Exploiting Thrips Aggregation Pheromones to Develop a Lure-and-Kill Strategy for the Management of the Bean Flower Thrips
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Pheromones and Attractants Used
2.3. Blue Sticky Traps
2.4. Rubber Septa Dispensers
2.5. Fungal Culture
2.6. Effects of Thrips Aggregation Pheromones and Non-Pheromone Attractant on M. anisopliae Conidial Viability
2.7. Effect of Thrips Aggregation Pheromone and Non-Pheromone Attractants on M. anisopliae Conidial Germ Tube Growth
2.8. Attraction of M. sjostedti to the Major Compound of Its Aggregation Pheromone
2.9. Effect of Major Compound of M. sjostedti Aggregation Pheromone on M. anisopliae Persistence in an Autoinoculation Device
2.10. Statistical Analysis
3. Results
3.1. Effect of M. sjostedti Aggregation Pheromone Components on Conidial Viability and Germ Tube Growth
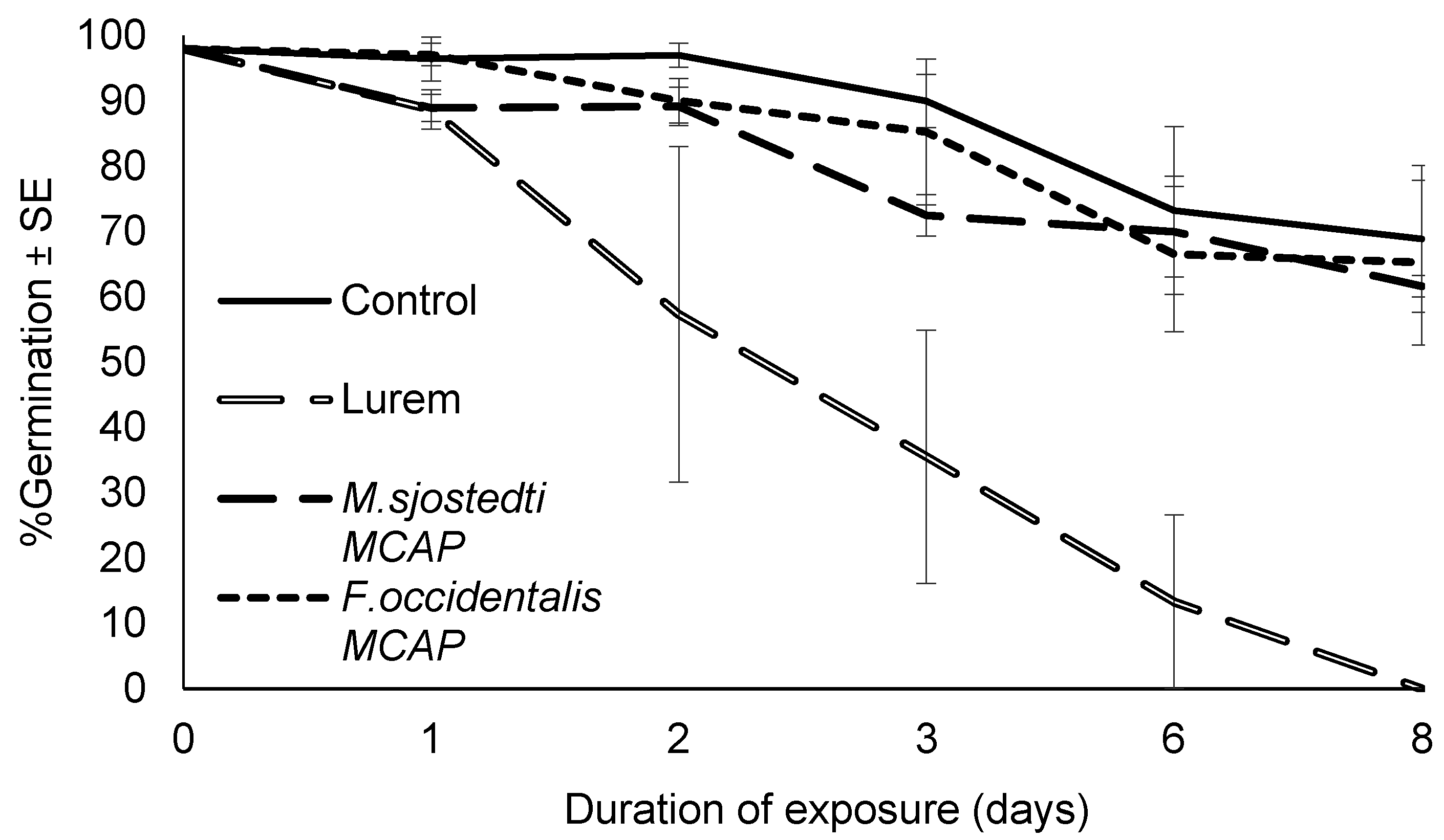
3.2. Effects of Thrips Aggregation Pheromones and Non-Pheromone Attractants on M. anisopliae Conidial Viability and Time Taken to Reach 50% Viability
3.3. Effect of Thrips Aggregation Pheromones and Non-Pheromone Attractant on M. anisopliae Germ Tube Growth
3.4. Attraction of M. sjostedti to the Major Compound of Its Aggregation Pheromone
3.5. Effect of M. sjostedti Aggregation Pheromone on M. anisopliae Viability in an Autoinoculation Device
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abate, T.; Alene, A.D.; Bergvinson, D.; Shiferaw, B.; Silim, S.; Orr, A.; Asfaw, S. Tropical Grain Legumes in Africa and South Asia. Knowledge and Opportunities; ICRISAT-CIAT-IITA, Ed.; IITA: Nairobi, Kenya, 2012. [Google Scholar]
- Maphosa, Y.; Jideani, V.A. The Role of Legumes in Human Nutrition. 2017. Available online: http://dx.doi.org/10.5772/intechopen.69127 (accessed on 2 June 2021).
- Mfuti, D.K.; Niassy, S.; Subramanian, S.; du Plessis, H.; Ekesi, S.; Maniania, N.K. Lure and infect strategy for application of entomopathogenic fungus for the control of bean flower thrips in cowpea. Biol. Control 2017, 107, 70–76. [Google Scholar] [CrossRef]
- Sobda, G.; Boukar, O.; Tongoona, P.B.; Ayertey, J.; Offei, K.S. Quantitative trait loci (QTL) for cowpea resistance to flower bud thrips (Megalurothrips sjostedti Trybom). Int. J. Plant Breed. Genet. 2017, 4, 292–299. [Google Scholar]
- Tamò, M.; Baumgärtner, J.; Delucchi, V.; Herren, H.R. Assessment of key factors responsible for the pest status of the bean flower thrips Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae) in West Africa. Bull. Entomol. Res. 1993, 83, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Ngakou, A.; Tamò, M.; Parh, I.A.; Nwaga, D.; Ntonifor, N.N.; Korie, S.; Nebane, C.L.N. Management of cowpea flower thrips, Megalurothrips sjostedti (Thysanoptera, Thripidae), in Cameroon. Crop Prot. 2008, 27, 481–488. [Google Scholar] [CrossRef]
- Niassy, S.; Ekesi, S.; Maniania, N.K.; Orindi, B.; Moritz, G.B.; de Kogel, W.J.; Subramanian, S. Active aggregation among sexes in bean flower thrips (Megalurothrips sjostedti) on cowpea (Vigna unguiculata). Entomol. Exp. Appl. 2016, 158, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamò, M.; Arodokoun, D.Y.; Zenz, N.; Tindo, M.; Agboton, C.; Adeoti, R. The Importance of Alternative Host plants for the Biological Control of Two Key Cowpea Insect Pests, the Pod Borer Maruca vitrata (Fabricius) and the Flower Thrips, Megalurothrips sjostedti (Trybom). In Proceedings of the 3rd World Cowpea Conference, Ibadan, Nigeria, 5–10 September 2000; pp. 81–93. [Google Scholar]
- Abtew, A.; Niassy, S.; Affognon, H.; Subramanian, S.; Kreiter, S.; Garzia, G.T.; Martin, T. Farmers’ knowledge and perception of grain legume pests and their management in the eastern province of Kenya. Crop Prot. 2016, 87, 90–97. [Google Scholar] [CrossRef]
- Jackai, L.E.N.; Adalla, C.B. Pest Management Practices in Cowpea: A Review. In Advances in Cowpea Research; Singh, B.B., Mohan Raj, D.R., Dashiella, K.E., Jackai, L.E.N., Eds.; Co-Publication of International Institute of Tropical Agriculture (IITA) and Japan International Research Centre for Agricultural Sciences (JIRCAS): Ibadan, Nigeria, 1997; pp. 240–258. [Google Scholar]
- Ekesi, S.; Chabi-Olaye, A.; Subramanian, S.; Borgemeister, C. Horticultural pest management and the African economy: Successes, challenges and opportunities in a changing global environment. In Proceedings of the All Africa Horticultural Congress, Nairobi, Kenya, 31 August 2009; pp. 165–183. [Google Scholar]
- Maniania, N.K. A low-cost contamination device for infecting adult tsetse flies, Glossina spp., with the entomopathogenic fungus Metarhizium anisopliae in the field. Biocontrol Sci. Technol. 2002, 12, 59–66. [Google Scholar] [CrossRef]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, L.M.; Mburu, D.M.; Masiga, D.; Ekesi, S. Selection of promising fungal biological control agent of the western flower thrips Frankliniella occidentalis (Pergande). Lett. Appl. Microbiol. 2012, 54, 487–493. [Google Scholar] [CrossRef]
- Niassy, S.; Tamiru, A.; Hamilton, J.G.C.; Kirk, W.D.J.; Mumm, R.; Sims, C.; de Kogel, W.J.; Ekesi, S.; Maniania, N.K.; Bandi, K.; et al. Characterization of male-produced aggregation pheromone of the bean flower thrips Megalurothrips sjostedti (Thysanoptera: Thripidae). J. Chem. Ecol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Dimbi, S.; Maniania, N.K.; Ekesi, S. Horizontal transmission of Metarhizium anisopliae in fruit flies and effect of fungal infection on egg laying and fertility. J. Insects 2013, 4, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Hedström, I.; Monge-Nájera, J. Is sexually transmitted fungal infection evidence for size-related mating success in neotropical guava fruit flies? J. Trop. Biol. 1998, 46, 1129–1132. [Google Scholar]
- Dimbi, S.; Maniania, N.K.; Lux, S.A.; Ekesi, S.; Mueke, J.M. Pathogenicity of Metarhizium anisopliae (Metsch.) Sorokin and Beauveria bassiana (Balsamo) Vuillemin to three adult fruit ßy species: Ceratitis capitata (Weidemann), C. rosa var. fasciventris Karsch and C.cosyra (Walker) (Diptera: Tephritidae). Mycopathologia 2003, 156, 375–382. [Google Scholar] [CrossRef]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, L.M.; Ekesi, S. Performance of a semiochemical-baited autoinoculation device treated with Metarhizium anisopliae for control of Frankliniella occidentalis on French bean in field cages. Entomol. Exp. Appl. 2012, 142, 97–103. [Google Scholar] [CrossRef]
- Mfuti, D.K.; Subramanian, S.; van Tol, R.W.H.M.; Wiegers, G.L.; De Kogel, W.J.; Niassy, S.; Du Plessis, H.; Ekesi, S.; Maniania, N.K. Spatial separation of semiochemical Lurem-TR and entomopathogenic fungi to enhance their compatibility and infectivity in an autoinoculation system for thrips management. Pest Manag. Sci. 2016, 72, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Mfuti, D.K.; Subramanian, S.; Niassy, S.; Salifu, D.; du Plessis, H.; Ekesi, S.; Maniania, N.K. Screening for attractants compatible with entomopathogenic fungus Metarhizium anisopliae for use in thrips management. Afr. J. Biotechnol. 2016, 15, 714–721. [Google Scholar]
- Pepper, H.P.; Tulip, S.J.; Nakano, Y.; George, J.H. Biomimetic total synthesis of (±)-doitunggarcinone A and (+)-garcibracteatone. J. Org. Chem. 2014, 79, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Sampson, C.; Kirk, W.D.J. Can mass trapping reduce thrips damage and is it economically viable? Management of the western flower thrips in strawberry. PLoS ONE 2013, 8, e80787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirk, W.D.J. The aggregation pheromones of thrips (Thysanoptera) and their potential for pest management. Int. J. Trop. Insect Sci. 2017, 37, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Mfuti, D.K.; Subramanian, S.; Niassy, S.; du Plessis, H.; Ekesi, S.; Maniania, N.K. Semiochemical-baited autodissemination device for managing BFT on cowpea. In Sustainable Management of Invasive Pests in Africa; Niassy, S., Ekesi, S., Migiro, L., Otieno, W., Eds.; Springer: Cham, Switzerland, 2020; pp. 253–266. [Google Scholar]
- Davidson, M.M.; Butler, R.C.; Winkler, S.; Teulon, D.A.J. Pyridine compounds increase trap capture of Frankliniella occidentalis (Pergande) in a covered crop. N. Z. Plant Prot. 2007, 60, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Muvea, A.M.; Waiganjo, M.M.; Kutima, H.L.; Osiemo, Z.; Nyasani, J.O.; Subramanian, S. Attraction of pest thrips (Thysanoptera: Thripidae) infesting French beans to coloured sticky traps with Lurem-TR and its utility for monitoring thrips populations. Int. J. Trop. Insect Sci. 2014, 34, 197–206. [Google Scholar]
- Teulon, D.A.J.; Davidson, M.M.; Perry, N.B.; Nielsen, M.-C.; Castañé, C.; Bosch, D.; Riudavets, J.; van Tol, R.W.H.M.; de Kogel, W.J. Methyl isonicotinate—A non-pheromone thrips semiochemical and its potential for pest management. Int. J. Trop. Insect Sci. 2017, 37, 50–56. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Ayuso, G.L.E. Soxhlet Extraction, Environmental Applications. 2000. Available online: https://www.sciencedirect.com/science/article/pii/B0122267702066813 (accessed on 2 June 2021).
- Cooke, V.M.; Miles, R.J.; Price, R.G.; Midgley, G.; Khamri, W.; Richardson, A.C. New chromogenic agar medium for the identification of Candida spp. Appl. Environ. Microbiol. 2002, 68, 3622–3627. [Google Scholar] [CrossRef] [Green Version]
- Maniania, N.K. A laboratory technique for infecting adult Tsetse with a fungal pathogen. Insect Sci. Its Appl. 1994, 15, 421–426. [Google Scholar] [CrossRef]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 189–253. [Google Scholar]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 1980, 34, 216–221. [Google Scholar]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Migiro, L.N.; Maniania, N.K.; Chabi-Olaye, A.; Vandenberg, J. Pathogenicity of entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana (Hypocreales: Clavicipitaceae) isolates to the adult pea leafminer (Diptera: Agromyzidae) and prospects of an autoinoculation device for infection in the field. Environ. Entomol. 2010, 39, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.K.; Blum, M.S.; Roncadori, R.W. Antifungal properties of the insect alarm pheromones, citral, 2-heptanone, and 4-methyl-3-heptanone. Mycologia 1975, 67, 701–708. [Google Scholar] [CrossRef]
- Sharma, A.; Sandhi, R.K.; Reddy, G.V.P. A Review of interactions between insect biological control agents and semiochemicals. Insects 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opisa, S.; Du Plessis, H.; Akutse, K.S.; Fiaboe, K.K.M.; Ekesi, S. Horizontal transmission of Metarhizium anisopliae between Spoladea recurvalis (Lepidoptera: Crambidae) adults and compatibility of the fungus with the attractant phenylacetaldehyde. Microb. Pathog. 2019, 131, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Nana, P.; Maniania, N.K.; Maranga, R.O.; Boga, H.I.; Kutima, H.L.; Eloff, J.N. Compatibility between Calpurnia aurea leaf extract, attraction aggregation and attachment pheromone and entomopathogenic fungus Metarhizium anisopliae on viability, growth and virulence of the pathogen. J. Pest Sci. 2012, 85, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Davidson, M.M.; Perry, N.B.; Larsen, L.; Green, V.C.; Butler, R.C.; Teulon, D.A.J. 4-Pyridyl carbonyl compounds as thrips lures: Effectiveness for western flower thrips in Y-tube bioassays. J. Agric. Food Chem. 2008, 56, 6554–6561. [Google Scholar] [CrossRef]
- Akella, S.V.S.; Kirk, W.D.J.; Lu, Y.-B.; Murai, T.; Walters, K.F.A.; Hamilton, J.G.C. Identification of the aggregation pheromone of the melon thrips, Thrips palmi. PLoS ONE 2014, 9, e103315. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.G.C.; Hall, D.R.; Kirk, W.D.J. Identification of a male-produced aggregation pheromone in the western flower thrips Frankliniella occidentalis. J. Chem. Ecol. 2005, 31, 1369–1379. [Google Scholar] [CrossRef]
- Liu, P.; Qin, Z.; Feng, M.; Huang, X.; Shi, W. The male-produced aggregation pheromone of the bean flower thrips Megalurothrips usitatus in China: Identification and attraction of conspecifics in the laboratory and field. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Irfan, S.; Kamil, M.; Sheikh, S.; Hasan, A.; Ahmad, A.; Lakshmi, V.; Nasir, A.; Mir, S.S. Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae. Microbiology 2016, 85, 436–443. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.; Battistia, F.; Silvab, F.K.; Lanab, A.D.; Pippib, B.; Casanovac, B.; Gnoattoc, S.; Fuentefriab, A.; Mayorgad, P.; Schapovala, E.E.S. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the tor pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [Green Version]




| Treatments | % Germination ± SE | Growth (µm/Day) ± SE | ||||
|---|---|---|---|---|---|---|
| Dose | Dose | |||||
| 1 µL | 10 µL | 100 µL | 1 µL | 10 µL | 100 µL | |
| Control | 87.5 ± 0.9 aA | 88.5 ± 2.0 aA | 86.2 ± 2.5 aA | 111.0 ± 6.0 aA | 95.1 ± 5.6 aA | 92.6 ± 6.8 aA |
| Major | 86.7 ± 1.1 aA | 49.1 ± 6.5 bB | 38.1 ± 9.8 bB | 108.0 ± 9.8 aA | 42.6 ± 6.2 bB | 22.9 ± 8.0 cC |
| Minor | 85.8 ± 1.2 aA | 47.2 ± 6.8 bB | 42.0 ± 10.9 bB | 114.0 ± 7.0 aA | 36.7 ± 7.3 bB | 34.7 ± 15.2 bB |
| F2,15 = 3.4; p = 0.08 | F2,15 = 42.1; p < 0.001 | F2,15 = 8.9; p < 0.001 | F2,9 = 0.3; p = 0.74 | F2,9 = 9.9; p < 0.001 | F2,9 = 6.5; p < 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mfuti, D.K.; Tamiru, A.; Kirk, W.D.J.; Akinyemi, A.O.; Campbell, H.; O’Brien, M.; Drijfhout, F.P.; Pope, T.W.; Niassy, S.; Subramanian, S. Exploiting Thrips Aggregation Pheromones to Develop a Lure-and-Kill Strategy for the Management of the Bean Flower Thrips. Agronomy 2021, 11, 1269. https://doi.org/10.3390/agronomy11071269
Mfuti DK, Tamiru A, Kirk WDJ, Akinyemi AO, Campbell H, O’Brien M, Drijfhout FP, Pope TW, Niassy S, Subramanian S. Exploiting Thrips Aggregation Pheromones to Develop a Lure-and-Kill Strategy for the Management of the Bean Flower Thrips. Agronomy. 2021; 11(7):1269. https://doi.org/10.3390/agronomy11071269
Chicago/Turabian StyleMfuti, David K., Amanuel Tamiru, William D. J. Kirk, Adeyemi O. Akinyemi, Heather Campbell, Matthew O’Brien, Falko P. Drijfhout, Tom W. Pope, Saliou Niassy, and Sevgan Subramanian. 2021. "Exploiting Thrips Aggregation Pheromones to Develop a Lure-and-Kill Strategy for the Management of the Bean Flower Thrips" Agronomy 11, no. 7: 1269. https://doi.org/10.3390/agronomy11071269
APA StyleMfuti, D. K., Tamiru, A., Kirk, W. D. J., Akinyemi, A. O., Campbell, H., O’Brien, M., Drijfhout, F. P., Pope, T. W., Niassy, S., & Subramanian, S. (2021). Exploiting Thrips Aggregation Pheromones to Develop a Lure-and-Kill Strategy for the Management of the Bean Flower Thrips. Agronomy, 11(7), 1269. https://doi.org/10.3390/agronomy11071269







