]
- Atzori, G.; Mancuso, S.; Masi, E. Seawater Potential Use in Soilless Culture: A Review. Sci. Hortic. 2019, 249, 199–207. [Google Scholar] [CrossRef]
- Clarkson, G.J.J.; O’Byrne, E.E.; Rothwell, S.D.; Taylor, G. Identifying Traits to Improve Postharvest Processability in Baby Leaf Salad. Postharvest Biol. Technol. 2003, 30, 287–298. [Google Scholar] [CrossRef]
- Conversa, G.; Lazzizera, C.; Bonasia, A.; Elia, A. Yield and Phosphorus Uptake of a Processing Tomato Crop Grown at Different Phosphorus Levels in a Calcareous Soil as Affected by Mycorrhizal Inoculation under Field Conditions. Biol. Fertil. Soils 2013, 49, 691–703. [Google Scholar] [CrossRef]
- Garrido, Y.; Tudela, J.A.; Marín, A.; Mestre, T.; Martínez, V.; Gil, M.I. Physiological, Phytochemical and Structural Changes of Multi-Leaf Lettuce Caused by Salt Stress. J. Sci. Food Agric. 2014, 94, 1592–1599. [Google Scholar] [CrossRef]
- Taârit, M.B.; Msaada, K.; Hosni, K.; Marzouk, B. Physiological Changes, Phenolic Content and Antioxidant Activity of Salvia officinalis L. Grown under Saline Conditions. J. Sci. Food Agric. 2012, 92, 1614–1619. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting Responses of Photosynthesis to Salt Stress in the Glycophyte Arabidopsis and the Halophyte Thellungiella: Role of the Plastid Terminal Oxidase as an Alternative Electron Sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; Gonnella, M.; Buono, V.; Ayala, O.; Santamaria, P. Agronomic, Physiological and Quality Response of Romaine and Red Oak-Leaf Lettuce to Nitrogen Input. Ital. J. Agron. 2017, 12. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M.; Serio, F. Yield and Quality of Greenhouse Multi-Leaf Lettuce Cultivars Grown in Soil and Soilless Culture under Mediterranean Conditions. Italus Hortus 2020, 27, 18–30. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. The Importance of Cl− Exclusion and Vacuolar Cl− Sequestration: Revisiting the Role of Cl− Transport in Plant Salt Tolerance. Front. Plant Sci. 2019, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A Review of Environment Effects on Nitrate Accumulation in Leafy Vegetables Grown in Controlled Environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High Concentrations of Na+ and Cl− Ions in Soil Solution Have Simultaneous Detrimental Effects on Growth of Faba Bean under Salinity Stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Effect of NaCl Stress on Health-Promoting Compounds and Antioxidant Activity in the Sprouts of Three Broccoli Cultivars. Int. J. Food Sci. Nutr. 2014, 65, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Alvino, A.; Barbieri, G. Vegetables of Temperate Climates: Leafy Vegetables. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 393–400. ISBN 978-0-12-384953-3. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).