Decreasing Defoliation Frequency Enhances Bromus valdivianus Phil. Growth under Low Soil Water Levels and Interspecific Competition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Experimental Design
2.2. Experimental Stages
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Tiller Components
3.2. Plant Morphological Traits at Final Harvest
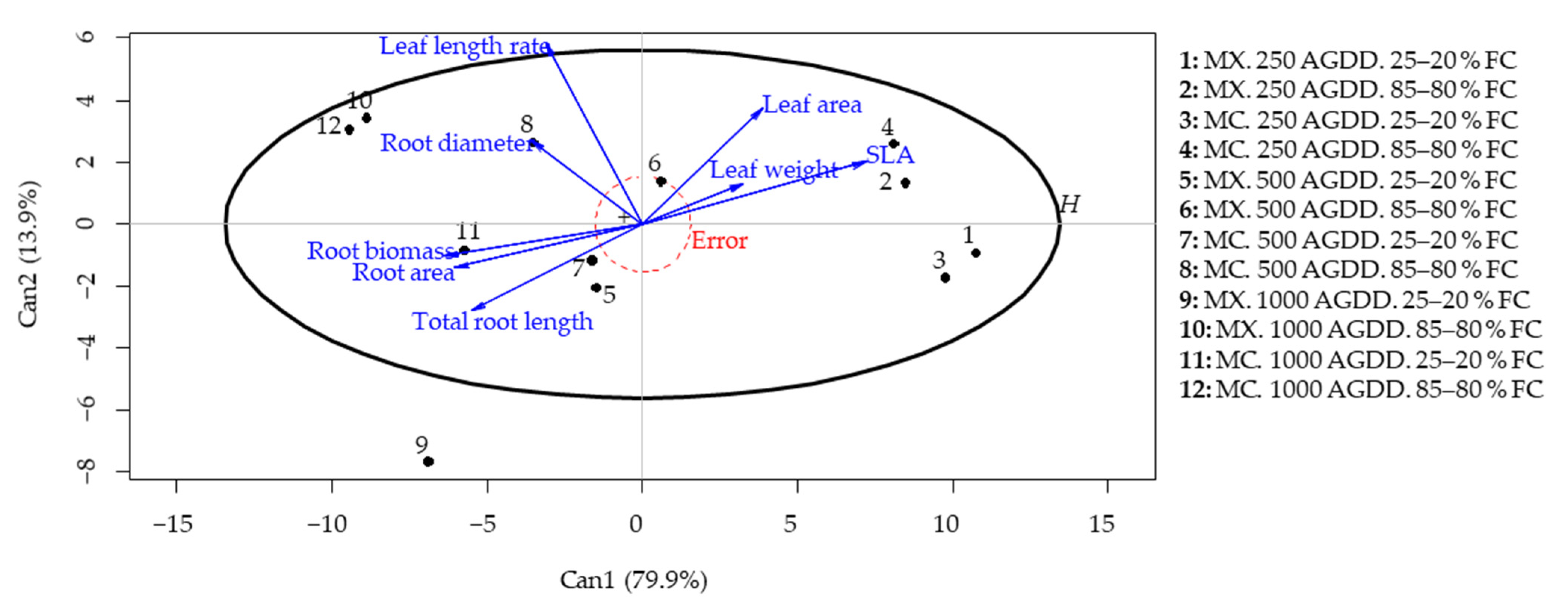
3.3. Effects on Plant Growth Morphology under Different Defoliation Frequencies
3.4. Changes in Stubble WSC, Starch and Leaf MDA Concentration
4. Discussion
4.1. Tiller Components
4.2. Plant Morphological Traits at Final Harvest and Effects on the Plant Growth Morphology under Different Defoliation Frequencies
4.3. Changes in Stubble Water Soluble Carbohydrate, Starch and Leaf MDA Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, D.J.; Newman, J.A. Grasslands and climate change: An overview. In Grasslands and Climate Change; Gibson, D.J., Newman, J.A., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 3–18. [Google Scholar]
- Diaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I. Plant trait responses to grazing–A global synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Tilman, D. Functional diversity. In Encyclopaedia of Biodiversity; Levin, S.A., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 3, pp. 109–120. [Google Scholar]
- Sanderson, M.A. Stability of production and plant species diversity in managed grasslands: A retrospective study. Basic Appl. Ecol. 2010, 11, 216–224. [Google Scholar] [CrossRef]
- Proulx, R.; Wirth, C.; Voigt, W.; Weigelt, A.; Roscher, C.; Attinger, S.; Baade, J.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Diversity promotes temporal stability across levels of ecosystem organization in experimental grasslands. PLoS ONE 2010, 5, e13382. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Fort, F.; Jouany, C.; Cruz, P. Root and leaf functional trait relations in Poaceae species: Implications of differing resource-acquisition strategies. J. Plant Ecol. 2013, 6, 211–219. [Google Scholar] [CrossRef]
- Turner, N. Adaptation to Water Deficits: A Changing Perspective. Funct. Plant Biol. 1986, 13, 175–190. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species; Springer: New York, NY, USA, 2014. [Google Scholar]
- Clement, A.; Dalley, D.; Chapman, D.; Edwards, G.; Bryant, R. Effect of grazing system on nitrogen partitioning in lactating dairy cows grazing irrigated pastures in Canterbury, New Zealand. Proc. N. Z. Soc. Anim. Prod. 2016, 76, 94–99. [Google Scholar]
- Kemp, P.D.; López, I.F. Hill country pastures in the southern North Island of New Zealand: An overview. NZGA Res. Pract. Ser. 2016, 16, 289–297. [Google Scholar] [CrossRef]
- Hofer, D.; Suter, M.; Haughey, E.; Finn, J.A.; Hoekstra, N.J.; Buchmann, N.; Lüscher, A. Yield of temperate forage grassland species is either largely resistant or resilient to experimental summer drought. J. Appl. Ecol. 2016, 53, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Henessy, K.; Fitzharris, B.; Bates, N.; Harvey, S.; Howden, M.; Hughes, L.; Salinger, J.; Warrick, R. Australia and New Zealand. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contributions of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambrige, UK, 2007; pp. 507–540. [Google Scholar]
- Woodward, S.J.R.; Van Oijen, M.; Griffiths, W.M.; Beukes, P.C.; Chapman, D.F. Identifying causes of low persistence of perennial ryegrass (Lolium perenne) dairy pasture using the Basic Grassland model (BASGRA). Grass Forage Sci. 2020, 75, 45–63. [Google Scholar] [CrossRef]
- Teughels, H.; Nijs, I.; Van Hecke, P.; Impens, I. Competition in a global change environment: The importance of different plant traits for competitive success. J. Biogeogr. 1995, 22, 297–305. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Zhang, J.; Song, H.; Liang, Q.; Tao, J.; Cornelissen, J.H.C.; Liu, J. Do shallow soil, low water availability, or their combination increase the competition between grasses with different root systems in karst soil? Environ. Sci. Pollut. Res. 2017, 24, 10640–10651. [Google Scholar] [CrossRef]
- Lopez, I.; Balocchi, O.; Lailhacar, P.; Oyarzun, C. Characterization of the growing sites of six native and naturalized pasture species in the Dominio Humedo of Chile. Agro Sur 1997, 25, 62–80. [Google Scholar] [CrossRef]
- Ramírez, M.; Keim, J.P.; López, I.F.; Balocchi, O. Vegetational dynamics of sown pastures with native and naturalized species with and without fertilizer application. Agro. Sur. 2014, 42, 3–14. [Google Scholar] [CrossRef]
- Calvache, I.; Balocchi, O.; Alonso, M.; Keim, J.P.F.; López, I.F. Thermal Time as a Parameter to Determine Optimal Defoliation Frequency of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.). Agronomy 2020, 10, 620. [Google Scholar] [CrossRef]
- López, I.F.; Kemp, P.D.; Dörner, J.; Descalzi, C.A.; Balocchi, O.A.; García, S. Competitive Strategies and Growth of Neighbouring Bromus valdivianus Phil. and Lolium perenne L. Plants Under Water Restriction. J. Agron. Crop Sci. 2013, 199, 449–459. [Google Scholar] [CrossRef]
- Keim, J.P.; López, I.F.; Balocchi, O.A. Sward herbage accumulation and nutritive value as affected by pasture renovation strategy. Grass Forage Sci. 2015, 70, 283–295. [Google Scholar] [CrossRef]
- Ordoñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J.; Herrmann, P.; Hernández, F.; Bhatia, S. Pasture brome (Bromus valdivianus) leaf growth physiology: A six-leaf grass species. Agron. N. Z. 2017, 47, 13–22. [Google Scholar]
- Turner, L.R.; Donaghy, D.J.; Lane, P.A.; Rawnsley, R.P. Effect of defoliation management, based on leaf stage, on perennial ryegrass (Lolium perenne L.), prairie grass (Bromus willdenowii Kunth.) and cocksfoot (Dactylis glomerata L.) under dryland conditions. 1. Regrowth, tillering and water-soluble carbohydrate concentration. Grass Forage Sci. 2006, 61, 164–174. [Google Scholar]
- Fulkerson, W.J.; Donaghy, D.J. Plant-soluble carbohydrate reserves and senescence—Key criteria for developing an effective grazing management system for ryegrass-based pastures: A review. Aust. J. Exp. Agric. 2001, 41, 261–275. [Google Scholar] [CrossRef]
- Turner, L.R.; Donaghy, D.J.; Lane, P.A.; Rawnsley, R.P. Effect of defoliation management, based on leaf stage, on perennial ryegrass (Lolium perenne L.), prairie grass (Bromus willdenowii Kunth.) and cocksfoot (Dactylis glomerata L.) under dryland conditions. 2. Nutritive value. Grass Forage Sci. 2006, 61, 175–181. [Google Scholar] [CrossRef]
- Harris, W.; Forde, B.; Hardacre, A. Temperature and cutting effects on the growth and competitive interaction of ryegrass and paspalum: 1. Dry matter production, tiller numbers, and light interception. N. Z. J. Agric. Res. 1981, 24, 299–307. [Google Scholar] [CrossRef]
- Tow, P.; Lazenby, A. Competition and Succession in Pastures; CABI: Wallingford, UK, 2000. [Google Scholar]
- Sanderson, M.A.; Skinner, R.H.; Barker, D.J.; Edwards, G.R.; Tracy, B.F.; Wedin, D.A. Plant Species Diversity and Management of Temperate Forage and Grazing Land Ecosystem. Crop Sci. 2004, 44, 1132–1144. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Fulkerson, W.; Slack, K. Leaf number as a criterion for determining defoliation time for Lolium perenne: 2. Effect of defoliation frequency and height. Grass Forage Sci. 1995, 50, 16–20. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: https://www.r-project.org/ (accessed on 3 June 2019).
- López, I.F.; Lambert, M.G.; Mackay, A.D.; Valentine, I. The influence of topography and pasture management on soil characteristics and herbage accumulation in hill pasture in the North Island of New Zealand. Plant Soil 2003, 255, 421–434. [Google Scholar] [CrossRef]
- Mommer, L.; Van Ruijven, J.; De Caluwe, H.; Smit-Tiekstra, A.E.; Wagemaker, C.A.M.; Joop Ouborg, N.; Bögemann, G.M.; Van Der Weerden, G.M.; Berendse, F.; De Kroon, H. Unveiling below-ground species abundance in a biodiversity experiment: A test of vertical niche differentiation among grassland species. J. Ecol. 2010, 98, 1117–1127. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Turner, L.R.; Donaghy, D.J.; Lane, P.A.; Rawnsley, R.P. Patterns of leaf and root regrowth, and allocation of water-soluble carbohydrate reserves following defoliation of plants of prairie grass (Bromus willdenowii Kunth.). Grass Forage Sci. 2007, 62, 497–506. [Google Scholar] [CrossRef]
- Chapman, D.; Lemaire, G. Morphogenetic and structural determinants of plant regrowth after defoliation. In Proceedings of the XVII International Grassland Congress; Baker, M.J., Ed.; Keeling and Mundy Ltd.: Palmerston North, New Zealand, 1993; pp. 94–104. [Google Scholar]
- Gastal, F.; Lemaire, G. Defoliation, Shoot Plasticity, Sward Structure and Herbage Utilization in Pasture: Review of the Underlying Ecophysiological Processes. Agriculture 2015, 5, 1146–1171. [Google Scholar] [CrossRef] [Green Version]
- Durand, J.-L.; Onillon, B.; Schnyder, H.; Rademacher, I. Drought effects on cellular and spatial parameters of leaf growth in tall fescue. J. Exp. Bot. 1995, 46, 1147–1155. [Google Scholar] [CrossRef]
- Durand, J.-L.; Schäufele, R.; Gastal, F. Grass leaf elongation rate as a function of developmental stage and temperature: Morphological analysis and modelling. Ann. Bot. 1999, 83, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Bartholomew, P.W.; Williams, R.D. Effects of exposure to below-freezing temperatures, soil moisture content and nitrogen application on phyllochron in cool-season grasses. Grass Forage Sci. 2006, 61, 146–153. [Google Scholar] [CrossRef]
- Descalzi, C.; Balocchi, O.; López, I.; Kemp, P.; Dörner, J. Different soil structure and water conditions affect the growing response of Lolium perenne L. and Bromus valdivianus Phil. growing alone or in mixture. J. Soil Sci. Plant Nutr. 2018, 18, 617–635. [Google Scholar] [CrossRef] [Green Version]
- Kemp, D.R.; Culvenor, R.A. Improving the grazing and drought tolerance of temperate perennial grasses. N. Z. J. Agric. Res. 2010, 37, 365–378. [Google Scholar] [CrossRef]
- Hall, R. The analysis and significance of competitive and non-competitive interference between species. In Plant Relations in Pastures; Wilson, J.R., Ed.; CSIRO: Melbourne, Australia, 1978; pp. 163–174. [Google Scholar]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and environmental regulation of root development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bristiel, P.; Roumet, C.; Violle, C.; Volaire, F. Coping with drought: Root trait variability within the perennial grass Dactylis glomerata captures a trade-off between dehydration avoidance and dehydration tolerance. Plant Soil 2018, 434, 327–342. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 2006, 224, 1080–1090. [Google Scholar] [CrossRef]
- Ogweno, J.O.; Hu, W.H.; Song, X.S.; Shi, K.; Mao, W.H.; Zhou, Y.H.; Yu, J.Q. Photoinhibition-induced reduction in photosynthesis is alleviated by abscisic acid, cytokinin and brassinosteroid in detached tomato leaves. Plant Growth Regul. 2010, 60, 175–182. [Google Scholar] [CrossRef]
- Donaghy, D.J.; Fulkerson, W.J. Priority for allocation of water-soluble carbohydrate reserves during regrowth of Lolium perenne. Grass Forage Sci. 1998, 53, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Volaire, F.; Norton, M.R.; Norton, G.M.; Lelievre, F. Seasonal patterns of growth, dehydrins and water-soluble carbohydrates in genotypes of Dactylis glomerata varying in summer dormancy. Ann. Bot. 2005, 95, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Volaire, F.; Thomas, H.; Lelièvre, F. Survival and recovery of perennial forage grasses under prolonged Mediterranean drought: I. Growth, death, water relations and solute content in herbage and stubble. New Phytol. 1998, 140, 439–449. [Google Scholar] [CrossRef]
- Smith, D. The nonstructural carbohydrates. In Chemistry and Biochemistry of Herbage; Butler, G., Bailey, R., Eds.; Academic Press: London, UK, 1973; pp. 105–155. [Google Scholar]
- Miyake, H. Starch accumulation in the bundle sheaths of C3 plants: A possible pre-condition for C4 photosynthesis. Plant Cell Physiol. 2016, 57, 890–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Leaf Area/Tiller (mm2 °Cd−1) | Leaf Weight/Tiller (mg °Cd−1) | Leaf Regrowth Stage | Specific Leaf Area (mm2 mg−1 °Cd−1) | Lamina Length Rate (mm °Cd−1) | Accumulated Leaf Length (mm) | Tillers No. plant−1 | |
|---|---|---|---|---|---|---|---|
| Pasture type | |||||||
| Bv Monoculture | 1.86 ± 0.14 a | 0.15 ± 0.01 a | 2.04 ± 0.17 a | 0.03 ± 0.01 | 0.34 ± 0.03 | 258.79 ± 21.14 | 4.38 ± 0.25 |
| Bv Mixture | 1.49 ± 0.15 b | 0.12 ± 0.01 b | 1.80 ± 0.18 b | 0.03 ± 0.01 | 0.34 ± 0.03 | 258.44 ± 24.65 | 3.92 ± 0.28 |
| Significance | * | * | * | ns | ns | ns | ns |
| Defoliation frequency (AGDD) | |||||||
| 250 | 2.13 ± 0.17 a | 0.16 ± 0.01 a | 1.15 ± 0.10 c | 0.05 ± 0.01 a | 0.30 ± 0.03 b | 210.53 ± 23.56 b | 4.28 ± 0.32 |
| 500 | 1.60 ± 0.92 b | 0.12 ± 0.01 b | 1.71 ± 0.08 b | 0.03 ± 0.01 b | 0.34 ± 0.03 ab | 290.31 ± 27.94 a | 4.00 ± 0.31 |
| 1000 | 1.30 ± 0.11 b | 0.12 ± 0.01 b | 2.84 ± 0.16 a | 0.01 ± 0.01 c | 0.39 ± 0.04 a | 275.00 ± 29.17 a | 4.16 ± 0.36 |
| Significance | *** | ** | *** | *** | ** | ** | ns |
| Water level | |||||||
| 20–25% FC | 1.39 ± 0.11 b | 0.13 ± 0.01 | 1.61 ± 0.13 b | 0.02 ± 0.01 b | 0.22 ± 0.02 b | 170.13 ± 12.95 b | 4.21 ± 0.25 |
| 80–85% FC | 1.96 ± 0.16 a | 0.14 ± 0.01 | 2.20 ± 0.19 a | 0.03 ± 0.01 a | 0.46 ± 0.02 a | 347.10 ± 14.35 a | 4.08 ± 0.28 |
| Significance | *** | ns | *** | ** | *** | *** | ns |
| Defoliation Frequency × Water level | |||||||
| 250 × 20–25% FC | 1.74 ± 0.08 | 0.15 ± 0.01 | 0.95 ± 0.14 e | 0.05 ± 0.01 | 0.18 ± 0.02 | 131.38 ± 14.26 | 4.25 ± 0.57 |
| 250 × 80–85% FC | 2.52 ± 0.26 | 0.18 ± 0.01 | 1.33 ± 0.12 d | 0.06 ± 0.01 | 0.41 ± 0.03 | 289.69 ± 9.62 | 4.17 ± 0.59 |
| 500 × 20–25% FC | 1.35 ± 0.26 | 0.12 ± 0.02 | 1.53 ± 0.10 d | 0.02 ± 0.01 | 0.24 ± 0.03 | 204.13 ± 28.03 | 3.58 ± 0.20 |
| 500 × 80–85% FC | 1.84 ± 0.24 | 0.12 ± 0.02 | 1.89 ± 0.08 c | 0.04 ± 0.01 | 0.44 ± 0.02 | 376.50 ± 20.93 | 4.08 ± 0.49 |
| 1000 × 20–25% FC | 1.08 ± 0.08 | 0.12 ± 0.01 | 2.28 ± 0.09 b | 0.01 ± 0.01 | 0.25 ± 0.02 | 174.88 ± 16.80 | 4.58 ± 0.78 |
| 1000 × 80–85% FC | 1.52 ± 0.17 | 0.12 ± 0.02 | 3.39 ± 0.13 a | 0.01 ± 0.01 | 0.53 ± 0.03 | 375.13 ± 22.35 | 3.33 ± 0.44 |
| Significance | ns | ns | ** | ns | ns | ns | ns |
| Above Ground Biomass (g DM plant−1) | Root Biomass (g DM plant−1) | Total Root Length (cm) | Root Area (cm2) | Root Diameter (mm) | Root Volume (cm3) | |
|---|---|---|---|---|---|---|
| Pasture type | ||||||
| Bv Monoculture | 1.06 ± 0.19 a | 0.36 ± 0.05 a | 2589.15 ± 374.82 | 204.73 ± 28.51 | 0.23 ± 0.01 a | 1.18 ± 0.18 |
| BV Mixture | 0.47 ± 0.06 b | 0.25 ± 0.04 b | 3135.07 ± 469.16 | 191.83 ± 29.42 | 0.20 ± 0.01 b | 0.96 ± 0.16 |
| Significance | *** | ** | ns | ns | ** | ns |
| Defoliation frequency (AGDD) | ||||||
| 250 | 0.38 ± 0.07 b | 0.14 ± 0.02 c | 1679.95 ± 191.45 c | 100.00 ± 10.91 c | 0.19 ± 0.01 | 0.49 ± 0.06 c |
| 500 | 0.85 ± 0.20 a | 0.29 ± 0.04 b | 2698.91 ± 358.41 b | 183.97 ± 27.05 b | 0.22 ± 0.01 | 1.02 ± 0.17 b |
| 1000 | 1.05 ± 0.19 a | 0.50 ± 0.05 a | 4612.46 ± 522.49 a | 310.87 ± 31.64 a | 0.22 ± 0.01 | 1.71 ± 0.17 a |
| Significance | *** | *** | *** | *** | ns | *** |
| Water level | ||||||
| 20–25% FC | 0.67 ± 0.10 | 0.30 ± 0.05 | 3018.90 ± 512.45 | 187.19 ± 31.29 | 0.20 ± 0.01 | 0.94 ± 0.16 |
| 80–85% FC | 0.86 ± 0.16 | 0.32 ± 0.04 | 2975.32 ± 316.51 | 209.37 ± 26.25 | 0.22 ± 0.01 | 1.20 ± 0.17 |
| Significance | ns | ns | ns | ns | ns | ns |
| Pasture type × Water level | ||||||
| Bv Monoculture × 20−25 FC | 0.80 ± 0.17 b | 0.31 ± 0.06 b | 2280.40 ± 457.28 c | 156.07 ± 30.85 b | 0.22 ± 0.01 | 0.86 ± 0.17 b |
| Bv Monoculture × 80−85 FC | 1.32 ± 0.24 a | 0.42 ± 0.07 a | 3437.90 ± 551.58 ab | 253.39 ± 43.71 a | 0.23 ± 0.01 | 1.51 ± 0.28 a |
| Bv Mixture × 20−25 FC | 0.54 ± 0.10 bc | 0.29 ± 0.08 b | 3757.40 ± 877.86 a | 218.13 ± 54.48 ab | 0.19 ± 0.01 | 1.02 ± 0.27 b |
| Bv Mixture × 80−85 FC | 0.39 ± 0.06 c | 0.22 ± 0.04 b | 2515.74 ± 260.81 bc | 165.36 ± 23.11 b | 0.21 ± 0.01 | 0.89 ± 0.16 b |
| Significance | * | ** | ** | ** | ns | ** |
| Defoliation frequency × Water level | ||||||
| 250 × 20–25 FC | 0.28 ± 0.06 | 0.11 ± 0.04 | 1421.85 ± 262.75 d | 81.67 ± 10.52 | 0.19 ± 0.01 | 0.38 ± 0.04 |
| 250 × 80–85 FC | 0.49 ± 0.10 | 0.17 ± 0.02 | 1938.04 ± 256.10 cd | 118.33 ± 16.70 | 0.20 ± 0.01 | 0.59 ± 0.10 |
| 500 × 20–25 FC | 0.61 ± 0.03 | 0.25 ± 0.03 | 2275.26 ± 478.84 cd | 144.87 ± 25.70 | 0.21 ± 0.01 | 0.74 ± 0.11 |
| 500 × 80–85 FC | 1.10 ± 0.38 | 0.33 ± 0.08 | 3122.56 ± 513.95 bc | 223.08 ± 44.14 | 0.22 ± 0.02 | 1.30 ± 0.30 |
| 1000 × 20–25 FC | 1.12 ± 0.18 | 0.54 ± 0.05 | 5359.58 ± 814.73 a | 335.02 ± 47.56 | 0.21 ± 0.01 | 1.69 ± 0.24 |
| 1000 × 80–85 FC | 0.98 ± 0.28 | 0.45 ± 0.08 | 3865.35 ± 560.47 b | 286.72 ± 43.71 | 0.24 ± 0.01 | 1.72 ± 0.71 |
| Significance | ns | ns | * | ns | ns | ns |
| WSC (g kg−1 DM) | WSC (mg tiller−1) | Starch (g kg−1 DM) | Starch (mg tiller−1) | MDA (µmol L−1) | |
|---|---|---|---|---|---|
| Pasture type | |||||
| Bv Monoculture | 58.49 ± 6.49 a | 5.54 ± 1.18 a | 1.57 ± 0.06 | 0.14 ± 0.02 a | 2.45 ± 0.26 |
| Bv Mixture | 52.49 ± 4.95 b | 4.17 ± 0.71 b | 1.66 ± 0.08 | 0.11 ± 0.01 b | 2.86 ± 0.29 |
| Significance | *** | *** | ns | *** | ns |
| Defoliation frequency | |||||
| 250 | 39.92 ± 4.50 c | 1.86 ± 0.30 c | 1.50 ± 0.04 b | 0.07 ± 0.01 c | 3.65 ± 0.6 a |
| 500 | 44.93 ± 4.11 b | 3.07 ± 0.41 b | 1.56 ± 0.07 ab | 0.10 ± 0.01 b | 2.14 ± 0.14 b |
| 1000 | 83.73 ± 3.51 a | 10.00 ± 0.83 a | 1.78 ± 0.12 a | 0.21 ± 0.02 a | 2.35 ± 0.32 b |
| Significance | *** | *** | * | *** | *** |
| Water level | |||||
| 20–25% FC | 64.59 ± 3.98 a | 5.68 ± 0.91 a | 1.54 ± 0.05 | 0.13 ± 0.01 | 3.12 ± 0.26 a |
| 80–85% FC | 46.74 ± 6.31 b | 4.04 ± 0.99 b | 1.68 ± 0.08 | 0.12 ± 0.02 | 2.11 ± 0.25 b |
| Significance | *** | *** | ns | ns | *** |
| Defoliation frequency × Water level | |||||
| 250 × 20–25% FC | 53.96 ± 1.85 c | 2.78 ± 0.20 | 1.58 ± 0.07 b | 0.08 ± 0.01 d | 4.40 ± 0.49 |
| 250 × 80–85 % FC | 25.87 ± 2.63 e | 0.94 ± 0.12 | 1.41 ± 0.03 b | 0.05 ± 0.01 e | 2.90 ± 0.24 |
| 500 × 20–25% FC | 56.43 ± 3.59 c | 4.29 ± 0.32 | 1.49 ± 0.07 b | 0.11 ± 0.01 c | 2.28 ± 0.25 |
| 500 × 80–85% FC | 33.44 ± 2.92 d | 1.85 ± 0.20 | 1.63 ± 0.12 b | 0.09 ± 0.01 d | 2.01 ± 0.16 |
| 1000 × 20–25% FC | 87.13 ± 3.10 a | 10.80 ± 1.14 | 1.54 ± 0.15 b | 0.19 ± 0.03 b | 2.88 ± 0.25 |
| 1000 × 80–85% FC | 80.90 ± 5.94 b | 9.34 ± 1.21 | 1.98 ± 0.13 a | 0.22 ± 0.02 a | 1.55 ± 0.58 |
| Significance | *** | ns | * | *** | ns |
| Pasture type × Defoliation frequency | |||||
| Bv Monoculture × 250 | 41.63 ± 6.38 cd | 2.04 ± 0.45 d | 1.45 ± 0.06 b | 0.07 ± 0.01 d | 3.49 ± 0.49 |
| Bv Monoculture × 500 | 46.07 ± 6.85 c | 3.22 ± 0.59 c | 1.56 ± 0.08 b | 0.11 ± 0.01 c | 2.24 ± 0.14 |
| Bv Monoculture × 1000 | 93.63 ± 1.40 a | 12.53 ± 0.70 a | 2.02 ± 0.14 a | 0.27 ± 0.01 a | 1.97 ± 0.45 |
| Bv Mixture × 250 | 38.20 ± 6.88 d | 1.69 ± 0.43 d | 1.54 ± 0.06 b | 0.06 ± 0.01 d | 3.81 ± 0.57 |
| Bv Mixture × 500 | 43.79 ± 5.19 cd | 2.93 ± 0.62 c | 1.57 ± 0.12 b | 0.10 ± 0.01 c | 2.01 ± 0.28 |
| Bv Mixture × 1000 | 75.49 ± 3.74 b | 7.90 ± 0.50 b | 1.58 ± 0.14 b | 0.16 ± 0.01 b | 2.79 ± 0.42 |
| Significance | *** | *** | * | *** | ns |
| Pasture type × Defoliation frequency × Water level | |||||
| Bv Monoculture × 250 × 20–25 | 55.25 ± 1.83 d | 3.01 ± 0.13 | 1.51 ± 0.11 | 0.08 ± 0.01 | 3.95 ± 0.62 |
| Bv Monoculture × 250 × 80–85 | 28.02 ± 3.87 ef | 1.06 ± 0.21 | 1.39 ± 0.06 | 0.05 ± 0.01 | 2.80 ± 0.64 |
| Bv Monoculture × 500 × 20–25 | 60.55 ± 2.84 cd | 4.5 ± 0.25 | 1.58 ± 0.1 | 0.12 ± 0.01 | 2.33 ± 0.16 |
| Bv Monoculture × 500 × 80–85 | 31.59 ± 4.11 ef | 1.93 ± 0.13 | 1.53 ± 0.14 | 0.09 ± 0.1 | 2.16 ± 0.24 |
| Bv Monoculture × 1000 × 20–25 | 93.89 ± 0.58 a | 13.52 ± 0.84 | 1.83 ± 0.07 | 0.26 ± 0.01 | 2.77 ± 0.35 |
| Bv Monoculture × 1000 × 80–85 | 93.46 ± 2.53 a | 11.86 ± 0.92 | 2.15 ± 0.19 | 0.27 ± 0.01 | 0.62 ± 0.03 |
| Bv Mixture × 250 × 20–25 | 52.68 ± 3.48 d | 2.56 ± 0.37 | 1.65 ± 0.08 | 0.08 ± 0.01 | 5.09 ± 0.64 |
| Bv Mixture × 250 × 80–85 | 23.72 ± 3.85 f | 0.81 ± 0.11 | 1.43 ± 0.02 | 0.05 ± 0.01 | 2.96 ± 0.24 |
| Bv Mixture × 500 × 20–25 | 52.31 ± 6.29 d | 4.09 ± 0.65 | 1.41 ± 0.07 | 0.11 ± 0.01 | 2.21 ± 0.74 |
| Bv Mixture × 500 × 80–85 | 35.28 ± 4.74 e | 1.77 ± 0.42 | 1.73 ± 0.2 | 0.08 ± 0.01 | 1.87 ± 0.23 |
| Bv Mixture × 1000 × 20–25 | 82.63 ± 2.55 b | 8.99 ± 0.12 | 1.35 ± 0.16 | 0.15 ± 0.02 | 3.02 ± 0.39 |
| Bv Mixture × 1000 × 80–85 | 68.35 ± 3.52 c | 6.81 ± 0.26 | 1.81 ± 0.15 | 0.18 ± 0.01 | 2.48 ± 0.91 |
| Significance | * | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Favre, J.; Zhang, Y.; López, I.F.; Donaghy, D.J.; Cranston, L.M.; Kemp, P.D. Decreasing Defoliation Frequency Enhances Bromus valdivianus Phil. Growth under Low Soil Water Levels and Interspecific Competition. Agronomy 2021, 11, 1333. https://doi.org/10.3390/agronomy11071333
García-Favre J, Zhang Y, López IF, Donaghy DJ, Cranston LM, Kemp PD. Decreasing Defoliation Frequency Enhances Bromus valdivianus Phil. Growth under Low Soil Water Levels and Interspecific Competition. Agronomy. 2021; 11(7):1333. https://doi.org/10.3390/agronomy11071333
Chicago/Turabian StyleGarcía-Favre, Javier, Yongmei Zhang, Ignacio F. López, Daniel J. Donaghy, Lydia M. Cranston, and Peter D. Kemp. 2021. "Decreasing Defoliation Frequency Enhances Bromus valdivianus Phil. Growth under Low Soil Water Levels and Interspecific Competition" Agronomy 11, no. 7: 1333. https://doi.org/10.3390/agronomy11071333






