Poriella subacida Gen. & Comb Nov. for Perenniporia subacida (Peck) Donk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological Studies
2.2. Molecular Techniques and Phylogenetic Analyses
3. Results
3.1. Molecular Phylogeny
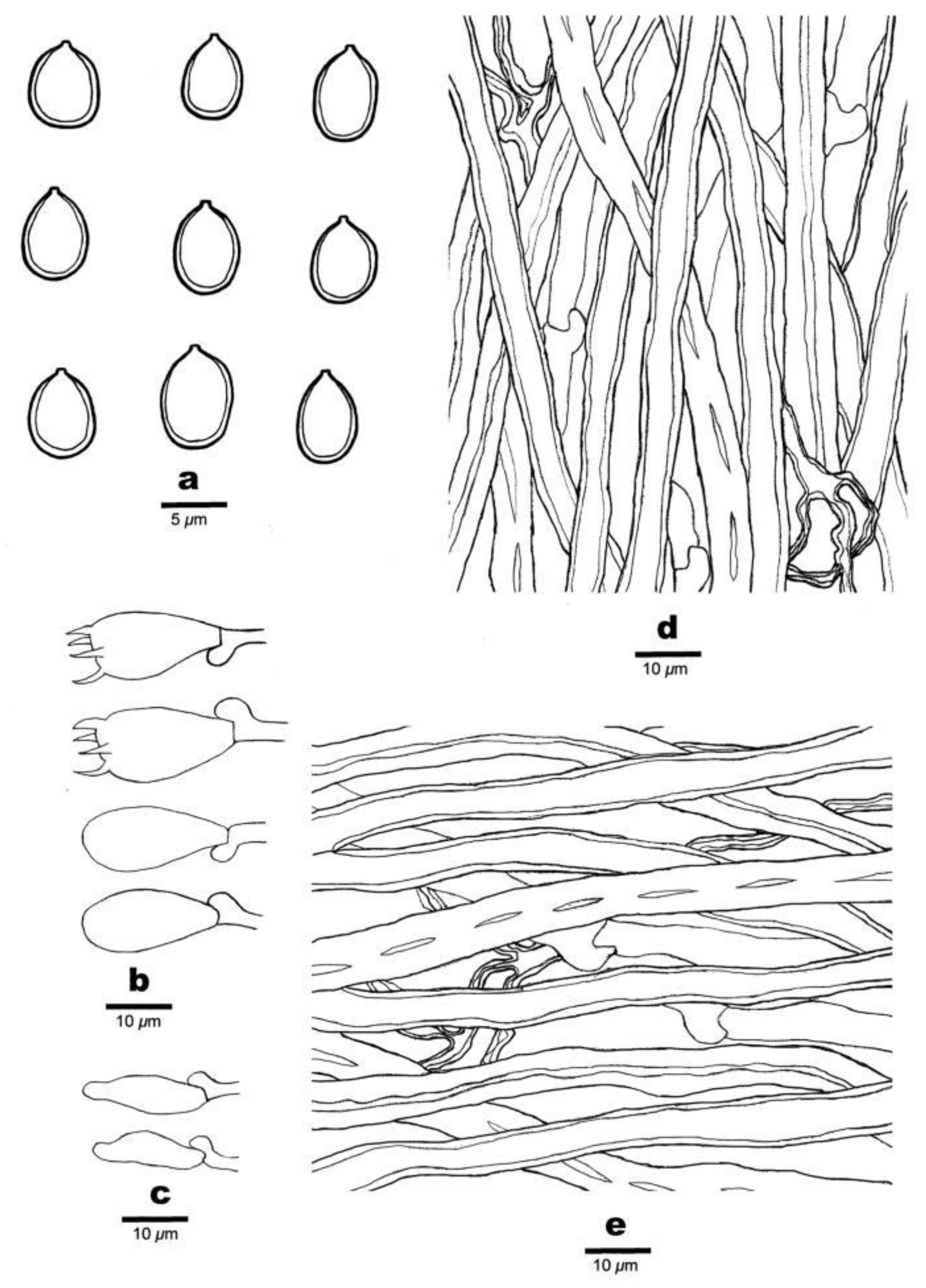
3.2. Taxonomy
- MycoBank: 840062.
- Basionym: Polyporus subacidus Peck, Ann. Rep. N.Y. St. Mus. nat. Hist. 38: 92, 1885.
- =Poria subacida (Peck) Sacc., Syll. fung. (Abellini) 6: 325 (1888).
- =Chaetoporus subacidus (Peck) Bondartsev & Singer, Annls mycol. 39(1): 51 (1941).
- =Oxyporus subacidus (Peck) Komarova, Mycoth. Eston. 3: 13 (1961).
- =Perenniporia subacida (Peck) Donk, Persoonia 5(1): 76 (1967).
- =Poria colorea Overh. & Englerth, Bull. Yale Univ. School For. 50: 21 (1942).
- =Poria fuscomarginata Berk. ex Cooke, Grevillea 15(no. 73): 24 (1886).
- =Poria subaurantia Berk. ex Cooke, Grevillea 15(no. 73): 27 (1886).
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, T.Y.; Stajich, J.E.; Hittinger, C.T.; Rokas, A. Toward a fully resolved fungal tree of life. Annu. Rev. Microbiol. 2020, 15, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjökvist, E.; Lindner, D.; Nakasone, K.; Niemelä, T.; Larsson, K.H.; Ryvarden, L.; et al. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 2017, 121, 798–824. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.M.; Cannon, P.F.; David, J.C.; Minter, D.W.; Stalpers, J.A. Ainsworth and Bisby’s Dictionary of the Fungi, 10th ed.; CAB International Press: Wallingford, UK, 2008. [Google Scholar] [CrossRef]
- Cui, B.K.; Li, H.J.; Ji, X.; Zhou, J.L.; Song, J.; Si, J.; Yang, Z.L.; Dai, Y.C. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 2019, 97, 137–392. [Google Scholar] [CrossRef]
- El-Gharabawy, H.M.; Leal-Dutra, C.A.; Griffith, G.W. Crystallicutis gen. nov. (Irpicaceae, Basidiomycota), including C. damiettensis sp. nov., found on Phoenix dactylifera (date palm) trunks in the Nile Delta of Egypt. Fungal Biol. 2021, 125, 447–458. [Google Scholar] [CrossRef]
- Matozaki, T.; Hattori, T.; Maekawa, N.; Nakagiri, A.; Ishikawa, N.K.; Sotome, K. Hirticrusta gen. nov. segregated from Neofomitella in Polyporaceae (Polyporales). Mycoscience 2020, 5, 240–248. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, C.L. Crepatura ellipsospora gen. et sp. nov. in Phanerochaetaceae (Polyporales, Basidiomycota) bearing a tuberculate hymenial surface. Mycol. Prog. 2019, 18, 785–793. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid fungi of Europe. Syn. Fung. 2014, 31, 1–455. [Google Scholar]
- Peck, C.H. Report of the botanist. Ann. Rep. N. Y. State Mus. Nat. Hist. 1885, 38, 77–138. [Google Scholar]
- Gramss, G.R.; Gilbertson, L.; Ryvarden, L. North American Polypores. Volume 1: Abortiporus—Lindtneria. 433 S., 209 Abb. Oslo 1986. Fungiflora A/S. J. Basic Microbiol. 1987. [Google Scholar] [CrossRef]
- Núñez, M.; Ryvarden, L. East Asian polypores 2. Syn. Fung. 2001, 14, 165–522. [Google Scholar]
- Dai, Y.C.; Niemelä, T.; Kinnunen, J. The polypore genera Abundisporus and Perenniporia (Basidiomycota) in China, with notes on Haploporus. Ann. Bot. Fenn. 2002, 39, 169–182. [Google Scholar]
- Decock, C.; Stalpers, J. Studies in Perenniporia: Polyporus unitus, Boletus medulla-panis, the nomenclature of Perenniporia, Poria and Physisporus, and a note on European Perenniporia with a resupinate basidiome. Taxon 2006, 53, 759–778. [Google Scholar] [CrossRef]
- Dai, Y.C. Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 2012, 53, 49–80. [Google Scholar] [CrossRef]
- Zhao, C.L.; Cui, B.K.; Dai, Y.C. New species and phylogeny of Perenniporia based on morphological and molecular characters. Fungal Divers. 2013, 58, 47–60. [Google Scholar] [CrossRef]
- Shen, S.; Xu, T.M.; Jason, K.; Zhao, C.L. Morphological and molecular identification of a new species of Perenniporia (Polyporales, Basidiomycota) in North America. Phytotaxa 2018, 351, 63–71. [Google Scholar] [CrossRef]
- Saccardo, P.A. Sylloge hymenomycetum, Vol. II. Polyporeae, Hydneae, Thelephoreae, Clavarieae, Tremellineae. Sylloge Fungorum 1888, 6, 1–928. [Google Scholar]
- Bondartsev, A.; Singer, R. Zur systematik der Polyporaceae. Ann. Mycol. 1941, 39, 43–65. [Google Scholar]
- Parmasto, E. Eesti seente eksikaat. Mycotheca Est. 1961, 3, 51–75. [Google Scholar]
- Donk, M.A. Notes on European polypores II. Persoonia 1967, 5, 47–130. [Google Scholar]
- Larsson, K.H. Re-thinking the classification of corticioid fungi. Mycol. Res. 2007, 111, 1040–1063. [Google Scholar] [CrossRef]
- Miettinen, O.; Larsson, K.H.; Sjökvist, E.; Larsson, K.L. Comprehensive taxon sampling reveals unaccounted diversity and morphological plasticity in a group of dimitic polypores (Polyporales, Basidiomycota). Cladistics 2012, 28, 251–270. [Google Scholar] [CrossRef] [Green Version]
- Binder, M.; Justo, A.; Riley, R.; Salamov, A.; López-Giráldez, F.; Sjökvist, E.; Copeland, A.; Foster, B.; Sun, H.; Larsson, E.; et al. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 2013, 105, 1350–1373. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.C.; Cui, B.K.; Si, J.; He, S.H.; Hyde, K.D.; Yuan, H.S.; Lui, X.Y.; Zhou, L.W. Dynamics of the worldwide number of fungi with emphasis on fungal diversity in China. Mycol. Prog. 2015, 14, 62. [Google Scholar] [CrossRef]
- Miettinen, O.; Spirin, V.; Vlasák, J.; Rivoire, B.; Stenroos, S.; Hibbett, D. Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota). MycoKeys 2016, 17, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Dai, Y.C.; Vlasák, J.; Yuan, Y. Molecular phylogeny and global diversity of the genus Haploporus (Polyporales, Basidiomycota). J. Fungi 2021, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.B.; Zhou, M.; Yuan, Y.; Dai, Y.C. Global diversity and taxonomy of Sidera (Hymenochaetales, Basidiomycota): Four new species and keys to species of the genus. J. Fungi. 2021, 7, 251. [Google Scholar] [CrossRef]
- Robledo, G.L.; Amalfi, M.; Castillo, G.; Rajchenberg, M.; Decock, C. Perenniporiella chaquenia sp. nov. and further notes on Perenniporiella and its relationships with Perenniporia (Poriales, Basidiomycota). Mycologia 2009, 101, 657–673. [Google Scholar] [CrossRef]
- Petersen, J.H. Farvekort: The Danish Mycological Society’s Colour Chart; Foreningen til Svampekundskabens Fremme: Greve, Denmark, 1996. [Google Scholar]
- Zhao, C.L.; Wu, Z.Q. Ceriporiopsis kunmingensis sp. nov. (Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Mycol Prog. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Huang, R.X.; Zhao, C.L. Three new species of Phlebia (Polyporales, Basidiomycota) based on the evidence from morphology and DNA sequence data. Mycol. Prog. 2020, 19, 753–767. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Lutzoni Lab. Available online: http://lutzonilab.org (accessed on 4 June 2021).
- Rehner, S.A.; Buckley, E.A. Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [PubMed]
- Kim, K.M.; Lee, J.S.; Jung, H.S. Fomitopsis incarnatus sp. nov. based on generic evaluation of Fomitopsis and Rhodofomes. Mycologia 2007, 99, 833–841. [Google Scholar] [CrossRef]
- Vlasak, J.; Vlasa, K.J., Jr.; Cui, B.K. Antrodia kmetii, a new European polypore similar to Antrodia variiformis. Cryptogam. Mycol. 2013, 34, 203–209. [Google Scholar] [CrossRef]
- Tomšovský, M.; Menkis, A.; Vasaitis, R. Phylogenetic relationships in European Ceriporiopsis species inferred from nuclear and mitochondrial ribosomal DNA sequences. Fungal Biol. 2010, 114, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Justo, A.; Hibbett, D.S. Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset. Taxon 2011, 60, 1567–1583. [Google Scholar] [CrossRef]
- Zhao, C.L.; Cui, B.K.; Steffen, K.T. Yuchengia, a new polypore genus segregated from Perenniporia (Polyporales, Basidiomycota) based on morphological and molecular characters. Nord. J. Bot. 2013, 31, 331–338. [Google Scholar] [CrossRef]
- Sotome, K.; Hattori, T.; Ota, Y.; Toanun, C.; Salleh, B.; Kakishima, M. Phylogenetic relationships of Polyporus and morphologically allied genera. Mycologia 2008, 100, 603–615. [Google Scholar] [CrossRef]
- Brazee, N.J.; Lindner, D.L.; Fraver, S.; D’Amato, A.W.; Milo, A.M. Wood-inhabiting, polyporoid fungi in aspen-dominated forests managed for biomass in the U.S. Lake States. Fungal Ecol. 2012, 5, 600–609. [Google Scholar] [CrossRef]
- Tabata, M.; Harrington, T.C.; Chen, W.; Abe, Y. Molecular phylogeny of species in the genera Amylostereum and Echinodontium. Mycoscience 2000, 41, 585–593. [Google Scholar] [CrossRef]
- Pildain, M.B.; Rajchenberg, M. The phylogenetic position of Postia s.l. (Polyporales, Basidiomycota) from Patagonia, Argentina. Mycologia 2013, 105, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Holder, M.T.; Vos, R.; Midford, P.E.; Liebowitz, T.; Chan, L.; Hoover, P.; Warnow, T. The CIPRES Portals. (Archived by WebCite(r)). 4 August 2009. Available online: http://www.phylo.org/sub_sections/portal; http://www.webcitation.org/5imQlJeQa (accessed on 4 June 2021).
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [Green Version]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author. Evolutionary Biology Centre; Uppsala University: Uppsala, Sweeden, 2004. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ipulet, P.; Ryvarden, L. New and interesting polypores from Uganda. Syn. Fung. 2005, 20, 87–99. [Google Scholar]
- Decock, C.; Ryvarden, L. Perenniporiella gen. nov. segregated from Perenniporia, including key to neotropical Perenniporia species with pileate basidiomes. Mycol. Res. 2003, 107, 93–103. [Google Scholar] [CrossRef]
- Murrill, W.A. Some described species of Poria. Mycologia 1919, 11, 231–244. [Google Scholar] [CrossRef]
- Murrill, W.A. Light-colored resupinate polypores II. Mycologia 1920, 12, 299–308. [Google Scholar]
- Pilát, A. Additamenta ad floram Sibiriae Asiaeque orientalis mycologicam. Pars Tertia. Bull. Soc. Mycolog. 1936, 51, 351–426. [Google Scholar]
- Pouzar, Z. Notes on four European polypores. Česká Mykol. 1984, 38, 203–204. [Google Scholar]
- Decock, C.; Ryvarden, L. Studies in neotropical polypores. Some coloured resupinate Perenniporia species. Mycol. Res. 1999, 103, 1138–1144. [Google Scholar] [CrossRef]
- Ryvarden, L.; Johansen, I. A Preliminary Polypore Flora of East Africa; Fungiflora: Oslo, Norway, 1980. [Google Scholar]
- Ryvarden, L. Genera of polypores: Nomenclature and taxonomy. Syn. Fung. 1991, 5, 1–363. [Google Scholar]



| Species Name | Sample No. | GenBank Accessions | References | |||
|---|---|---|---|---|---|---|
| ITS | LSU | mtSSU | TEF1 | |||
| Abortiporus biennis | TFRI 274 | EU232187 | EU232235 | [21] | ||
| A. biennis | EL65-03 | JN649325 | JN649325 | [21] | ||
| Abundisporus roseoalbus | Dai 12269 | KC415908 | KC415910 | KF051037 | KF181131 | [5] |
| A. pubertatis | Cui 5776 | KC787565 | KC787572 | KF051029 | KF181129 | [5] |
| A. sclerosetosus | MUCL 41438 | FJ411101 | FJ393868 | [28] | ||
| A. violaceus | Ryvarden 10775 | KF018126 | KF018134 | KF051058 | KF181152 | [5] |
| Amylocystis lapponica | KHL 11755 | EU118603 | EU118603 | [21] | ||
| Antrodia albida | CBS 308.82 | DQ491414 | [35] | |||
| A. albida | FP 105979 | EU232272 | EU232272 | [36] | ||
| A. heteromorpha | CBS 200.91 | DQ491415 | AY515350 | [35] | ||
| A. macra | MUAF 887 | EU340898 | [23] | |||
| Bjerkandera adusta | NBRC 4983 | AB733156 | AB733333 | [23] | ||
| Cinereomyces lindbladii | FBCC 177 | HQ659223 | HQ659223 | [23] | ||
| Climacocystis borealis | KH 13318 | JQ031126 | JQ031126 | [23] | ||
| Coriolopsis caperata | LE(BIN)-0677 | AB158316 | AB158316 | [37] | ||
| Donkioporia expansa | MUCL 35116 | FJ411104 | FJ393872 | [28] | ||
| Earliella scabrosa | PR1209 | JN165009 | JN164793 | [38] | ||
| Fragiliporia fragilis | Dai 13080 | KJ734260 | KJ734264 | KJ734268 | KJ790245 | [5] |
| F. fragilis | Dai 13559 | KJ734261 | KJ734265 | KJ734269 | KJ790246 | [5] |
| F. fragilis | Dai 13561 | KJ734262 | KJ734266 | KJ734270 | KJ790247 | [5] |
| Ganoderma sichuanense | Wu 1006-38 | JQ781858 | JX029989 | JX029976 | [5] | |
| G. sichuanense | Dai 12479 | JQ781864 | JX029988 | JX029975 | [5] | |
| G. australe | Cui 9511 | JN048773 | JN048792 | [39] | ||
| G. sinense | Wei 5327 | KF494998 | KF495008 | KF494976 | [5] | |
| G. applanatum | Dai 12483 | KF494999 | KF495009 | KF494977 | [5] | |
| Gelatoporia subvermispora | BRNU 592909 | FJ496694 | FJ496706 | [37] | ||
| Grammetheliopsis subtropica | Cui 9041 | JQ845096 | JQ845099 | KF051039 | KF181133 | [39] |
| Heterobasidion annosum | PFC 5252 | KC492906 | KC492906 | [23] | ||
| Hornodermoporus latissima | Cui 6625 | HQ876604 | JF706340 | KF051040 | KF181134 | [5] |
| H. martius | Cui 7992 | HQ876603 | HQ654114 | KF051041 | KF181135 | [5] |
| H. martius | MUCL 41677 | FJ411092 | FJ393859 | [28] | ||
| H. martius | MUCL 41678 | FJ411093 | FJ393860 | [28] | ||
| Hydnopolyporus fimbriatus | LR 40855 | JN649347 | JN649347 | [23] | ||
| Hypochnicium lyndoniae | NL 041031 | JX124704 | JX124704 | [23] | ||
| Lentinus tigrinus | DSH93-181 | AY218419 | AF518627 | U27050 | [38] | |
| Microporellus violaceocinerascens | MUCL 45229 | FJ411106 | FJ393874 | [28] | ||
| M. violaceocinerascens | Cui 8459 | HQ876606 | HQ654113 | KF051042 | KF181136 | [5] |
| Obba rivulosa | KCTC 6892 | FJ496693 | FJ496710 | [4] | ||
| Perenniporia hainaniana | Cui 6364 | JQ861743 | JQ861759 | KF051044 | KF181138 | [5] |
| P. hainaniana | Cui 6365 | JQ861744 | JQ861760 | KF051045 | KF181139 | [5] |
| P. hainaniana | Cui 6366 | JQ861745 | JQ861761 | KF494996 | KF494981 | [5] |
| P. medulla-panis | MUCL 49581 | FJ411088 | FJ393876 | [28] | ||
| P. medulla-panis | MUCL 43250 | FJ411087 | FJ393875 | [28] | ||
| P. medulla-panis | Cui 3274 | JN112792 | JN112793 | KF051043 | KF181137 | [5] |
| P. substraminea | Cui 10177 | JQ001852 | JQ001844 | KF051046 | KF181140 | [5] |
| P. substraminea | Cui 10191 | JQ001853 | JQ001845 | KF051047 | KF181141 | [5] |
| P. substraminea | Dai 10781 | KF495007 | KF495018 | KF494995 | KF494983 | [5] |
| Perenniporiella chaquenia | MUCL 47647 | FJ411083 | FJ393855 | HM467609 | [28] | |
| P. chaquenia | MUCL 47648 | FJ411084 | FJ393856 | HM467610 | [28] | |
| P. micropora | MUCL43581 | FJ411086 | FJ393858 | HM467608 | [28] | |
| P. neofulva | MUCL 45091 | FJ411080 | FJ393852 | HM467599 | [28] | |
| P. pendula | MUCL 46034 | FJ411082 | FJ393853 | HM467601 | [28] | |
| Phanerochaete chrysosporium | BKM-F-1767 | HQ188436 | GQ470643 | [37] | ||
| Phlebia unica | KHL 11786 | EU118657 | EU118657 | [37] | ||
| Physisporinus sanguinolentus | BRNM 699576 | FJ496671 | FJ496725 | [37] | ||
| Piloporia sajanensis | Mannine 2733a | HQ659239 | HQ659239 | [37] | ||
| Podoscypha venustula | CBS 65684 | JN649367 | JN649367 | [23] | ||
| Polyporus tuberaster | CulTENN 8976 | AF516598 | AJ488116 | [40] | ||
| Poriella subacida | Dai 8224 | HQ876605 | JF713024 | KF218322 | KF286328 | [15] |
| P. subacida | Cui 3643 | FJ613655 | AY336753 | KF218320 | KF286326 | [15] |
| P. subacida | Cui 10053 | KF495006 | KF495017 | KF218321 | KF286327 | [15] |
| P. subacida | MUCL 31402 | FJ411103 | FJ393880 | [28] | ||
| P. subacida | CBS 463.50 | FJ805245 | Direct submission | |||
| P. subacida | DLL 2009-125 | JQ673136 | [41] | |||
| P. subacida | DLL 2009-150 | JQ673014 | [41] | |||
| P. subacida | DLL 2009-154 | JQ673015 | [41] | |||
| P. subacida | Dai 8859 | FJ613656 | [15] | |||
| P. subacida | HHb-14877-T | AY089739 | AY089739 | Direct submission | ||
| P. subacida | B 37 | AF218403 | [42] | |||
| Postia alni | X 1400 | KC595932 | KC595932 | [23] | ||
| P. caesia | CIEFAP 174 | JX090109 | JX090129 | [23] | ||
| P. guttulata | KHL 11739 | EU11865 | EU11865 | [43] | ||
| P. venata | CIEFAP 346 | JX090113 | JX090133 | [43] | ||
| P. lactea | X 1391 | KC595939 | KC595939 | [23] | ||
| Pyrofomes demidoffii | MUCL 41034 | FJ411105 | FJ393873 | [28] | ||
| Sebipora aquosa | Miettinen 8680 | HQ659240 | HQ659240 | [22] | ||
| Skeletocutis amorpha | Miettinen 11038 | FN907913 | FN907913 | [37] | ||
| Stereum hirsutum | NBRC 6520 | AB733150 | AB733325 | [23] | ||
| Trametes elegans | FP105679 | JN048766 | JN048785 | [15] | ||
| T. hirsuta | Cui 7784 | JN048768 | JN048787 | [15] | ||
| T. hirsuta | RLG5133T | JN164854 | JN164801 | AF042154 | JN164891 | [38] |
| T. pubescens | PRM 900586 | AY684173 | AY855906 | [37] | ||
| Truncospora ochroleuca | Dai 11486 | HQ654105 | JF706349 | KF051048 | KF181142 | [5] |
| T. ochroleuca | MUCL 39726 | FJ411098 | FJ393865 | [28] | ||
| T. ochroleuca | Cui 5671 | JX941584 | JX941602 | KF218309 | KF286315 | [5] |
| T. ochroleuca | Cui 5673 | JX941585 | JX941603 | KF218308 | KF286314 | [5] |
| T. ornata | Cui 5714 | HQ654103 | HQ654116 | KF051056 | KF181150 | [5] |
| T. ohiensis | MUCL 41036 | FJ411096 | FJ393863 | [28] | ||
| Tyromyces chioneus | Cui 10225 | KF698745 | KF698756 | [5] | ||
| T. kmetii | Penttila 13474 | KF705040 | KF705041 | [5] | ||
| Vanderbylia delavayi | Dai 6891 | JQ861738 | KF495019 | KF218287 | KF286293 | [5] |
| V. fraxinea | DP 83 | AM269789 | AM269853 | [28] | ||
| V. fraxinea | Cui 7154 | HQ654095 | HQ654110 | KF218288 | KF286294 | [5] |
| V. fraxinea | Cui 8885 | HQ876611 | JF706344 | KF218289 | KF286295 | [5] |
| V. fraxinea | Cui 8871 | JF706329 | JF706345 | KF051050 | KF181144 | [5] |
| V. robiniophila | Cui 5644 | HQ876609 | JF706342 | KF051051 | KF181145 | [5] |
| V. vicina | MUCL 44779 | FJ411095 | AF518666 | [28] | ||
| Yuchengia narymica | Dai 7050 | JN048776 | JN048795 | KF051053 | KF181147 | [39] |
| Y. narymica | Dai 10510 | HQ654101 | JF706346 | KF051054 | KF181148 | [39] |
| Y. narymica | Dai 6998 | JN048775 | JN048794 | KF051055 | KF181149 | [5] |
| Y. narymica | 0709/42 | JN641258 | JN641265 | [39] | ||
| Y. narymica | 0709/157 | JN641259 | JN641266 | [39] | ||
| Y. narymica | 0809/3 | JN641261 | JN641268 | KF051049 | KF181143 | [39] |
| Genera | Hyphal System | Basidiospore Morphology | Chemical Reactions | Reference | |
|---|---|---|---|---|---|
| Skeletal Hyphae | Basidiospores | ||||
| Perenniporia s.s. | dimitic | ellipsoid, truncate or not | dextrinoid or not, CB+ | variable dextrinoid, CB+ | [13] |
| Perenniporiopsis | trimitic | oblong-ellipsoid, truncate | dextrinoid, CB– in the context, CB+ in the trama | dextrinoid, CB+ | [6] |
| Perenniporiella | dimitic | globose to subglobose, non-truncate | non-dextrinoid to strongly dextrinoid, slightly to distinctly cyanophilous | slightly dextrinoid, CB+ | [51] |
| Poriella subacida | di-trimitic | ellipsoid, non-truncate | strongly dextrinoid, CB+ | non-dextrinoid, CB+ | This study |
| Yuchengia narymica | dimitic | ellipsoid, non-truncate | amyloid, CB– | IKI–, CB+ | [38] |
| Specimens | Locality | Basidiospores (µm) | Average | Q | Pores/mm | Substrate |
|---|---|---|---|---|---|---|
| BPI 844697 (Type) | USA, NY | (4.2–)4.4–5.7(–6.2) × (3.1–)3.5–4.3(–4.5) | 5.2 × 3.9 | 1.35 | 4–5 | on Tsuga Carr. |
| BPI 844698 | USA, NY | (4.7–)5–6(–6.4) × (3.6–)3.9–4.6(–4.8) | 5.4 × 4.1 | 1.36 | 4–5 | on Tsuga Carr. |
| BPI 844699 | USA, NY | (4.3–)4.5–5.7(–6.2) × (3.2–)3.6–4.4(–4.6) | 5.2 × 3.9 | 1.35 | 4–5 | on Tsuga Carr. |
| BPI 885858 | USA, NY | (4.9–)5.1–6.2(–6.5) × (3.4–)3.7–4.6(–4.8) | 5.6 × 4.2 | 1.3 | 4–5 | on Tsuga Carr. |
| FH0053717 | USA, NY | (4.8–)5–5.6(–5.8) × (3.7–)3.9–4.5(–4.7) | 5.3 × 4.2 | 1.33 | 4–5 | on the log of Picea asperata Mast. |
| Hesler 11907 | USA, TN | (4.4–)4.6–5.3(–5.5) × (3.5–)3.7–4.3(–4.6) | 4.9 × 4 | 1.2 | 4–5 | on the log of Pinus Linn |
| 00605389 | USA, KS | (4.1–)4.5–5.1(–5.3) × (3–)3.4–4.5(–4.8) | 4.8 × 3.8 | 1.3 | 4–6 | on underside of old log |
| Lowe 9407 | USA, AZ | (4.6–)4.9–5.8(–6) × (3.9–)4.3–4.9(–5.1) | 5.4 × 4.5 | 1.2 | 4–6 | on the log of Picea asperata Mast. |
| 00605344 | USA, ME | (4.3–)4.6–5.2(–5.4) × (3.4–)3.6–4.2(–4.6) | 4.9 × 3.9 | 1.2 | 4–5 | on fallen Populus L. log |
| 00605372 | Canada, Ontario | (4.4–)4.6–5.8(–6) × (3–)3.3–4.2(–4.5) | 5.3 × 3.8 | 1.2 | 4–6 | on fallen trunk of Abies Mill |
| Cui 9849 | China, Heilongjiang | (4.9–)5.2–5.7(–5.9) × (3.9–)4.1–4.4(–4.6) | 5.4 × 4.2 | 1.3 | 4–5 | on fallen trunk of Picea Dietr. |
| Yuan 3854 | China, Yunnan | (5–)5.2–5.8(–6) × (3.9–)4.1–4.4(–4.5) | 5.6 × 4.2 | 1.3 | 4–6 | on angiosperm trunk |
| Cui 2712 | China, Zhejiang | (4.9–)5.1–5.7(–6) × (3.9–)4–4.4(–4.6) | 5.4 × 4.2 | 1.3 | 4–5 | on fallen trunk of Picea Dietr. |
| Dai 2648 | Finland, Pohjanmaa | (4.3–)4.7–5.7(–6.1) × (3–)3.3–3.9(–4.1) | 5.3 × 3.6 | 1.3 | 4–6 | on fallen trunk of Picea Dietr. |
| Dai 12619 | Finland, Pohjanmaa | (4.5–)4.6–5.8(–6.4) × (3.1–)3.5–4.1(–4.6) | 5.2 × 3.8 | 1.3 | 4–5 | on Picea Dietr. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Karunarathna, S.C.; Zhao, C.-L. Poriella subacida Gen. & Comb Nov. for Perenniporia subacida (Peck) Donk. Agronomy 2021, 11, 1308. https://doi.org/10.3390/agronomy11071308
Chen R, Karunarathna SC, Zhao C-L. Poriella subacida Gen. & Comb Nov. for Perenniporia subacida (Peck) Donk. Agronomy. 2021; 11(7):1308. https://doi.org/10.3390/agronomy11071308
Chicago/Turabian StyleChen, Rui, Samantha C. Karunarathna, and Chang-Lin Zhao. 2021. "Poriella subacida Gen. & Comb Nov. for Perenniporia subacida (Peck) Donk" Agronomy 11, no. 7: 1308. https://doi.org/10.3390/agronomy11071308
APA StyleChen, R., Karunarathna, S. C., & Zhao, C.-L. (2021). Poriella subacida Gen. & Comb Nov. for Perenniporia subacida (Peck) Donk. Agronomy, 11(7), 1308. https://doi.org/10.3390/agronomy11071308








