Mixed Growth with Weeds Promotes Mycorrhizal Colonization and Increases the Plant-Availability of Phosphorus under Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil Sampling and Analyses
2.3. Plant Harvest and Analyses
2.4. Statistical Analyses
3. Results
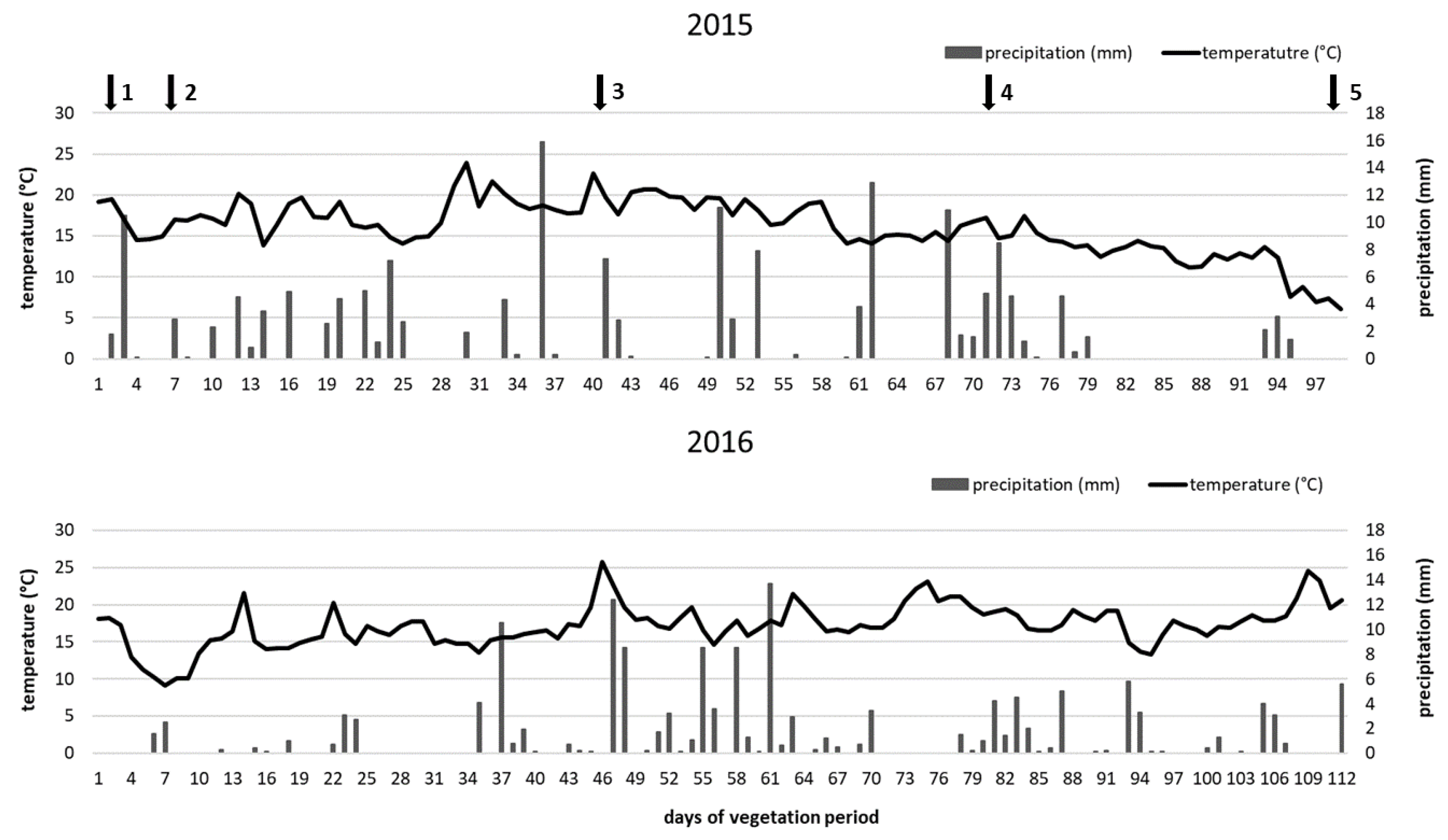
3.1. Weather Conditions
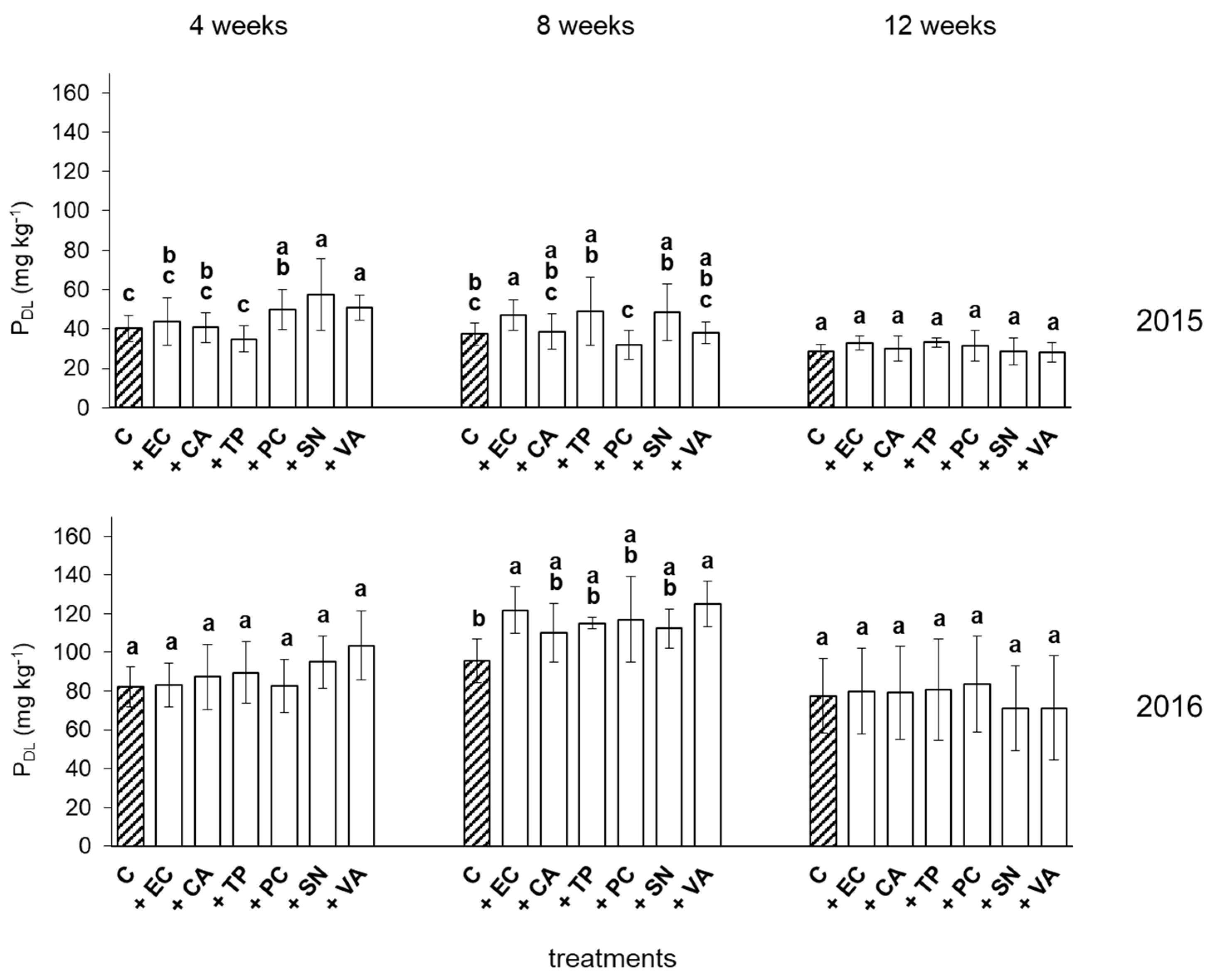
3.2. Plant-Available P
3.3. Microbial Biomass P in the Soil
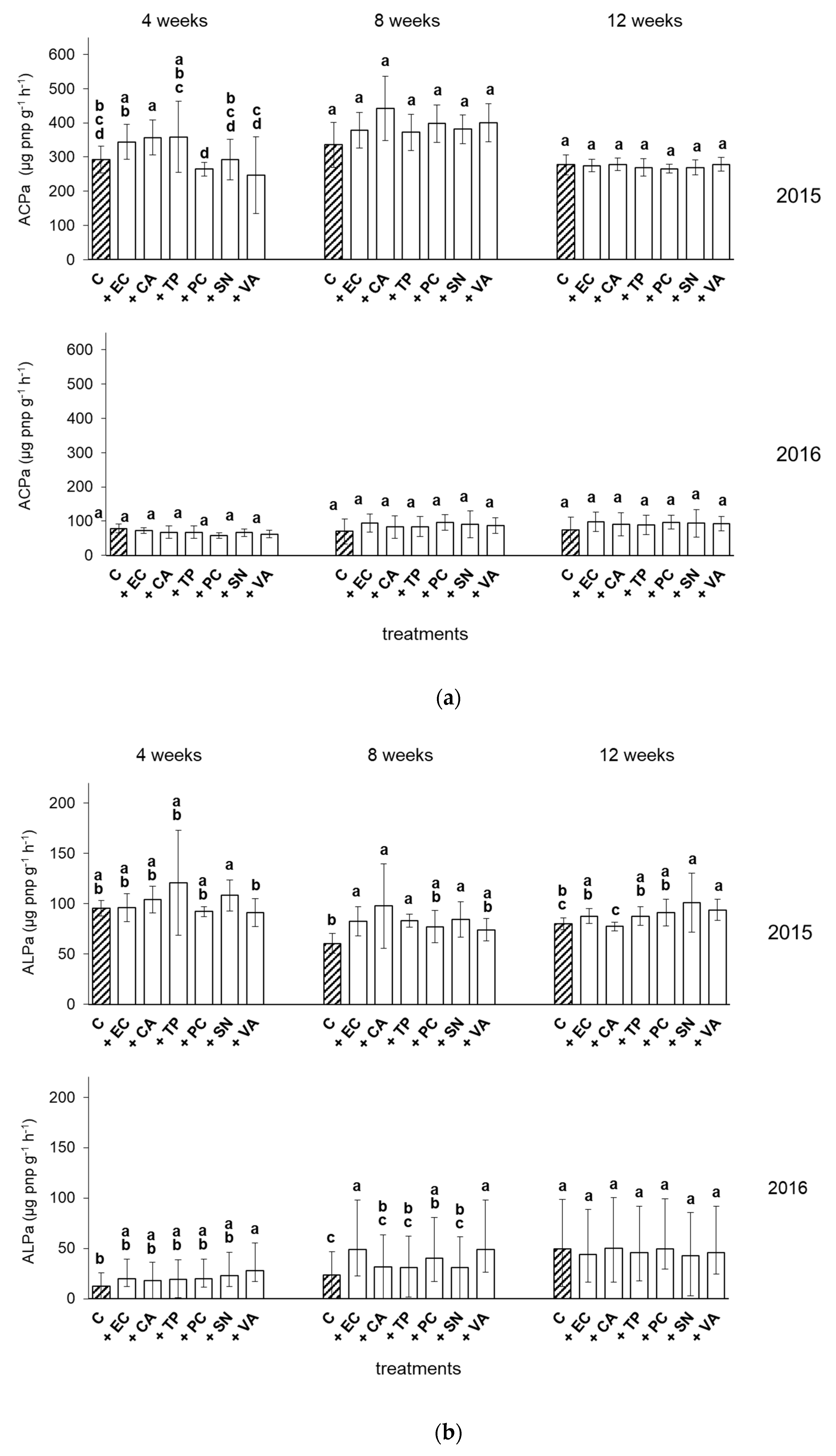
3.4. Soil Enzyme Activities
3.5. Mycorrhizal Colonization of Weeds
3.6. Plant Traits of Maize
3.7. Nutrient Concentrations in the Shoots of Maize
3.8. Mycorrhizal Colonization of Maize
3.9. Weed Species-Specific Mycorrhizal Dependency of Maize
3.10. Correlation of Plant Available P and Soil Enzyme Activities
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, 14–22. [Google Scholar] [CrossRef]
- Heckenmüller, M.; Narita, D.; Klepper, G. Global Availability of Phosphorus and Its Implications for Global Food Supply: An Economic Overview; EconStor, Kiel Working Papers 1897; Kiel Institute for the World Economy: Kiel, Germany, 2014. [Google Scholar]
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; et al. Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. J. Plant Nutr. Soil Sci. 2011, 349, 89–120. [Google Scholar] [CrossRef]
- Roberts, T.L.; Johnston, A.E. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- Brümmer, G.W. Phosphor. In Scheffer Schachtschabel Lehrbuch der Bodenkunde, 16th ed.; Blume, H.P., Brümmer, G.W., Horn, R., Kandeler, E., Kögel-Knabner, I., Kretzschmar, R., Stahr, K., Wilke, B.-M., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2009; pp. 412–421. [Google Scholar]
- Treseder, K.K. The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 2013, 371, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar]
- Dinkelaker, B.; Marschner, H. In vivo demonstration of acid phosphatase activity in the rhizosphere of soil-grown plants. Plant Soil 1992, 144, 199–205. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Marschner, H. Phosphatase activity in the rhizosphere of VA-mycorrhizal wheat supplied with inorganic and organic phosphorous. Soil Biol. Biochem. 1994, 26, 387–395. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Claassen, N. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol. Fertil. Soils 1988, 5, 308–312. [Google Scholar] [CrossRef]
- Ho, I. Acid phosphatase activity in forest soil. For. Sci. 1979, 25, 567–568. [Google Scholar]
- Herbien, S.A.; Neal, J.L. Soil pH and phosphatase activity. Commun. Soil Sci. Plant Anal. 1990, 21, 439–456. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.C.; Rowland, D.L.; Corkidi, L.; Egerton-Warburton, L.M.; Allen, E.B. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 2003, 84, 1895–1908. [Google Scholar] [CrossRef]
- Khan, K.S.; Joergensen, R.G. Changes in microbial biomass and P fractions in biogenic household waste compost amended with inorganic P fertilizers. Biores. Technol. 2009, 100, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Sugito, T.; Yoshida, K.; Takebe, M.; Shinano, T.; Toyota, K. Soil microbial biomass phosphorus as an indicator of phosphorus availability in a Gleyic Andosol. Soil Sci. Plant Nutr. 2010, 56, 390–398. [Google Scholar] [CrossRef]
- Kouno, K.; Wu, J.; Brookes, P.C. Turnover of biomass C and P in soil following incorporation of glucose or ryegrass. Soil Biol. Biochem. 2001, 34, 617–622. [Google Scholar] [CrossRef]
- Palomo, L.; Claassen, N.; Jones, D.L. Differential mobilization of P in the maize rhizosphere by citric acid and potassium citrate. Soil Biol. Biochem. 2006, 38, 683–692. [Google Scholar] [CrossRef]
- Mat Hassan, H.; Marschner, P.; Mc Neill, A. Growth, P uptake in grain legumes and changes in rhizosphere soil P pools. Biol. Fertil. Soils 2012, 48, 151–159. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Scow, K.; Brennan, E. Soil phosphorus mobilization in the rhizosphere of cover crops has little effect on phosphorus cycling in California agricultural soils. Soil Biol. Biochem. 2014, 78, 255–262. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Weed-Crop Competition: A Review, 2nd ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008. [Google Scholar]
- Hacker, N.; Ebeling, A.; Gessler, A.; Gleixner, G.; González Macé, O.; de Kroon, H.; Lange, M.; Kommer, L.; Eisenhauer, N.; Ravenek, J.; et al. Plant diversity shapes microbe-rhizosphere effects on P mobilisation from organic matter in soil. Ecol. Lett. 2015, 18, 1356–1365. [Google Scholar] [CrossRef]
- Weil, R.R. Maize-weed competition and soil erosion in unweeded maize. Trop. Agric. 1982, 59, 207–213. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Browman, M.G.; Tabatabai, M.A. Phosphodiesterase activity of soils. Soil Sci. Soc. Am. J. 1978, 42, 284–290. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Part 2; American Society of Agronomy: Madison, WI, USA, 1982; pp. 903–948. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powelson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Riehm, H. Arbeitsvorschrift zur Bestimmung der Phosphorsäure und des Kaliums nach Lactatverfahren. J. Plant Nutr. Soil Sci. 1948, 40, 61–74. [Google Scholar]
- VDLUFA. Methodenbuch des Verbandes Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten 2002/5; VDLUFA-V: Darmstadt, Germany, 2005. [Google Scholar]
- Brundrett, M.C.; Piche, Y.; Peterson, R.L. A new method for observing the morphology of vesicular–arbuscular mycorrhizae. Can. J. Bot. 1984, 62, 2128–2134. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. In R-Foundation for Statistical Computing; Version 3.3.2.; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.2-4; 2016. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 25 June 2021).
- Khan, K.S.; Joergensen, R.G. Compost and phosphorus amendments for stimulating microorganisms and growth of ryegrass in a Ferrasol und a Luvisol. J. Plant Nutr. Soil Sci. 2012, 175, 108–114. [Google Scholar] [CrossRef]
- Blume, H.P.; Leinweber, P. Beurteilung des Bodens als Wurzel- und Lebensraum. In Bodenkundliches Praktikum, 3rd ed.; Blume, H.P., Stahr, K., Leinweber, P., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2009; p. 221. [Google Scholar]
- Eichler, B.; Caus, M.; Schnug, E.; Köppen, D. Soil acid and alkaline phosphatase activities in regulation to crop species and fungal treatment. Landbauforsch. Volkenrode 2004, 54, 1–5. [Google Scholar]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef] [Green Version]
- Gavito, M.E.; Miller, M.H. Early phosphorus nutrition, mycorrhizae development, dry matter partitioning and yield of maize. Plant Soil 1998, 199, 177–186. [Google Scholar] [CrossRef]
- Chu, Q.; Wang, X.; Yang, Y.; Chen, F.; Zhang, F.; Feng, G. Mycorrhizal responsiveness of maize (Zea mays L.) genotypes as related to releasing date and available P content in soil. Mycorrhiza 2013, 23, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2014, 88, 41–49. [Google Scholar] [CrossRef]
- Hao, X.J.; Hong, J.P.; Zhang, T.Q.; Li, R.; Gao, W.J.; Zheng, Z.M. Effects of arbuscular mycorrhizal fungal inoculation and phosphorus (P) addition on maize P utilization and growth in reclaimed soil of a mining area. Commun. Soil Sci. Plant Anal. 2014, 45, 2413–2428. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Lehmann, A.; Veresoglou, S.D.; Leifheit, E.F.; Rillig, M.C. Arbuscular mycorrhizal influence on zinc nutrition in crop plants-a meta-analysis. Soil Biol. Biochem. 2014, 69, 123–131. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.X.; Liu, Y.M.; Liu, D.Y.; Chen, X.P.; Zou, C.Q. Zinc uptake by roots and accumulation in maize plants as affected by phosphorus application and arbuscular mycorrhizal colonization. Plant Soil. 2017, 413, 59–71. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropping species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [Green Version]



| Year | Sampling (Weeks of Growth) | Treatment | Mycorrhizal Root Length (%) | |
|---|---|---|---|---|
| 2015 | 4 weeks | EC | 25.7 (7.7) | a |
| CA | 0.0 (0) | c | ||
| TP | 33.3 (15.2) | a | ||
| PC | 0.0 (0) | c | ||
| SN | 5.4 (1.4) | b | ||
| VA | 5.1 (1.2) | b | ||
| 8 weeks | EC | 17.3 (6.4) | b | |
| CA | 4.4 (2.3) | b | ||
| TP | 14.7 (6.9) | a | ||
| PC | 0.6 (0.9) | c | ||
| SN | 4.7 (4.0) | b | ||
| VA | 4.4 (2.8) | b | ||
| 2016 | 4 weeks | EC | 20.3 (4.2) | a |
| CA | 5.2 (3.9) | b | ||
| TP | 19.3 (2.4) | a | ||
| PC | 0.3 (0.6) | c | ||
| SN | 0.3 (0.8) | b | ||
| VA | 1.4 (1.0) | b | ||
| 8 weeks | EC | 16.4 (2.6) | a | |
| CA | 1.3 (1.0) | c | ||
| TP | 10.0 (1.9) | b | ||
| PC | 0.0 (0) | d | ||
| SN | 1.0 (1.8) | cd | ||
| VA | 1.8 (1.5) | c | ||
| Year | Treatment | Shoot Dry Matter | Root Dry Matter | Root to Shoot Ratio | Root Length Density | ||||
|---|---|---|---|---|---|---|---|---|---|
| (g) | (g) | (cm cm−3) | |||||||
| 2015 | C | 11.27 (3.33) | a | 8.80 (1.78) | a | 0.82 (0.26) | b | 1.41 (0.40) | ab |
| +EC | 7.41 (5.94) | d | 6.62 (4.08) | bc | 1.03 (0.84) | b | 1.57 (0.36) | ab | |
| +CA | 3.73 (0.87) | e | 4.05 (0.57) | c | 1.09 (0.13) | a | 0.99 (0.25) | abc | |
| +TP | 5.87 (1.31) | d | 6.10 (2.81) | bc | 1.10 (0.66) | ab | 0.96 (0.33) | bc | |
| +PC | 7.13 (4.20) | cd | 4.56 (1.90) | bc | 0.78 (0.23) | b | 0.92 (0.33) | bc | |
| +SN | 8.09 (2.41) | bc | 5.57 (1.16) | b | 0.74 (0.17) | b | 0.65 (0.21) | c | |
| +VA | 8.41 (1.91) | ab | 5.71 (1.05) | b | 0.70 (0.19) | b | 1.20 (0.36) | abc | |
| 2016 | C | 26.52 (25.91) | ab | 12.50 (6.40) | a | 0.58 (0.21) | a | 3.46 (1.03) | ab |
| + EC | 37.38 (36.29) | b | 9.42 (5.06) | ab | 0.29 (0.06) | bc | 4.00 (1.05) | ab | |
| +CA | 26.67 (30.53) | c | 12.31 (13.97) | ab | 0.49 (0.12) | a | 2.27 (0.99) | b | |
| +TP | 18.17 (23.15) | bc | 6.43 (4.96) | b | 0.41 (0.13) | ab | 3.35 (0.90) | ab | |
| +PC | 34.86 (37.54) | b | 10.19 (6.01) | ab | 0.34 (0.12) | b | 2.26 (0.99) | b | |
| +SN | 29.59 (25.23) | ab | 8.04 (2.66) | ab | 0.32 (0.11) | b | 4.01 (0.96) | a | |
| +VA | 53.45 (35.97) | a | 10.98 (4.63) | ab | 0.21 (0.05) | c | 4.07 (2.10) | a |
| Year | Treatment | Nutrient Concentration in Maize Shoots | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Zn | ||||||||||||||
| (g kg−1) | |||||||||||||||||||
| 2015 | C | 8.74 | (0.86) | abc | 2.13 | (0.38) | d | 18.33 | (1.58) | a | 2.95 | (0.45) | a | 2.11 | (0.20) | ab | 0.018 | (0.005) | c |
| +EC | 9.64 | (1.96) | a | 2.49 | (0.49) | bc | 18.88 | (1.88) | ab | 2.61 | (0.77) | a | 2.07 | (0.32) | ab | 0.020 | (0.003) | b | |
| +CA | 9.65 | (1.26) | a | 2.95 | (0.36) | a | 18.06 | (1.51) | a | 2.57 | (0.54) | a | 2.17 | (0.25) | a | 0.042 | (0.036) | a | |
| +TP | 9.11 | (0.76) | ab | 2.31 | (0.29) | bcd | 18.35 | (2.16) | a | 2.65 | (0.39) | a | 2.12 | (0.21) | ab | 0.020 | (0.001) | b | |
| +PC | 8.96 | (1.55) | abc | 2.57 | (0.39) | b | 17.29 | (2.18) | abc | 2.44 | (0.58) | a | 2.11 | (0.22) | ab | 0.022 | (0.003) | b | |
| +SN | 8.46 | (1.01) | bc | 2.20 | (0.30) | cd | 16.05 | (1.84) | bc | 2.32 | (0.56) | a | 1.90 | (0.22) | ab | 0.018 | (0.001) | b | |
| +VA | 8.42 | (1.06) | c | 2.11 | (0.22) | c | 15.54 | (1.62) | c | 2.40 | (0.65) | a | 1.87 | (0.16) | b | 0.018 | (0.002) | b | |
| 2016 | C | 6.89 | (0.87) | a | 2.25 | (0.52) | c | 10.46 | (1.66) | a | 2.22 | (0.88) | a | 1.56 | (0.51) | ab | 0.020 | (0.003) | b |
| +EC | 7.28 | (1.43) | a | 2.65 | (0.60) | ab | 10.03 | (1.40) | a | 2.42 | (0.68) | a | 1.71 | (0.46) | a | 0.022 | (0.008) | ab | |
| +CA | 7.38 | (1.06) | a | 2.37 | (0.59) | a | 10.49 | (1.16) | a | 1.98 | (0.68) | a | 1.62 | (0.49) | a | 0.019 | (0.006) | a | |
| +TP | 6.99 | (1.93) | a | 2.34 | (0.38) | bc | 9.86 | (1.03) | a | 2.23 | (0.32) | a | 1.69 | (0.36) | a | 0.019 | (0.003) | ab | |
| +PC | 7.14 | (1.42) | a | 2.46 | (0.35) | ab | 11.62 | (1.64) | a | 2.03 | (0.60) | a | 1.55 | (0.43) | ab | 0.017 | (0.002) | b | |
| +SN | 7.11 | (1.44) | a | 2.32 | (0.51) | bc | 9.97 | (1.73) | a | 2.36 | (0.50) | a | 1.53 | (0.35) | ab | 0.019 | (0.004) | ab | |
| +VA | 7.90 | (1.38) | a | 2.42 | (0.56) | c | 10.44 | (1.63) | a | 2.01 | (0.57) | a | 1.35 | (0.39) | b | 0.020 | (0.003) | ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacher, A.; Baum, C.; de Mol, F.; Dehmer, K.J.; Gerowitt, B. Mixed Growth with Weeds Promotes Mycorrhizal Colonization and Increases the Plant-Availability of Phosphorus under Maize (Zea mays L.). Agronomy 2021, 11, 1304. https://doi.org/10.3390/agronomy11071304
Zacher A, Baum C, de Mol F, Dehmer KJ, Gerowitt B. Mixed Growth with Weeds Promotes Mycorrhizal Colonization and Increases the Plant-Availability of Phosphorus under Maize (Zea mays L.). Agronomy. 2021; 11(7):1304. https://doi.org/10.3390/agronomy11071304
Chicago/Turabian StyleZacher, Anika, Christel Baum, Friederike de Mol, Klaus J. Dehmer, and Bärbel Gerowitt. 2021. "Mixed Growth with Weeds Promotes Mycorrhizal Colonization and Increases the Plant-Availability of Phosphorus under Maize (Zea mays L.)" Agronomy 11, no. 7: 1304. https://doi.org/10.3390/agronomy11071304
APA StyleZacher, A., Baum, C., de Mol, F., Dehmer, K. J., & Gerowitt, B. (2021). Mixed Growth with Weeds Promotes Mycorrhizal Colonization and Increases the Plant-Availability of Phosphorus under Maize (Zea mays L.). Agronomy, 11(7), 1304. https://doi.org/10.3390/agronomy11071304





