Helichrysum italicum (Roth) G. Don Essential Oil from Serbia: Chemical Composition, Classification and Biological Activity—May It Be a Suitable New Crop for Serbia?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. EO Extraction and Analysis
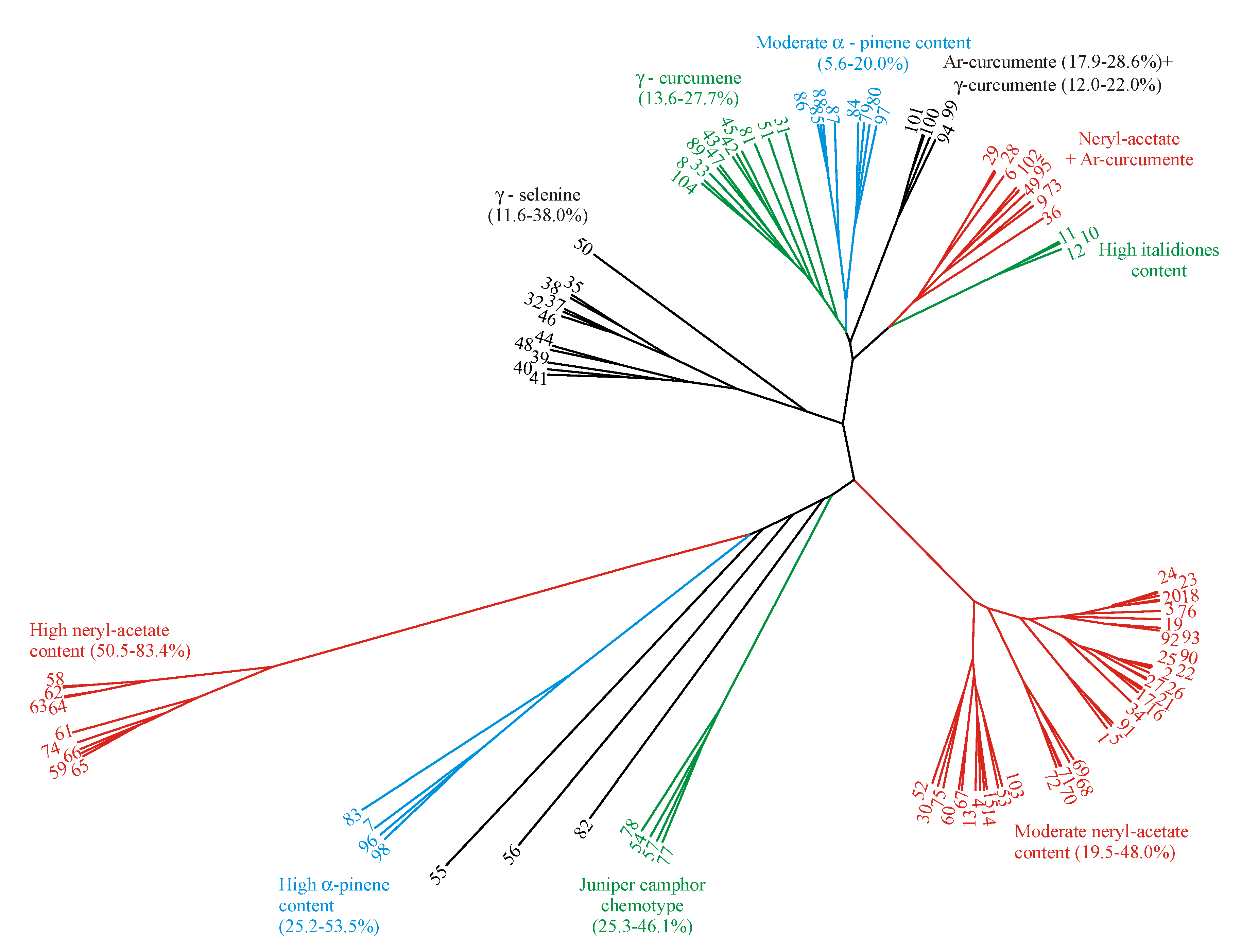
2.3. Phylogenetic Tree Diagram
2.4. QSRR Analysis
2.5. Artificial Neural Network (ANN)
2.6. Global Sensitivity Analysis
2.7. Biological Activity
2.7.1. Antimicrobial Activity
2.7.2. Antioxidant Activity
2.7.3. In Vitro α-Glucosidase Inhibitory Potential
2.7.4. Anti-Inflammatory Activity
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baser, K.H.C.; Demirci, B.; Kirimer, N. Compositions of the Essential Oils of FourHelichrysumSpecies from Madagascar. J. Essent. Oil Res. 2002, 14, 53–55. [Google Scholar] [CrossRef]
- Schmidt-Lebuhn, A.N.; Bruhl, J.J.; Telford, I.R.; Wilson, P.G. Phylogenetic relationships of Coronidium, Xerochrysum and several neglected Australian species of “Helichrysum” (Asteraceae:Gnaphalieae). TAXON 2015, 64, 96–109. [Google Scholar] [CrossRef]
- Smissen, R.D.; Breitwieser, I.; Ward, J.M. Genetic diversity in the New Zealand endemic species Helichrysum lanceolatum (Asteraceae: Gnaphalieae). N. Z. J. Bot. 2006, 44, 237–247. [Google Scholar] [CrossRef]
- Galbany-Casals, M.; Garcia-Jacas, N.; Sáez, L.; Benedí, C.; Susanna, A. Phylogeny, Biogeography, and Character Evolution in Mediterranean, Asiatic, and Macaronesian Helichrysum (Asteraceae, Gnaphalieae) Inferred from Nuclear Phylogenetic Analyses. Int. J. Plant Sci. 2009, 170, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Şenol, S.G.; Secmen, O.; Ozturk, B.; Galbany-Casals, M. Helichrysum unicapitatum (Asteraceae), a New Species from Turkey. Ann. Bot. Fenn. 2011, 48, 145–154. [Google Scholar] [CrossRef]
- Azizi, N.; Sheidai, M.; Mozaffarian, V.; Nourmohammadi, Z. Karyotype and genome size analyses in species of Helichrysum (Asteraceae). Acta Bot. Bras. 2014, 28, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Aghababyan, M.; Greuter, W.; Mazzola, P.; Raimondo, F.M. Typification of Sicilian Helichrysum (Compositae) revisited. TAXON 2007, 56, 1285–1288. [Google Scholar] [CrossRef]
- Ninčević, T.; Grdiša, M.; Šatović, Z.; Jug-Dujaković, M. Helichrysum italicum (Roth) G. Don: Taxonomy, biological activity, biochemical and genetic diversity. Ind. Crop. Prod. 2019, 138, 111487. [Google Scholar] [CrossRef]
- Perrino, E.V.; Tomaselli, V.; Costa, R.; Pavone, P. Conservation status of habitats (Directive 92/43 EEC) of coastal and low hill belts in a Mediterranean biodiversity hot spot (Gargano—Italy). Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2013, 147, 1006–1028. [Google Scholar] [CrossRef]
- European Commission DG Environment. Interpretation Manual of European Union Habitats (Version EUR27); European Commission DG Environment: Brussels, Belgium, 2007. [Google Scholar]
- Mancini, E.; de Martino, L.; Marandino, A.; Scognamiglio, M.R.; de Feo, V. Chemical Composition and Possible in Vitro Phytotoxic Activity of Helichrsyum italicum (Roth) Don ssp. italicum. Molecules 2011, 16, 7725. [Google Scholar] [CrossRef] [Green Version]
- Melito, S.; Sias, A.; Petretto, G.L.; Chessa, M.; Pintore, G.A.M.; Porceddu, A. Genetic and Metabolite Diversity of Sardinian Populations of Helichrysum italicum. PLoS ONE 2013, 8, e79043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, G.; Ruta, C.; Traversa, A.; D’Ambruoso, G.; Tarraf, W.; De Mastro, F.; De Mastro, G.; Cocozza, C. Remediation of a heavy metals contaminated soil using mycorrhized and non-mycorrhized Helichrysum italicum (Roth) Don. Land Degrad. Dev. 2018, 29, 91–104. [Google Scholar] [CrossRef]
- Han, X.; Beaumont, C.; Stevens, N. Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochim. Open 2017, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Melito, S.; Petretto, G.; Podani, J.; Foddai, M.; Maldini, M.; Chessa, M.; Pintore, G. Altitude and climate influence Helichrysum italicum subsp. microphyllum essential oils composition. Ind. Crop. Prod. 2016, 80, 242–250. [Google Scholar] [CrossRef]
- Roussis, V.; Tsoukatou, M.; Petrakis, P.V.; Chinou, I.; Skoula, M.; Harborne, J.B. Volatile constituents of four Helichrysum species growing in Greece. Biochem. Syst. Ecol. 2000, 28, 163–175. [Google Scholar] [CrossRef]
- Perrino, E.; Valerio, F.; Gannouchi, A.; Trani, A.; Mezzapesa, G. Ecological and Plant Community Implication on Essential Oils Composition in Useful Wild Officinal Species: A Pilot Case Study in Apulia (Italy). Plants 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Usai, M.; Foddai, M.; Bernardini, A.F.; Muselli, A.; Costa, J.; Marchetti, M. Chemical Composition and Variation of the Essential Oil of Wild SardinianHelichrysum ItalicumG. Don subsp. Microphyllum (Willd.) Nym from Vegetative Period to Post-blooming. J. Essent. Oil Res. 2010, 22, 373–380. [Google Scholar] [CrossRef]
- Reidel, R.V.B.; Cioni, P.L.; Majo, L.; Pistelli, L. Evolution of Volatile Emission in Rhus coriaria Organs During Different Stages of Growth and Evaluation of the Essential Oil Composition. Chem. Biodivers. 2017, 14, e1700270. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, B.; Piras, A.; Desogus, E.; Porcedda, S.; Ballero, M. Analysis of the Volatile Concentrate of the Leaves and Flowers ofHelichrysum italicum(Roth) Don ssp.microphyllum (Willd.) Nyman (Asteraceae) by Supercritical Fluid Extraction and Their Essential Oils. J. Essent. Oil Res. 2003, 15, 120–126. [Google Scholar] [CrossRef]
- Andreani, S.; Uehara, A.; Blagojević, P.; Radulović, N.; Muselli, A.; Baldovini, N. Key odorants of industrially-produced Helichrysum italicum subsp. italicum essential oil. Ind. Crop. Prod. 2019, 132, 275–282. [Google Scholar] [CrossRef]
- Dobrnjac, M.; Hodžić, A.; Dobrnjac, S.; Dobrnjac, D. The new solution of the substance flow system in the steam distillation process of essential oil. Intern. J. Aer. Innov. 2017, 5, 153–155. [Google Scholar]
- Yap, C.W. PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2010, 32, 1466–1474. [Google Scholar] [CrossRef]
- Nekoei, M.; Mohammadhosseini, M. Chemical Compositions of the Essential Oils from the Aerial Parts ofAchillea wilhelmsiiUsing Traditional Hydrodistillation, Microwave Assisted Hydro- distillation and Solvent-Free Microwave Extraction Methods: Comparison with the Volatile Compounds Obtained by Headspace Solid-Phase Microextraction. J. Essent. Oil Bear. Plants 2016, 19, 59–75. [Google Scholar] [CrossRef]
- Goldberg, D.E. Genetic Algorithms in Search, Optimization, and Machine Learning; Addison-Wesley Professional: Longman, MA, USA, 1989; pp. 55–60. [Google Scholar]
- Gramatica, P. Principles of QSAR models validation: Internal and external. QSAR Comb. Sci. 2007, 26, 694–701. [Google Scholar] [CrossRef]
- Hu, X.; Weng, Q. Estimating impervious surfaces from medium spatial resolution imagery using the self-organizing map and multi-layer perceptron neural networks. Remote. Sens. Environ. 2009, 113, 2089–2102. [Google Scholar] [CrossRef]
- Acimovic, M.; Pezo, L.; Jeremic, J.S.; Cvetkovic, M.; Rat, M.; Cabarkapa, I.; Tesevic, V. QSRR Model for Predicting Retention Indices of Geraniol Chemotype of Thymus serpyllum Essential Oil. J. Essent. Oil Bear. Plants 2020, 23, 464–473. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics. Drug Sel. 2009, 41, 1–962. [Google Scholar]
- Aćimović, M.; Pezo, L.; Tešević, V.; Čabarkapa, I.; Todosijević, M. QSRR Model for predicting retention indices of Satureja kitaibelii Wierzb. ex Heuff. essential oil composition. Ind. Crop. Prod. 2020, 154, 112752. [Google Scholar] [CrossRef]
- Xu, S.-G.; Zhao, Y.-J.; Liao, J.-H.; Yang, X.-B. Understanding the stable boron clusters: A bond model and first-principles calculations based on high-throughput screening. J. Chem. Phys. 2015, 142, 214307. [Google Scholar] [CrossRef]
- Yoon, P.; Yoon, H.; Kim, Y.; Kim, G.-B. A Comparative Study on Forecasting Groundwater Level Fluctuations of National Groundwater Monitoring Networks using TFNM, ANN, and ANFIS. J. Soil Groundw. Environ. 2014, 19, 123–133. [Google Scholar] [CrossRef]
- Varga, A.; Kocic-Tanackov, S.; Cabarkapa, I.; Acimovic, M.; Tomicic, Z. Chemical composition and antibacterial activity of spice essential oils against Escherichia coli and Salmonella Typhimurium. J. Food Saf. Food Qual. 2019, 70, 157–194. [Google Scholar] [CrossRef]
- Girones-Vilaplana, A.; Mena, P.; Moreno, D.A.; Garcia-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food Agric. 2013, 94, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Al-Saikhan, M.S.; Howard, L.R.; Miller, J.C. Antioxidant Activity and Total Phenolics in Different Genotypes of Potato (Solanum tuberosum, L.). J. Food Sci. 1995, 60, 341–343. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Krunic, M. Anthocyanin profiles and biological properties of caneberry (Rubusspp.) press residues. J. Sci. Food Agric. 2014, 94, 2393–2400. [Google Scholar] [CrossRef]
- Ullah, H.A.; Zaman, S.; Juhara, F.; Akter, L.; Tareq, S.M.; Masum, E.H.; Bhattacharjee, R. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement. Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zeljkovic, S.C.; Šolić, M.E.; Maksimović, M. Volatiles ofHelichrysum italicum(Roth) G. Don from Croatia. Nat. Prod. Res. 2015, 29, 1874–1877. [Google Scholar] [CrossRef]
- Leonardi, M.; Ambryszewska, K.E.; Melai, B.; Flamini, G.; Cioni, P.L.; Parri, F.; Pistelli, L. Essential-Oil Composition ofHelichrysum italicum(Roth) G. Don ssp. Italicum from Elba Island (Tuscany, Italy). Chem. Biodivers. 2013, 10, 343–355. [Google Scholar] [CrossRef]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Pedro, D.H.J.; Maria, A.E.; Mihoub, Z.M. Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, filamentous fungi and Candida albicans. Saudi Pharm. J. 2017, 25, 780–787. [Google Scholar] [CrossRef]
- Paolini, J.; Desjobert, J.-M.; Costa, J.; Bernardini, A.-F.; Castellini, C.B.; Cioni, P.-L.; Flamini, G.; Morelli, I. Composition of essential oils ofHelichrysum italicum (Roth) G. Don fil subsp. Italicum from Tuscan archipelago islands. Flavour Fragr. J. 2006, 21, 805–808. [Google Scholar] [CrossRef]
- Satta, M.; Tuberoso, C.; Angioni, A.; Pirisi, F.M.; Cabras, P. Analysis of the Essential Oil ofHelichrysum italicum G. Don ssp.Microphyllum (Willd) Nym. J. Essent. Oil Res. 1999, 11, 711–715. [Google Scholar] [CrossRef]
- Kladar, N.V.; Anačkov, G.T.; Rat, M.; Srđenović, B.U.; Grujić, N.N.; Šefer, E.I.; Bozin, B. Biochemical Characterization ofHelichrysum italicum(Roth) G. Don subsp. Italicum (Asteraceae) from Montenegro: Phytochemical Screening, Chemotaxonomy, and Antioxidant Properties. Chem. Biodivers. 2015, 12, 419–431. [Google Scholar] [CrossRef]
- Talić, S.; Odak, I.; Bevanda, A.M.; Crnjac, N.; Paštar, M. Helichrysum italicum (Roth) G. Don subsp. Italicum from Herzegovina. Croat. Chem. Acta 2019, 92, 69–77. [Google Scholar] [CrossRef]
- Bouchaala, M.; Ramdani, M.; Chalard, P.; Figueredo, G.; Lograda, T. Chemical Composition, Antibacterial Activity and Chromosome Number of Helichrysum italicum from Algeria. Int. J. Pharm. Phytoch. Res. 2016, 8, 1675–1683. [Google Scholar]
- Tucker, A.O.; Maciarello, M.J.; Charles, D.J.; Simon, J.E. Volatile Leaf Oil of the Curry PlantHelichrysum italicum(Roth) G. Don subsp. italicum and Dwarf Curry Plant subsp.microphyllum (Willd.) Nyman in the North American Herb Trade. J. Essent. Oil Res. 1997, 9, 583–585. [Google Scholar] [CrossRef]
- Mastelić, J.; Politeo, O.; Jerković, I. Contribution to the Analysis of the Essential Oil of Helichrysum italicum (Roth) G. Don. Determination of Ester Bonded Acids and Phenols. Molecules 2008, 13, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Cristofari, G.; Znini, M.; Majidi, L.; Costa, J.; Hammouti, B.; Paolini, J. Helichrysum italicum subsp. italicum essential oil as environmentally friendly inhibitor on the corrosion of mil steel in hydrochlorid acid. Int. J. Electrochem. Sci. 2012, 7, 9024–9041. [Google Scholar]
- Blazevic, N.; Petricic, J.; Stanic, G.; Males, Z. Variations in yields and composition of immortelle (Helichrysum italicum, Roth Guss.) essential oil from different locations and vegetation periods along Adriatic coast. Acta Pharm. 1995, 45, 517–522. [Google Scholar]
- Fraternale, D.; Flamini, G.; Ascrizzi, R. In Vitro Anticollagenase and Antielastase Activities of Essential Oil of Helichrysum italicum subsp. italicum (Roth) G. Don. J. Med. Food 2019, 22, 1041–1046. [Google Scholar] [CrossRef]
- Stupar, M.; Ljaljevic-Grbic, M.; Dzamic, A.; Unkovic, N.; Ristic, M.; Vukojevic, J. Antifungal activity of Helichrysum italicum (Roth) G. Don (Asteraceae) essential oil against fungi isolated from cultural heritage objects. Arch. Biol. Sci. 2014, 66, 1539–1545. [Google Scholar] [CrossRef]
- Tzanova, M.; Grozeva, N.; Gerdzhikova, M.; Atanasov, V.; Terzieva, S.; Prodanova, R. Biochemical composition of essential oil of Corsican Helichrysum italicum (Roth) G. Don, introduced and cultivated in South Bulgaria. Bulg. J. Agric. Sci. 2018, 24, 1071–1077. [Google Scholar]
- Bianchini, A.; Tomi, P.; Costa, J.; Bernardini, A.F. Composition of Helichrysum italicum (Roth) G. Don fil. subsp. italicum essential oils from Corsica (France). Flavour Fragr. J. 2001, 16, 30–34. [Google Scholar] [CrossRef]
- Morone-Fortunato, I.; Montemurro, C.; Ruta, C.; Perrini, R.; Sabetta, W.; Blanco, A.; Lorusso, E.; Avato, P. Essential oils, genetic relationships and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. Italicum from wild Mediterranean germplasm. Ind. Crop. Prod. 2010, 32, 639–649. [Google Scholar] [CrossRef]
- Dzamic, A.M.; Mileski, K.S.; Ciric, A.; Ristic, M.S.; Sokovic, M.; Marin, P.D. Essential Oil Composition, Antioxidant and Antimicrobial Properties of Essential Oil and Deodorized Extracts of Helichrysum italicum (Roth) G. Don. J. Essent. Oil Bear. Plants 2019, 22, 493–503. [Google Scholar] [CrossRef]
- Costa, P.; Loureiro, J.M.; Teixeira, M.A.; Rodrigues, A.E. Extraction of aromatic volatiles by hydrodistillation and supercritical fluid extraction with CO2 from Helichrysum italicum subsp. picardii growing in Portugal. Ind. Crop. Prod. 2015, 77, 680–683. [Google Scholar] [CrossRef]
- Oliva, A.; Garzoli, S.; Sabatino, M.; Tadić, V.; Costantini, S.; Ragno, R.; Božović, M. Chemical composition and antimicrobial activity of essential oil of Helichrysum italicum (Roth) G. Don fil. (Asteraceae) from Montenegro. Nat. Prod. Res. 2020, 34, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Assaeed, A.; ElShamy, A.; El Gendy, A.E.-N.; Dar, B.; Al-Rowaily, S.; Abd-Elgawad, A. Sesquiterpenes-Rich Essential Oil from Above Ground Parts of Pulicaria somalensis Exhibited Antioxidant Activity and Allelopathic Effect on Weeds. Agronomy 2020, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Chanotiya, C.S.; Yadav, A.; Kalra, A. Volatiles of Callicarpa macrophylla: A Rich Source of Selinene Isomers. Nat. Prod. Commun. 2010, 5, 269–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soh, Z.; Saito, M.; Kurita, Y.; Takiguchi, N.; Ohtake, H.; Tsuji, T. A Comparison between the Human Sense of Smell and Neural Activity in the Olfactory Bulb of Rats. Chem. Senses 2013, 39, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Bednarczyk, A.A.; Kramer, A. Identification and Evaluation of the Flavor-Significant Components of Ginger Essential Oil. Chem. Senses 1975, 1, 377–386. [Google Scholar] [CrossRef]
- Odak, I.; Lukic, T.; Talic, S. Impact of Storage Conditions on Alteration of Juniper and Immortelle Essential Oils. J. Essent. Oil Bear. Plants 2018, 21, 614–622. [Google Scholar] [CrossRef]
- Combes, C.; Legrix, M.; Rouquet, V.; Rivoire, S.; Grasset, S.; Cenizo, V.; Moga, A.; Portes, P. 166 Helichrysum italicum essential oil prevents skin lipids peroxidation caused by pollution and UV. J. Investig. Dermatol. 2017, 137, S221. [Google Scholar] [CrossRef]
- Riabov, P.A.; Micić, D.; Božović, R.B.; Jovanović, D.V.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Ostojić, S.; Blagojević, S.; et al. The chemical, biological and thermal characteristics and gastronomical perspectives of Laurus nobilis essential oil from different geographical origin. Ind. Crop. Prod. 2020, 151, 112498. [Google Scholar] [CrossRef]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food andin vivo: What they do and how they work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Zhu, X.; Casadesus, G.; Castellani, R.J.; Nunomura, A.; Smith, M.; Lee, H.-G.; Perry, G. Antioxidant approaches for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2010, 10, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Poli, G.; Leonarduzzi, G.M.; Biasi, F.; Chiarpotto, E. Oxidative Stress and Cell Signalling. Curr. Med. Chem. 2004, 11, 1163–1182. [Google Scholar] [CrossRef]
- Čakar, U.; Grozdanić, N.; Pejin, B.; Vasić, V.; Čakar, M.; Petrović, A.; Djordjević, B. Impact of vinification procedure on fruit wine inhibitory activity against α-glucosidase. Food Biosci. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.R.S.; Rao, K.V.B. Efficacy of Alpha Glucosidase Inhibitor from Marine Actino bacterium in the Control of Post-prandial Hyperglycaemia in Streptozotocin (STZ) Induced Diabetic Male Albino Wister Rats. Iran J. Pharm. Res. 2018, 17, 202–214. [Google Scholar]
- Opie, E.L. On the Relation of Necrosis and Inflammation to Denaturation of Proteins. J. Exp. Med. 1962, 115, 597–608. [Google Scholar] [CrossRef]
- Viegas, D.A.; Palmeira-De-Oliveira, A.; Salgueiro, L.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.D.C.; Giner, R.M.; Máñez, S.; Tournier, H.; Schinella, G.; Ríos, J.-L. Anti-inflammatory and antioxidant properties of Helichrysum italicum. J. Pharm. Pharmacol. 2010, 54, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, K.; Pražnikar, Z.J.; Arbeiter, A.B.; Petelin, A.; Bandelj, D.; Kenig, S. A Comparative Study of the Antioxidative Effects of Helichrysum italicum and Helichrysum arenarium Infusions. Antioxidants 2021, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Mollova, S.; Fidan, H.; Antonova, D.; Bozhilov, D.; Stanev, S.; Kostova, I.; Stoyanova, A. Chemical composition and antimicrobial and antioxidant activity of Helichrysumitalicum (Roth) G.Don subspecies essential oils. Turk. J. Agric. For. 2020, 44, 371–378. [Google Scholar] [CrossRef]
- Poli, F.; Muzzoli, M.; Sacchetti, G.; Tassinato, G.; Lazzarin, R.; Bruni, A. Antioxidant Activity of Supercritical CO2 Extracts of Helichrysum italicum. Pharm. Biol. 2003, 41, 379–383. [Google Scholar] [CrossRef]

| No | Component | Cycle | RIa | RIpred. | % |
|---|---|---|---|---|---|
| 1 | α-Pinene | Train. | 926 | 935.054 | 11.8 |
| 2 | α-Fenchene | Train. | 942 | 1055.410 | 0.2 |
| 3 | Camphene | Train. | 944 | 1177.543 | 0.1 |
| 4 | β-Pinene | Valid. | 970 | 1415.606 | 0.3 |
| 5 | α-Terpinene | Train. | 1015 | 1373.881 | 0.2 |
| 6 | p-Cymene | Test. | 1021 | 1467.734 | 0.2 |
| 7 | Limonene | Test. | 1024 | 1571.383 | 2.6 |
| 8 | 1,8-Cineole | Train. | 1027 | 928.460 | 0.3 |
| 9 | Isobutyl angelate | Train. | 1044 | 944.529 | 0.3 |
| 10 | γ-Terpinene | Train. | 1052 | 946.147 | 0.4 |
| 11 | Terpinolene | Train. | 1080 | 1033.521 | 0.2 |
| 12 | Linalool | Train. | 1091 | 1026.925 | 0.8 |
| 13 | 2-Methyl butyl-2-methyl butyrate | Valid. | 1095 | 1045.050 | 0.3 |
| 14 | Isoamyltiglate | Train. | 1146 | 1026.019 | 1.8 |
| 15 | Borneol | Train. | 1159 | 1080.790 | 0.2 |
| 16 | Menthol | Valid. | 1166 | 1094.205 | 0.4 |
| 17 | Terpinen-4-ol | Test. | 1172 | 1147.990 | 0.2 |
| 18 | 5,6-Decanedione | Train. | 1182 | 1155.678 | 0.2 |
| 19 | α-Terpineol | Train. | 1186 | 1183.886 | 0.2 |
| 20 | Nerol | Train. | 1225 | 1192.495 | 0.4 |
| 21 | NI-1 | 1283 | 0.5 | ||
| 22 | Menthyl acetate | Train. | 1291 | 1224.095 | 0.2 |
| 23 | Neryl acetate | Test. | 1362 | 1287.671 | 5.5 |
| 24 | NI-2 | 1364 | 0.4 | ||
| 25 | α-Ylangene | Train. | 1370 | 1393.100 | 0.4 |
| 26 | α-Copaene | Train. | 1374 | 1393.100 | 2.6 |
| 27 | iso-Italicene | Train. | 1398 | 1373.881 | 0.2 |
| 28 | Italicene | Test. | 1402 | 1452.010 | 4.2 |
| 29 | cis-α-Bergamotene | Valid. | 1413 | 1481.175 | 1.3 |
| 30 | β-Caryophyllene | Train. | 1418 | 1440.820 | 6.7 |
| 31 | trans-α-Bergamotene | Train. | 1434 | 1461.915 | 1.3 |
| 32 | Bisabolane | Train. | 1441 | 1452.106 | 1.0 |
| 33 | 6,9-Guaiadiene | Train. | 1442 | 1471.975 | 1.9 |
| 34 | NI-3 | 1447 | 1.4 | ||
| 35 | Nerylpropanoate | Train. | 1452 | 1471.975 | 1.7 |
| 36 | trans-β-Farnesene | Valid. | 1456 | 1467.734 | 0.7 |
| 37 | α-Acoradiene | Train. | 1464 | 1453.653 | 0.6 |
| 38 | β-Acoradiene | Train. | 1466 | 1461.323 | 0.5 |
| 39 | α-Selinene | Train. | 1475 | 1492.297 | 1.8 |
| 40 | γ-curcumene | Valid. | 1480 | 1472.205 | 13.6 |
| 41 | ar-Curcumene | Train. | 1483 | 1503.786 | 5.0 |
| 42 | β-Selinene | Train. | 1486 | 1543.763 | 12.2 |
| 43 | α-Selinene | Test. | 1495 | 1627.550 | 4.3 |
| 44 | α-Muurolene | Train. | 1500 | 1608.356 | 0.4 |
| 45 | NI-4 | 1507 | 0.5 | ||
| 46 | β-Curcumene | Test | 1511 | 1630.044 | 0.4 |
| 47 | γ-Cadinene | Train. | 1513 | 1630.137 | 0.5 |
| 48 | δ-Cadinene | Train. | 1523 | 1622.599 | 1.0 |
| 49 | NI-5 | 1532 | 0.1 | ||
| 50 | NI-6 | 1533 | 0.1 | ||
| 51 | NI-7 | 1537 | 0.1 | ||
| 52 | α-Calacorene | Train. | 1542 | 1666.463 | 0.3 |
| 53 | NI-8 | 1561 | 0.1 | ||
| 54 | NI-9 | 1580 | 3.0 | ||
| 55 | NI-10 | 1590 | 0.2 | ||
| 56 | Guaiol | Train. | 1594 | 937.385 | 0.3 |
| 57 | NI-11 | 1600 | 0.5 | ||
| 58 | Rosifoliol | Train. | 1604 | 1145.600 | 0.7 |
| 59 | NI-12 | 1610 | 0.3 | ||
| 60 | NI-13 | 1645 | 0.3 | ||
| 61 | 1-epi-Cubenol | Train. | 1629 | 1186.741 | 0.3 |
| 62 | NI-14 | 1631 | 0.3 | ||
| 63 | epi-α-Cadinol | Train. | 1638 | 1500.806 | 0.2 |
| 64 | β-Eudesmol | Train. | 1647 | 1578.032 | 0.2 |
| 65 | neo-Intermedeol | 1652 | 0.6 | ||
| 66 | epi-β-Bisabolol | Train. | 1665 | 1585.105 | 0.2 |
| 67 | NI-15 | 1693 | 0.1 | ||
| 68 | Geranyl hexanoate | Valid. | 1729 | 1965.463 | 0.1 |
| 69 | NI-16 | 1777 | 0.2 | ||
| 70 | NI-17 | 1801 | 0.2 |
| AATSC4c | MATS1c | VE3_Dzv | VABC | |
|---|---|---|---|---|
| AATS1m | −0.1315 | −0.2060 | 0.0211 | −0.1478 |
| p = 0.353 | p = 0.143 | p = 0.882 | p = 0.296 | |
| AATSC4c | 0.1945 | −0.0260 | 0.1840 | |
| p = 0.167 | p = 0.855 | p = 0.141 | ||
| MATS1 | 0.0165 | 0.1076 | ||
| p = 0.908 | p = 0.448 | |||
| VE3_Dzv | 0.0720 | |||
| p = 0.612 | ||||
| Net. Name | Performance | Error | Train. Algor. | Error Funct. | Hidden Activat. | Output Activat. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Train. | Test. | Valid. | Train. | Test. | Valid. | |||||
| MLP 4-8-1 | 0.997 | 0.978 | 0.997 | 156.604 | 1175.567 | 6687.007 | BFGS 117 | SOS | Tanh | Identity |
| Antibacterial Activity | |||
|---|---|---|---|
| Gram Negative | MIC | MBC | Gentamicin (MIC) |
| Escherichia coli (ATCC 8739) | >454.50 | >454.50 | 2.00 |
| Escherichia coli (ATCC 10536) | >454.50 | >454.50 | 1.00 |
| Pseudomonas aeruginosa (ATCC 27853) | >454.50 | >454.50 | 1.00 |
| Salmonella enteritidis (ATCC 13076) | >454.50 | >454.50 | 0.50 |
| Salmonella Typhimurium (ATCC 14028) | >454.50 | >454.50 | 0.50 |
| Klebsiellaaerogenes (ATCC 13048) | >454.50 | >454.50 | 0.50 |
| Proteus hauseri (ATCC 13315) | >454.50 | >454.50 | 1.00 |
| Gram Positive | |||
| Bacillus cereus (ATCC 11778) | 454.50 | 454.50 | 0.19 |
| Bacillus spizizenii (ATCC 6633) | >454.50 | >454.50 | 0.38 |
| Enterococcus faecalis (ATCC 29212) | >454.50 | >454.50 | 8.00 |
| Listeria monocytogenes (ATCC 19111) | 454.50 | 454.50 | 0.19 |
| Listeria innocua (ATCC 33090) | >454.50 | >454.50 | 0.50 |
| Listeria ivanovii (ATCC 19119) | >454.50 | >454.50 | 0.50 |
| Rhodococcusequi (ATCC 6939) | 454.50 | 454.50 | 0.38 |
| Staphylococcus aureus (ATCC 25923) | >454.50 | >454.50 | 0.38 |
| Staphylococcusepidermidis (ATCC 12228) | 454.50 | 454.50 | 0.094 |
| Antioxidant Activity | |||
| DPPH | 254.66 ± 19.01 | ||
| RP | 27.69 ± 0.40 | ||
| ABTS | 734.24 ± 42.62 | ||
| BCB | 96.58 ± 7.11 | ||
| α-Glucosidase Inhibitory Potential | |||
| AHgA | 66.02 ± 1.91 | ||
| Anti-Inflammatory Activity | |||
| AIA | 37.41 ± 0.42 | ||
| No. | Ref. | 4,6,9-Trimethyldec-8-ene-3,5-dione | ar-Curcumene | Carvacrol | Eudesm-5-en-11-ol | Eudesmen-7-(11)-en-4-ol | Italicene | Limonene | Linalool | Nerol | Neryl Acetate | Neryl Propanoate | α-Pinene | α-Terpineol | β-Caryophyllene | β-Eudesmol | β-Selinene | γ-Curcumene |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [42] | 0.0 | 2.4 | 0.0 | 10.1 | 0.0 | 0.5 | 2.5 | 1.3 | 4.4 | 26.5 | 3.9 | 1.7 | 1.7 | 1.1 | 1.5 | 0.0 | 3.3 |
| 2 | [42] | 2.2 | 3.2 | 0.0 | 4.7 | 0.0 | 2.1 | 3.4 | 2.3 | 6.7 | 31.0 | 7.5 | 3.4 | 1.5 | 0.4 | 0.6 | 0.0 | 5.7 |
| 3 | [42] | 0.8 | 4.5 | 0.0 | 4.0 | 0.0 | 2.5 | 4.0 | 2.8 | 4.3 | 41.3 | 5.5 | 1.9 | 1.1 | 0.2 | 0.3 | 0.0 | 3.2 |
| 4 | [42] | 1.3 | 2.6 | 0.0 | 4.2 | 0.0 | 1.1 | 8.5 | 2.1 | 5.3 | 24.3 | 6.7 | 11.4 | 1.8 | 0.3 | 0.3 | 0.0 | 4.6 |
| 5 | [42] | 3.7 | 3.9 | 0.0 | 13.7 | 0.0 | 0.3 | 4.6 | 2.4 | 6.8 | 26.6 | 4.5 | 1.6 | 2.3 | 0.9 | 1.2 | 0.0 | 1.9 |
| 6 | [42] | 3.4 | 2.6 | 0.0 | 6.7 | 0.0 | 0.3 | 10.9 | 5.6 | 10.6 | 11.5 | 3.2 | 12.8 | 2.5 | 0.6 | 0.4 | 0.0 | 1.4 |
| 7 | [42] | 0.2 | 0.9 | 0.0 | 2.7 | 0.0 | 0.5 | 1.1 | 2.5 | 4.2 | 7.1 | 2.2 | 31.6 | 2.2 | 0.3 | 0.1 | 0.0 | 2.1 |
| 8 | [54] | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 5.4 | 2.5 | 0.5 | 0.8 | 7.9 | 1.4 | 15.9 | 0.3 | 4.7 | 0.1 | 6.9 | 22.5 |
| 9 | [43] | 0.0 | 11.4 | 0.0 | 0.0 | 0.0 | 0.0 | 6.1 | 3.0 | 5.0 | 4.9 | 0.0 | 3.8 | 2.5 | 0.5 | 0.0 | 0.0 | 0.0 |
| 10 | [44] | 19.8 | 2.6 | 0.0 | 1.7 | 0.0 | 0.9 | 0.2 | 2.9 | 1.6 | 14.9 | 7.3 | 0.1 | 0.5 | 0.0 | 0.0 | 0.0 | 13.7 |
| 11 | [44] | 14.7 | 1.9 | 0.0 | 1.8 | 0.0 | 0.7 | 1.9 | 3.9 | 1.4 | 18.7 | 4.7 | 2.0 | 1.3 | 0.0 | 0.0 | 0.5 | 11.1 |
| 12 | [44] | 18.3 | 1.3 | 0.0 | 3.8 | 0.0 | 0.7 | 4.3 | 0.7 | 2.2 | 21.0 | 7.8 | 4.5 | 1.2 | 0.0 | 0.0 | 0.0 | 7.7 |
| 13 | [44] | 7.6 | 2.0 | 0.0 | 7.6 | 0.0 | 1.2 | 4.0 | 0.8 | 3.3 | 26.8 | 6.2 | 4.3 | 2.3 | 0.0 | 0.0 | 0.1 | 7.7 |
| 14 | [44] | 4.4 | 1.2 | 0.0 | 1.1 | 0.0 | 0.6 | 10.4 | 1.2 | 1.8 | 27.9 | 3.0 | 8.6 | 1.9 | 0.0 | 0.0 | 0.4 | 8.6 |
| 15 | [44] | 2.0 | 1.8 | 0.0 | 1.7 | 0.0 | 0.5 | 7.5 | 1.3 | 4.2 | 30.2 | 9.2 | 6.1 | 2.6 | 0.0 | 0.0 | 0.5 | 11.0 |
| 16 | [44] | 8.8 | 1.5 | 0.0 | 1.7 | 0.0 | 0.6 | 7.6 | 1.1 | 2.2 | 33.3 | 3.8 | 4.2 | 2.1 | 0.0 | 0.0 | 0.8 | 8.6 |
| 17 | [44] | 4.4 | 1.5 | 0.0 | 1.3 | 0.0 | 0.8 | 5.5 | 0.9 | 2.8 | 39.9 | 6.0 | 6.1 | 1.6 | 0.0 | 0.0 | 0.4 | 11.1 |
| 18 | [44] | 0.3 | 1.5 | 0.0 | 2.9 | 0.0 | 1.1 | 2.4 | 1.5 | 3.6 | 40.1 | 5.5 | 2.9 | 2.1 | 0.0 | 0.0 | 0.0 | 5.4 |
| 19 | [44] | 1.4 | 1.2 | 0.0 | 4.3 | 0.0 | 1.1 | 1.2 | 1.4 | 7.6 | 42.3 | 16.4 | 0.5 | 2.2 | 0.0 | 0.0 | 0.1 | 9.4 |
| 20 | [44] | 2.6 | 1.0 | 0.0 | 5.8 | 0.0 | 1.1 | 5.7 | 0.8 | 3.2 | 44.5 | 7.0 | 4.1 | 1.3 | 0.0 | 0.0 | 0.0 | 6.0 |
| 21 | [56] | 3.7 | 2.3 | 0.0 | 0.8 | 0.0 | 2.4 | 6.9 | 2.4 | 3.2 | 36.3 | 4.8 | 2.7 | 0.7 | 0.0 | 0.4 | 0.0 | 12.9 |
| 22 | [56] | 4.5 | 4.1 | 0.0 | 2.1 | 0.0 | 2.7 | 6.1 | 1.8 | 2.6 | 34.5 | 5.6 | 2.7 | 0.5 | 0.0 | 0.6 | 0.0 | 7.9 |
| 23 | [56] | 3.5 | 3.3 | 0.0 | 0.0 | 0.0 | 3.4 | 7.3 | 2.8 | 3.4 | 42.5 | 5.9 | 2.9 | 0.8 | 0.0 | 0.0 | 0.0 | 6.2 |
| 24 | [56] | 4.3 | 4.6 | 0.0 | 2.3 | 0.0 | 3.0 | 5.1 | 1.5 | 3.4 | 39.9 | 6.7 | 1.5 | 0.5 | 0.0 | 0.7 | 0.0 | 5.1 |
| 25 | [56] | 2.2 | 2.5 | 0.0 | 2.4 | 0.0 | 2.6 | 7.5 | 1.2 | 4.7 | 32.6 | 5.8 | 1.8 | 0.8 | 0.0 | 0.7 | 0.0 | 11.7 |
| 26 | [56] | 5.6 | 1.8 | 0.0 | 3.5 | 0.0 | 1.2 | 2.9 | 2.1 | 4.2 | 34.9 | 4.9 | 1.7 | 1.7 | 0.0 | 0.8 | 0.0 | 3.7 |
| 27 | [56] | 3.1 | 1.8 | 0.0 | 4.2 | 0.0 | 1.4 | 1.9 | 2.3 | 4.9 | 37.6 | 4.3 | 0.7 | 2.0 | 0.0 | 1.3 | 0.0 | 6.7 |
| 28 | [56] | 1.4 | 0.8 | 0.0 | 3.6 | 0.0 | 0.9 | 5.7 | 1.1 | 3.2 | 20.3 | 2.7 | 1.8 | 2.1 | 0.0 | 1.0 | 0.0 | 1.1 |
| 29 | [56] | 0.8 | 0.9 | 0.0 | 5.1 | 0.0 | 1.1 | 4.8 | 1.0 | 2.0 | 15.8 | 1.6 | 1.5 | 1.6 | 0.0 | 0.8 | 0.0 | 0.8 |
| 30 | [45] | 0.0 | 4.5 | 0.0 | 3.9 | 0.0 | 2.3 | 1.2 | 9.1 | 10.7 | 28.9 | 11.4 | 0.2 | 0.2 | 0.2 | 0.8 | 1.8 | 11.4 |
| 31 | [45] | 0.0 | 4.8 | 0.0 | 20.2 | 0.0 | 4.2 | 1.2 | 14.9 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 3.2 | 2.6 | 1.9 | 18.2 |
| 32 | [57] | 0.0 | 0.0 | 3.8 | 0.0 | 0.0 | 0.7 | 0.0 | 1.5 | 0.5 | 1.7 | 0.7 | 0.9 | 0.8 | 5.1 | 0.0 | 33.3 | 4.2 |
| 33 | [57] | 5.5 | 4.9 | 6.2 | 0.0 | 0.0 | 2.7 | 0.0 | 0.4 | 2.4 | 15.1 | 0.5 | 3.6 | 0.8 | 1.2 | 0.0 | 3.6 | 23.3 |
| 34 | [57] | 11.0 | 6.4 | 1.9 | 0.0 | 0.0 | 0.9 | 0.0 | 3.9 | 3.9 | 32.0 | 3.6 | 0.0 | 1.0 | 0.0 | 1.6 | 0.0 | 5.0 |
| 35 | [57] | 0.0 | 8.3 | 8.4 | 0.0 | 0.0 | 1.4 | 0.0 | 0.9 | 0.6 | 0.4 | 0.0 | 0.0 | 0.5 | 3.0 | 0.0 | 19.7 | 2.3 |
| 36 | [57] | 6.2 | 6.4 | 9.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 18.8 | 8.5 | 6.8 | 0.0 | 2.3 | 2.7 | 0.0 | 0.0 | 0.0 |
| 37 | [57] | 0.0 | 0.0 | 6.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 1.1 | 38.0 | 0.0 |
| 38 | [57] | 0.0 | 2.9 | 6.8 | 0.0 | 0.0 | 1.7 | 0.0 | 1.0 | 1.7 | 3.2 | 0.0 | 0.0 | 1.5 | 0.0 | 0.0 | 25.7 | 6.5 |
| 39 | [57] | 0.0 | 1.1 | 14.8 | 0.0 | 0.0 | 0.8 | 0.0 | 0.0 | 0.8 | 1.0 | 0.0 | 0.0 | 1.0 | 10.1 | 13.7 | 15.9 | 3.3 |
| 40 | [57] | 0.0 | 3.1 | 8.7 | 0.0 | 0.0 | 1.3 | 0.0 | 1.4 | 0.5 | 1.1 | 2.3 | 0.0 | 0.8 | 12.7 | 1.6 | 11.6 | 8.4 |
| 41 | [57] | 0.0 | 0.0 | 3.8 | 0.0 | 0.0 | 0.4 | 0.0 | 0.4 | 0.9 | 0.8 | 2.7 | 0.0 | 0.9 | 6.2 | 2.9 | 20.0 | 1.6 |
| 42 | [57] | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 | 1.0 | 1.8 | 0.0 | 1.2 | 0.5 | 8.6 | 0.0 | 2.8 | 15.0 |
| 43 | [57] | 0.0 | 0.0 | 9.5 | 0.0 | 0.0 | 1.9 | 0.0 | 1.5 | 2.0 | 4.5 | 0.2 | 0.0 | 2.6 | 6.5 | 1.5 | 3.8 | 14.3 |
| 44 | [57] | 0.0 | 0.0 | 7.4 | 0.0 | 0.0 | 0.6 | 0.0 | 0.5 | 1.3 | 1.4 | 0.0 | 0.0 | 0.4 | 14.4 | 0.0 | 24.5 | 3.4 |
| 45 | [57] | 0.0 | 2.7 | 9.8 | 0.0 | 0.0 | 2.5 | 0.0 | 0.0 | 0.9 | 1.0 | 0.0 | 0.0 | 1.0 | 10.9 | 0.0 | 2.3 | 27.7 |
| 46 | [57] | 0.0 | 0.0 | 5.4 | 0.0 | 0.0 | 0.7 | 0.0 | 1.5 | 2.7 | 2.8 | 1.5 | 0.8 | 0.9 | 4.5 | 0.0 | 28.9 | 0.0 |
| 47 | [57] | 0.0 | 0.0 | 2.6 | 0.0 | 0.0 | 1.9 | 0.0 | 1.9 | 1.9 | 4.7 | 0.2 | 0.0 | 2.9 | 7.3 | 1.5 | 15.3 | 17.1 |
| 48 | [57] | 0.0 | 0.0 | 5.8 | 0.0 | 0.0 | 1.5 | 0.0 | 0.0 | 0.4 | 1.4 | 0.0 | 0.5 | 0.0 | 18.6 | 0.0 | 26.7 | 14.7 |
| 49 | [57] | 0.0 | 6.1 | 3.3 | 0.0 | 0.0 | 0.0 | 0.0 | 5.1 | 1.7 | 11.4 | 0.9 | 0.0 | 1.2 | 7.8 | 2.4 | 6.1 | 7.7 |
| 50 | [57] | 0.0 | 0.0 | 3.2 | 0.0 | 0.0 | 6.5 | 0.0 | 1.2 | 0.5 | 8.1 | 0.0 | 1.1 | 0.4 | 2.6 | 0.0 | 22.0 | 41.0 |
| 51 | [57] | 0.0 | 2.2 | 3.6 | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 0.4 | 1.2 | 0.0 | 0.0 | 0.6 | 3.0 | 2.3 | 4.7 | 14.5 |
| 52 | [46] | 0.0 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 29.2 | 10.1 | 0.4 | 1.1 | 1.7 | 0.0 | 1.6 | 18.8 |
| 53 | [58] | 0.0 | 2.2 | 0.0 | 0.0 | 0.0 | 2.4 | 2.6 | 0.5 | 1.9 | 20.4 | 3.2 | 4.3 | 1.0 | 1.9 | 0.9 | 2.9 | 14.1 |
| 54 | [16] | 0.0 | 0.0 | 0.0 | 0.0 | 25.3 | 1.0 | 15.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.9 | 0.0 | 4.4 |
| 55 | [16] | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 1.8 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.7 |
| 56 | [16] | 0.0 | 0.0 | 0.0 | 0.0 | 4.4 | 0.0 | 2.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 57 | [16] | 0.0 | 2.1 | 0.0 | 0.0 | 32.1 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.5 | 0.0 | 3.3 |
| 58 | [16] | 0.0 | 1.0 | 0.0 | 0.0 | 2.8 | 1.1 | 3.5 | 1.7 | 0.0 | 73.7 | 3.7 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 1.1 |
| 59 | [16] | 0.0 | 0.7 | 0.0 | 0.0 | 5.0 | 0.4 | 6.6 | 0.0 | 0.0 | 59.9 | 1.1 | 0.0 | 0.0 | 0.0 | 3.2 | 0.0 | 1.6 |
| 60 | [16] | 0.0 | 1.7 | 0.0 | 0.0 | 8.3 | 0.6 | 4.2 | 0.2 | 0.0 | 33.1 | 7.2 | 0.0 | 0.0 | 0.0 | 2.2 | 0.0 | 3.6 |
| 61 | [16] | 0.0 | 0.0 | 0.0 | 0.0 | 17.8 | 0.0 | 6.7 | 4.0 | 0.0 | 52.8 | 14.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 62 | [16] | 0.0 | 0.4 | 0.0 | 0.0 | 4.8 | 0.0 | 2.5 | 0.0 | 0.0 | 70.0 | 4.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.0 | 1.3 |
| 63 | [16] | 0.0 | 0.1 | 0.0 | 0.0 | 1.6 | 0.0 | 1.8 | 0.4 | 0.0 | 77.7 | 6.7 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.5 |
| 64 | [16] | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.5 | 0.0 | 83.4 | 7.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 65 | [16] | 0.0 | 0.1 | 0.0 | 0.0 | 1.9 | 1.0 | 0.8 | 2.2 | 6.8 | 60.3 | 5.4 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 3.7 |
| 66 | [16] | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 1.8 | 5.9 | 0.0 | 4.1 | 50.5 | 7.2 | 0.0 | 0.0 | 0.0 | 1.3 | 0.0 | 1.3 |
| 67 | [16] | 0.0 | 1.3 | 0.0 | 0.0 | 4.2 | 1.0 | 0.4 | 1.5 | 1.9 | 28.4 | 8.2 | 0.0 | 0.0 | 0.0 | 7.1 | 0.0 | 1.4 |
| 68 | [16] | 0.0 | 2.9 | 0.0 | 0.0 | 15.7 | 1.1 | 0.0 | 0.0 | 0.0 | 43.9 | 11.5 | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 2.9 |
| 69 | [16] | 0.0 | 1.9 | 0.0 | 0.0 | 12.4 | 2.1 | 2.0 | 0.2 | 2.9 | 45.4 | 3.7 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 6.7 |
| 70 | [16] | 0.0 | 3.6 | 0.0 | 0.0 | 13.6 | 1.9 | 0.0 | 1.5 | 0.0 | 33.0 | 4.1 | 0.0 | 0.0 | 0.0 | 4.1 | 0.0 | 3.2 |
| 71 | [16] | 0.0 | 2.8 | 0.0 | 0.0 | 14.9 | 1.5 | 0.0 | 1.5 | 1.2 | 37.6 | 3.4 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 2.9 |
| 72 | [16] | 0.0 | 3.4 | 0.0 | 0.0 | 19.6 | 3.8 | 0.7 | 0.0 | 0.0 | 34.4 | 1.1 | 0.0 | 0.0 | 0.0 | 2.3 | 0.0 | 7.2 |
| 73 | [16] | 0.0 | 2.7 | 0.0 | 0.0 | 0.0 | 1.3 | 1.9 | 4.3 | 6.2 | 3.9 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.5 |
| 74 | [16] | 0.0 | 1.6 | 0.0 | 0.0 | 11.6 | 1.8 | 0.0 | 2.1 | 0.0 | 56.9 | 4.0 | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 | 8.7 |
| 75 | [16] | 0.0 | 3.2 | 0.0 | 0.0 | 7.2 | 2.6 | 2.8 | 0.0 | 9.7 | 22.7 | 12.5 | 0.0 | 0.0 | 0.0 | 2.5 | 0.0 | 10.6 |
| 76 | [16] | 0.0 | 2.0 | 0.0 | 0.0 | 0.3 | 2.5 | 8.6 | 1.2 | 13.6 | 42.9 | 3.7 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 6.0 |
| 77 | [16] | 0.0 | 3.2 | 0.0 | 0.0 | 31.5 | 2.8 | 4.9 | 0.0 | 0.7 | 7.3 | 2.0 | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 | 9.9 |
| 78 | [16] | 0.0 | 2.0 | 0.0 | 0.0 | 46.1 | 2.0 | 3.3 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.0 |
| 79 | [47] | 6.6 | 1.7 | 0.0 | 0.0 | 0.0 | 2.5 | 5.6 | 1.4 | 0.8 | 6.7 | 0.8 | 20.0 | 1.4 | 3.5 | 0.6 | 3.3 | 5.6 |
| 80 | [48] | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 12.0 | 0.0 | 9.2 | 0.0 | 0.7 | 0.0 |
| 81 | [49] | 0.0 | 1.1 | 0.0 | 0.0 | 0.0 | 1.8 | 1.1 | 1.0 | 4.5 | 0.0 | 6.5 | 3.4 | 0.0 | 1.9 | 0.4 | 0.0 | 16.0 |
| 82 | [49] | 0.0 | 20.8 | 0.0 | 0.0 | 0.0 | 1.1 | 10.7 | 17.3 | 14.6 | 0.0 | 1.8 | 0.5 | 0.0 | 0.0 | 0.3 | 0.5 | 0.0 |
| 83 | [59] | 0.0 | 2.8 | 0.0 | 0.0 | 0.0 | 1.2 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 | 53.5 | 0.4 | 0.0 | 0.0 | 0.0 | 27.4 |
| 84 | [50] | 0.0 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 4.0 | 0.0 | 1.1 | 10.4 | 0.7 | 12.8 | 2.0 | 2.0 | 3.6 | 2.0 | 0.0 |
| 85 | [60] | 0.0 | 0.0 | 0.0 | 5.7 | 0.0 | 0.0 | 1.9 | 0.9 | 2.3 | 17.1 | 0.0 | 5.6 | 2.8 | 4.9 | 3.1 | 0.0 | 18.1 |
| 86 | [60] | 0.0 | 0.0 | 0.0 | 5.0 | 0.0 | 0.0 | 2.9 | 1.9 | 2.1 | 20.6 | 0.0 | 8.8 | 2.7 | 4.8 | 5.6 | 0.0 | 12.5 |
| 87 | [60] | 0.0 | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 | 4.5 | 1.1 | 0.6 | 12.0 | 0.0 | 19.5 | 0.4 | 3.1 | 5.1 | 0.0 | 25.8 |
| 88 | [60] | 0.0 | 0.0 | 0.0 | 8.1 | 0.0 | 0.0 | 1.7 | 0.5 | 0.2 | 18.0 | 0.0 | 7.0 | 0.3 | 3.3 | 3.1 | 0.0 | 13.0 |
| 89 | [55] | 0.0 | 10.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.1 | 0.0 | 3.9 | 0.0 | 0.7 | 0.2 | 5.4 | 0.0 | 11.3 | 14.1 |
| 90 | [51] | 4.6 | 3.5 | 0.0 | 2.3 | 0.0 | 3.0 | 4.6 | 2.7 | 4.0 | 31.0 | 5.1 | 1.8 | 0.5 | 0.0 | 1.3 | 0.0 | 10.7 |
| 91 | [18] | 0.0 | 0.0 | 0.0 | 14.7 | 0.0 | 0.0 | 3.9 | 1.9 | 8.7 | 29.0 | 2.9 | 0.1 | 5.2 | 0.5 | 3.2 | 0.0 | 1.1 |
| 92 | [18] | 0.0 | 0.0 | 0.0 | 5.2 | 0.0 | 0.0 | 1.1 | 3.4 | 7.4 | 48.0 | 6.9 | 0.0 | 1.8 | 0.1 | 2.2 | 0.0 | 0.2 |
| 93 | [18] | 0.0 | 0.0 | 0.0 | 11.2 | 0.0 | 0.0 | 1.6 | 3.9 | 5.7 | 41.8 | 4.2 | 0.0 | 1.0 | 0.2 | 3.7 | 0.0 | 0.6 |
| 94 | [52] | 0.0 | 28.6 | 0.1 | 0.0 | 0.0 | 1.7 | 0.4 | 0.4 | 1.4 | 6.2 | 0.0 | 4.2 | 10.1 | 0.2 | 0.0 | 0.0 | 12.0 |
| 95 | [52] | 0.0 | 14.5 | 0.4 | 0.0 | 0.0 | 0.0 | 5.7 | 2.0 | 2.2 | 11.8 | 0.0 | 2.3 | 0.8 | 3.3 | 0.0 | 0.0 | 9.9 |
| 96 | [52] | 0.0 | 1.0 | 0.4 | 0.0 | 0.0 | 1.3 | 6.2 | 2.6 | 1.0 | 4.1 | 0.0 | 29.9 | 1.1 | 1.8 | 0.0 | 0.0 | 9.6 |
| 97 | [52] | 0.0 | 10.2 | 0.0 | 0.0 | 0.0 | 1.3 | 4.1 | 1.8 | 0.7 | 13.0 | 0.0 | 10.1 | 1.0 | 10.8 | 0.0 | 0.0 | 4.9 |
| 98 | [52] | 0.0 | 9.3 | 0.0 | 0.0 | 0.0 | 9.3 | 2.8 | 0.4 | 2.4 | 13.5 | 0.0 | 25.2 | 0.9 | 0.4 | 0.0 | 0.0 | 0.0 |
| 99 | [52] | 0.0 | 25.1 | 0.1 | 0.0 | 0.0 | 2.6 | 0.2 | 1.4 | 1.4 | 5.9 | 0.0 | 0.4 | 0.2 | 1.1 | 0.0 | 0.0 | 22.0 |
| 100 | [52] | 0.0 | 23.3 | 0.0 | 0.0 | 0.0 | 1.3 | 0.1 | 0.1 | 3.8 | 7.5 | 0.0 | 0.1 | 0.4 | 1.0 | 0.0 | 0.0 | 12.0 |
| 101 | [52] | 0.0 | 17.9 | 0.1 | 0.0 | 0.0 | 1.2 | 0.2 | 3.3 | 2.0 | 6.7 | 0.0 | 3.7 | 0.1 | 1.1 | 0.0 | 0.0 | 12.2 |
| 102 | [52] | 0.0 | 7.8 | 0.1 | 0.0 | 0.0 | 1.4 | 0.6 | 1.0 | 2.0 | 12.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 15.4 |
| 103 | [21] | 0.0 | 3.7 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 2.3 | 6.7 | 19.5 | 5.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.0 |
| 104 | TS * | 0.0 | 5.0 | 0.0 | 0.0 | 0.0 | 4.2 | 2.6 | 0.8 | 0.4 | 5.5 | 1.7 | 11.8 | 0.2 | 6.7 | 0.2 | 12.2 | 13.6 |
| Average | 1.5 | 3.5 | 1.2 | 1.8 | 2.7 | 1.4 | 2.8 | 1.7 | 2.8 | 21.7 | 3.4 | 3.8 | 1.0 | 2.0 | 1.1 | 3.4 | 7.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V. Helichrysum italicum (Roth) G. Don Essential Oil from Serbia: Chemical Composition, Classification and Biological Activity—May It Be a Suitable New Crop for Serbia? Agronomy 2021, 11, 1282. https://doi.org/10.3390/agronomy11071282
Aćimović M, Ljujić J, Vulić J, Zheljazkov VD, Pezo L, Varga A, Tumbas Šaponjac V. Helichrysum italicum (Roth) G. Don Essential Oil from Serbia: Chemical Composition, Classification and Biological Activity—May It Be a Suitable New Crop for Serbia? Agronomy. 2021; 11(7):1282. https://doi.org/10.3390/agronomy11071282
Chicago/Turabian StyleAćimović, Milica, Jovana Ljujić, Jelena Vulić, Valtcho D. Zheljazkov, Lato Pezo, Ana Varga, and Vesna Tumbas Šaponjac. 2021. "Helichrysum italicum (Roth) G. Don Essential Oil from Serbia: Chemical Composition, Classification and Biological Activity—May It Be a Suitable New Crop for Serbia?" Agronomy 11, no. 7: 1282. https://doi.org/10.3390/agronomy11071282








