The Effect of Alfalfa Mineral Fertilization and Times of Soil Sampling on Enzymatic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Treatments
2.2. Soil Sampling and Analyses
2.3. Biochemical Index of Soil Fertility
2.4. Weather Conditions
2.5. Statistical Analysis
3. Results
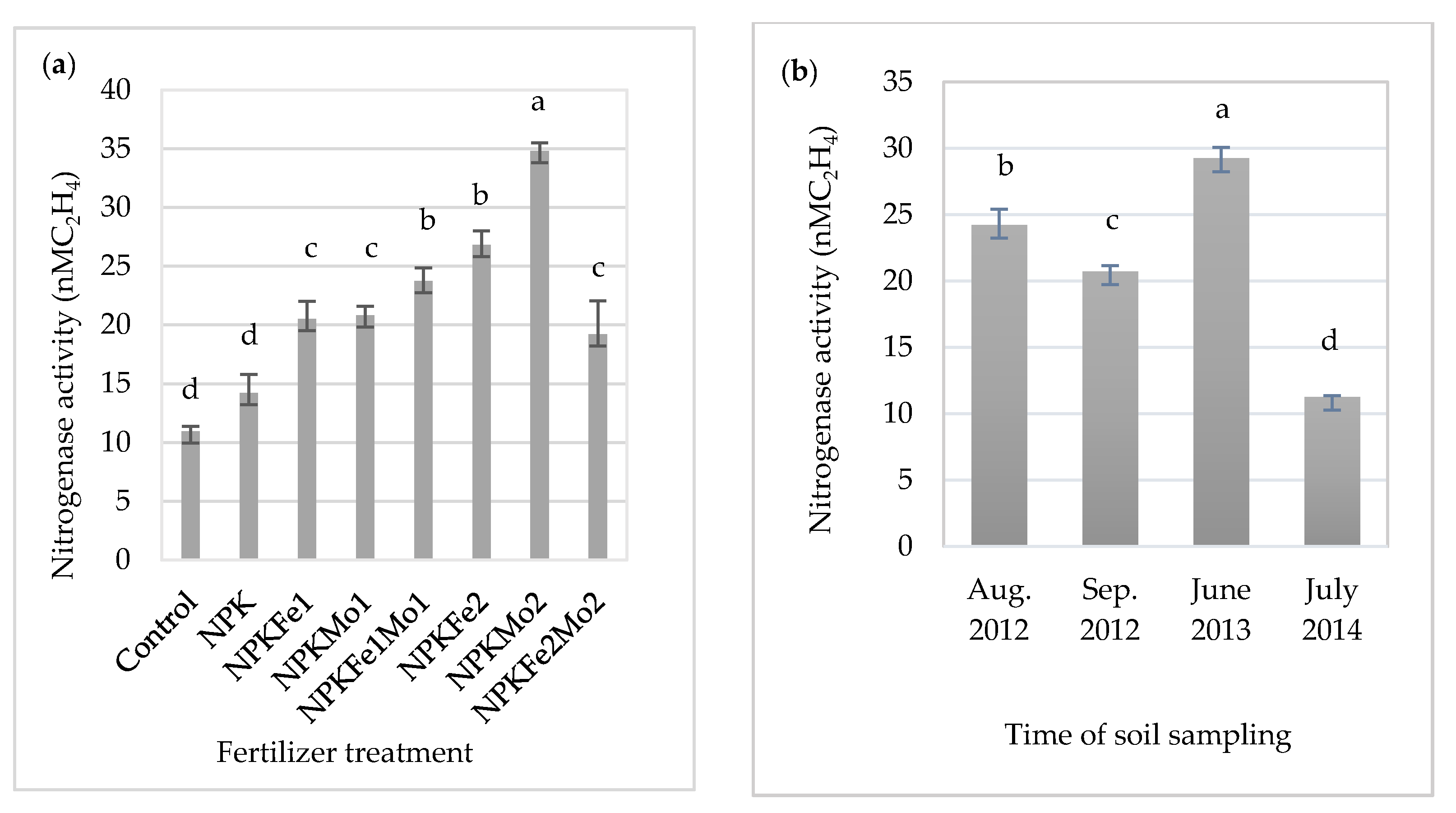
3.1. Nitrogenase Activity
3.2. Urease Activity
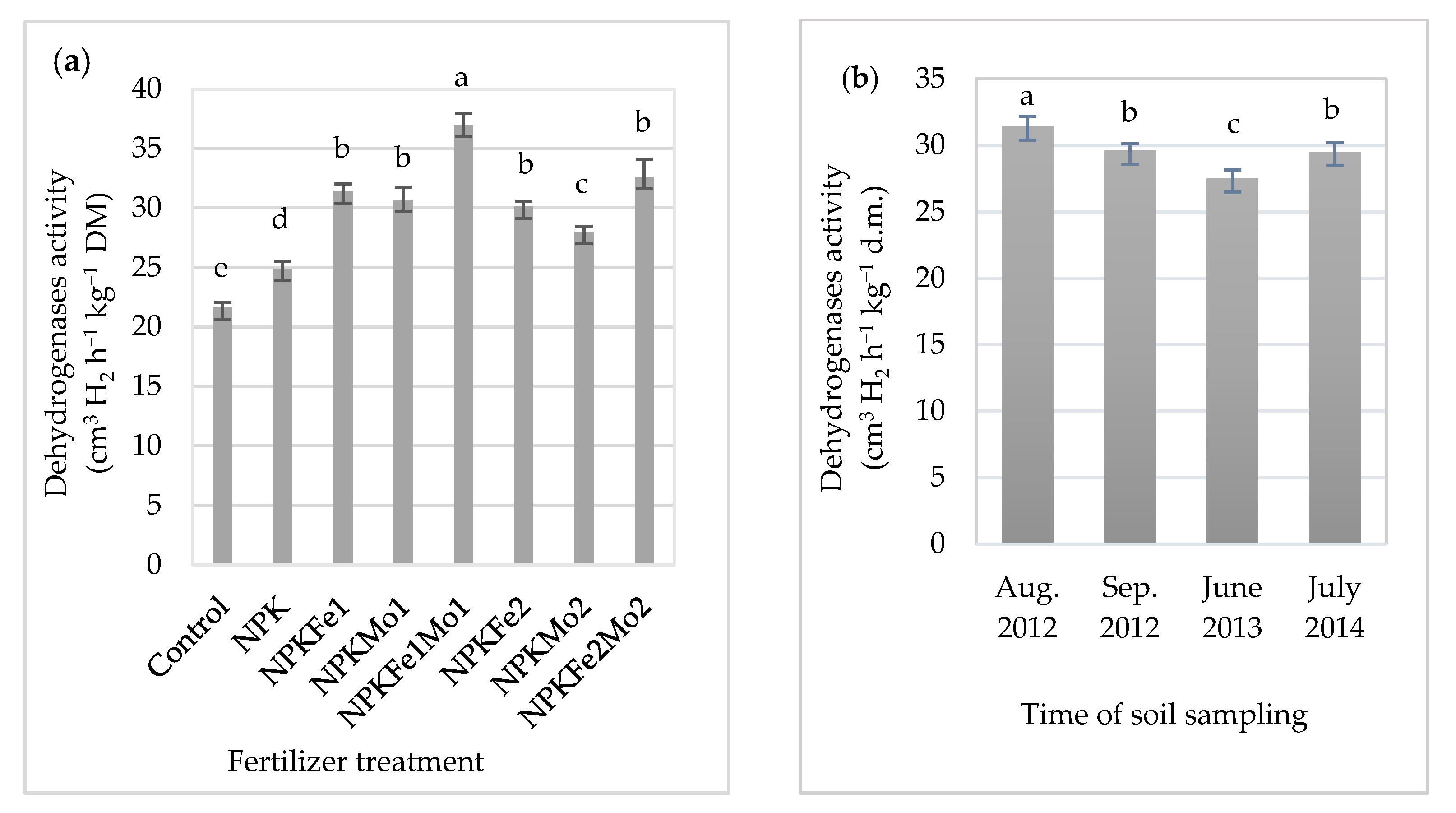
3.3. Dehydrogenases Activity
3.4. Alkaline Phosphatase Activity
3.5. Acid Phosphatase Activity
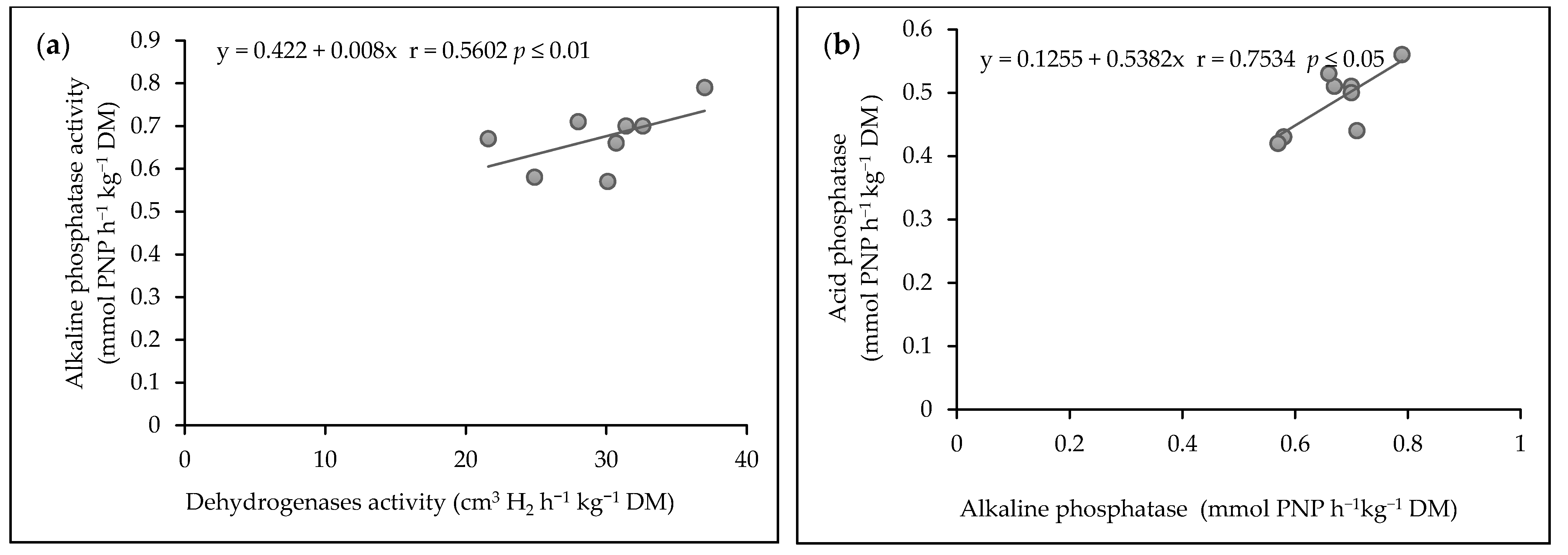
3.6. Correlation between Soil Enzyme Activities
3.7. Total Organic Carbon in Soil
3.8. Biochemical Index (BCHI) of Soil Fertility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adetunji, A.T.; Ncube, B.; Meyer, A.H.; Olatunji, O.S.; Mulidzi, R.; Lewu, F.B. Soil pH, nitrogen, phosphatase and urease activities in reponse to cover crop species, termination stage and termination method. Heliyon 2021, 7, eo5980. [Google Scholar] [CrossRef] [PubMed]
- Faligowska, A.; Szymańska, G.; Panasiewicz, K.; Szukała, J.; Koziara, W.; Ratajczak, K. The long-term effect of legumes as forecrops on the productivity of rotation (winter rape-winter wheat-winter wheat) with nitrogen fertilization. Plant Soil Environ. 2019, 65, 138–144. [Google Scholar] [CrossRef]
- Yang, L.; Li, T.; Li, F.; Lemcoff, J.H.; Cohen, S. Fertilization regulates soil enzymatic activity and fertility dynamics in cucumber field. Sci. Horticult. 2008, 116, 21–26. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Spychaj-Fabisiak, E.; Pior, N. Prediction of molybdenum availability to plants in differentiated soil conditions. Plant Soil Environ. 2017, 63, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, P.; Li, T.; Chen, Y.; Ying, X.; Liu, S. Tee effect of two organic manures on soil properties and crop yields on a temperate calcareous soil under a wheat-maize cropping system. Eur. J. Agron. 2009, 31, 36–42. [Google Scholar] [CrossRef]
- Kalembasa, S.; Symanowicz, B. Quantitative abilities of biological nitrogen reduction for Rhizobium galegae cultures by goat’s rue. Ecol. Chem. Eng. 2010, 17, 757–764. [Google Scholar]
- Shao, X.; Zheng, J. Soil organic carbon, black carbon, and enzyme activity under long-term fertilization. J. Integr. Agric. 2014, 13, 517–524. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Gryta, A. Forecrop effects abundance and diversity of soil microorganisms during the growth of the subsequent crop. Agronomy 2020, 10, 1971. [Google Scholar] [CrossRef]
- Symanowicz, B.; Kalembasa, S.; Niedbała, M.; Toczko, M.; Skwarek, K. Fertilisation of pea (Pisum sativum L.) with nitrogen and potassium and its effect on soil enzymatic activity. J. Elem. 2018, 23, 57–67. [Google Scholar] [CrossRef]
- Harasim, E.; Antonkiewicz, J.; Kwiatkowski, C.A. The effects of catch crops and tillage systems on selected physical properties and enzymatic activity of loess soil in a spring wheat monoculture. Agronomy 2020, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Mndzebele, B.; Ncube, B.; Fessehazion, M.; Mabhaudhi, T.; Amoo, S.; Plooy, C.; Venter, S.; Modi, A. Effects of cowpea-amaranth intercropping and fertilizer application on soil phosphatase activities, available soil phosphorus, and crop growth response. Agronomy 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Garbuz, S.A.; Yaroslavtseva, N.V.; Kholodov, V.A. Enzymatic activity inside and outside of water-stable aggregates in soil under different land use. Eurasian Soil Sci. 2016, 49, 367–375. [Google Scholar] [CrossRef]
- Swędrzyńska, D.; Grześ, S. Microbiological parameters of soil under sugar beet as a response to the long-term application of different tillage systems. Pol. J. Environ. Stud. 2015, 24, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Niu, D.; Kang, J.; Zhou, Y.; Li, X. Effects of livestock exclusion on soil physical and biochemical properties of a desert rangeland. Pol. J. Environ. Stud. 2015, 24, 2587–2595. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Wilczewski, E. Soil phosphatase activity and phosphorus content as influenced by catch crops cultivated as green manure. Pol. J. Environ. Stud. 2014, 23, 157–165. [Google Scholar]
- Lauber, C.I.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. App. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radulov, I.; Berbecea, A.; Sala, F.; Crista, F.; Lato, A. Mineral fertilization influence on soil pH, cationic exchange capacity and nutrient content. Res. J. Agric. Sci. 2011, 43, 160–165. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Kalembasa, S. Quick method of determination of organic carbon in soil. Pol. J. Soil Sci. 1991, 24, 17–22. [Google Scholar]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: Cambridge, MA, USA; Harcourt Brace & Company: London, UK, 1998; p. 576. [Google Scholar]
- Casida, L.E., Jr.; Klein, D.D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Wolińska, A.; Zapasek, M.; Stępniewska, Z. The optimal TTC dose and its chemical reduction level during soil dehydrogenase activity assay. Acta Agroph. 2016, 23, 303–314. [Google Scholar]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Book Series: Agronomy Monographs; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1983. [Google Scholar] [CrossRef] [Green Version]
- Kucharski, J.; Boros, E.; Wyszkowska, J. Biochemical activity of nickel—Contaminated soil. Pol. J. Environ. Stud. 2009, 18, 1039–1044. [Google Scholar]
- Skowera, B. Changes of conditions in the Polish area (1971–2010). Fragm. Agron. 2014, 31, 74–87. (In Polish) [Google Scholar]
- Symanowicz, B.; Skorupka, W. Effect of mineral fertilization on nitrogenase activity, yield, nitrogen content and uptake with alfalfa (Medicago sativa L.) yield. J. Elem. 2019, 24, 181–191. [Google Scholar] [CrossRef]
- Haki, J.; Kunzová, E.; Tocauerová, Š.; Menšik, L.; Mrázková, M.; Pozdišek, J. Impact of long-term manure and mineral fertilization on yield and nutritive value of Lucerne (Medicago sativa) in relation to changes in canopy structure. Eur. J. Agron. 2021, 123, 126219. [Google Scholar] [CrossRef]
- Symanowicz, B.; Kalembasa, S. Effect of iron, molybdenum and cobalt on the amount of nitrogen, biologically reduced by Rhizobium galegae. Ecol. Chem. Eng. 2012, 19, 1311–1320. [Google Scholar] [CrossRef]
- Symanowicz, B.; Kalembasa, S.; Skorupka, W.; Niedbała, M. The changes of enzymatic activity of soil under eastern galega (Galega orientalis Lam.) after NPKCa fertilization. Plant Soil Environ. 2014, 60, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Kalembasa, D.; Szukała, J.; Symanowicz, B.; Kalembasa, S.; Faligowska, S.; Becher, M. Amount of biologically nitrogen fixed by faba bean and its uptake by winter wheat determined by 15N ID method. Arch. Agron. Soil Sci. 2020. [Google Scholar] [CrossRef]
- Mocek-Płóciniak, A.; Niewiadomska, A.; Głuchowska, K. Effect of the culturing density of the Sinorhizobium meliloti Bp on the development of lucerne (Medicago sativa L.) and nitrogenase activity. Acta Sci. Pol. Agric. 2008, 7, 39–45. [Google Scholar]
- Swędrzyńska, D.; Małecka, I.; Blecharczyk, A.; Swędrzyński, A.; Starzyk, J. Effects of various long-term tillage systems on some chemical and biological properties of soil. Pol. J. Environ. Stud. 2013, 22, 1835–1844. [Google Scholar]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators. A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Sołek-Podwika, K.; Ciarkowska, K. Urease activity in soils anthropogenically enriched in sulphur compounds. Soil Sci. Ann. 2008, 59, 196–200. (In Polish) [Google Scholar]
- Kalembasa, S.; Symanowicz, B. The changes of molybdenum and cobalt contents in biomass of goat’s rue (Galega orientalis Lam.). Fres. Environ. Bull. 2009, 18, 1150–1153. [Google Scholar]
- Li, F.; Kautz, T.; Pude, R.; Köpke, U. Nodulation of lucerne (Medicago sativa L.) roots: Depth distribution and temporal variation. Plant Soil Environ. 2012, 58, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, A.; Wilczewski, E. Effects of catch crops cultivated for green manure and mineral nitrogen fertilization on soil enzyme activities and chemical properties. Geoderma 2012, 189–190, 72–80. [Google Scholar] [CrossRef]
- Pan, C.; Liu, C.; Zhao, H.; Wang, Y. Changes of soil physico-chemical properties and enzyme activities in relation to grassland salinization. Eur. J. Soil Biol. 2012, 55, 13–19. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J. The use of reed canary grass and giant miscanthus in the phytoremediation of municipal sewage sludge. Environ. Sci. Pollut. Res. 2016, 23, 9505–9517. [Google Scholar] [CrossRef]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Lemanowicz, J. Mineral fertilization as a factor determining selected sorption properties of soil against the activity of phosphatases. Plant Soil Environ. 2013, 59, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Biederbeck, V.O.; Zentner, R.P.; Campbell, C.A. Soil microbial populations and activities as influenced by legume green fallow in a semiarid climate. Soil Biol. Biochem. 2005, 37, 1775–1784. [Google Scholar] [CrossRef]
- Makoi, J.H.; Chimphango, S.B.; Dakora, F.D. Elevated levels of acid and alkaline phosphatase activity in roots and rhizosphere of cowpea (Vigna unguiculata L. Walp.) genotypes grown in mixed culture and at different densities with sorghum (Sorghum bicolor L.). Crop. Pasture Sci. 2010, 61, 279–286. [Google Scholar] [CrossRef]
- Tang, X.; Placella, S.A.; Daydé, F.; Bernard, L.; Robin, A.; Journet, E.P.; Hinsinger, P. Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertiliser gradient. Plant Soil 2016, 407, 119–134. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Bhavya, G.; Shaker, K.C.; Jayasree, G.; Reddy, M.M. Effect of integrate use of phosphorus, PSB and vermicompost on acid and alkaline phosphatase activity and yield of green gram (Vigna radiata L.). Int. J. Curr. Microbial. Appl. Sci. 2018, 7, 1465–1468. [Google Scholar] [CrossRef]



| Chemical Properties | Years | ||
|---|---|---|---|
| 2012 | 2013 | 2014 | |
| Ctot g kg−1 | 35.6 ± 0.8 | 34.1 ± 0.9 | 33.8 ± 0.7 |
| Ntot g kg−1 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.4 ± 0.2 |
| P * mg kg−1 | 264.8 ± 3.8 | 258.6 ± 2.1 | 249.9 ± 3.5 |
| K * mg kg−1 | 88.7 ± 2.7 | 92.6 ± 2.5 | 91.1 ± 3.3 |
| Mg * mg kg−1 | 91.3 ± 2.2 | 82.3 ± 1.9 | 78.2 ± 1.7 |
| Fe * mg kg−1 | 1391.5 ± 7.9 | 1349.7 ± 8.3 | 1356.8 ± 9.0 |
| Mo * mg kg−1 | 0.018 ± 0.003 | 0.021 ± 0.001 | 0.020 ± 0.001 |
| pH | 6.82 | 6.71 | 6.65 |
| Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | Mean/Sum |
| Temperature (°C) | |||||||||||||
| 2012 | −2.0 | −7.8 | 4.0 | 9.0 | 14.5 | 16.4 | 20.4 | 18.0 | 14.2 | 7.5 | 5.4 | −4.1 | 8.0 |
| 2013 | −4.2 | −8.0 | −2.5 | 7.5 | 15.3 | 17.7 | 18.8 | 18.3 | 11.4 | 9.6 | 5.2 | 1.7 | 7.6 |
| 2014 | −3.5 | 1.1 | 5.9 | 9.7 | 13.7 | 15.1 | 20.5 | 17.8 | 13.7 | 8.4 | 3.7 | −1.0 | 8.8 |
| Precipitation (mm) | |||||||||||||
| 2012 | 41.6 | 18.8 | 26.7 | 40.3 | 59.7 | 118.7 | 41.4 | 64.1 | 30.8 | 41.6 | 21.9 | 33.6 | 543.7 |
| 2013 | 45.8 | 37.7 | 34.8 | 57.6 | 145.8 | 11.9 | 49.1 | 44.1 | 86.6 | 18.0 | 35.5 | 15.5 | 682.4 |
| 2014 | 41.3 | 29.5 | 36.3 | 39.5 | 79.5 | 74.2 | 37.5 | 105.7 | 26.3 | 3.0 | 32.5 | 90.4 | 595.7 |
| Hydrothermal index Sielianinov K (mm/°C) | |||||||||||||
| 2012 | −6.93 | −0.86 | 2.22 | 1.49 | 1.37 | 2.41 | 0.68 | 1.19 | 0.72 | 1.85 | 1.35 | −2.64 | |
| 2013 | −3.63 | −1.32 | −4.49 | 2.56 | 3.18 | 2.11 | 0.87 | 0.80 | 2.53 | 0.62 | 2.27 | 2.94 | |
| 2014 | −3.93 | 9.58 | 1.99 | 1.36 | 1.93 | 1.64 | 0.61 | 1.98 | 0.64 | 0.12 | 2.93 | −2.92 | |
| Fertilizer Treatment | Time of Soil Sampling | |||
|---|---|---|---|---|
| August 2012 | September 2012 | June 2013 | July 2014 | |
| Control * | 9.18 ± 1.36 eB | 9.39 ± 1.28 dB | 14.66 ± 1.19 cA | 10.51 ± 1.48 bB |
| NPK | 10.81 ± 2.14 eB | 12.37 ± 0.71 dB | 24.65 ± 4.78 bA | 9.03 ± 0.89 bB |
| NPKFe1 | 18.73 ± 2.35 dB | 17.03 ± 2.23 cB | 40.51 ± 5.20 aA | 5.76 ± 0.40 cC |
| NPKMo1 | 22.24 ± 1.42 cB | 19.54 ± 1.28 cB | 29.16 ± 1.27 bA | 11.69 ± 0.24 bC |
| NPKFe1Mo1 | 41.78 ± 2.03 bA | 16.57 ± 0.33 cC | 27.97 ± 2.82 bB | 8.64 ± 1.03 bD |
| NPKFe2 | 25.90 ± 3.76 cB | 24.98 ± 3.19 bB | 36.74 ± 5.09 aA | 19.61 ± 1.96 aB |
| NPKMo2 | 51.47 ± 5.38 aA | 39.55 ± 1.69 aB | 40.19 ± 2.26 aB | 8.05 ± 1.39 bC |
| NPKFe2Mo2 | 13.81 ± 4.35 dB | 26.44 ± 2.53 bA | 19.58 ± 3.38 cB | 16.97 ± 2.74 aB |
| Fertilizer Treatment | Time of Soil Sampling | |||
|---|---|---|---|---|
| August 2012 | September 2012 | June 2013 | July 2014 | |
| Control * | 315.5 ± 2.70 bA | 319.4 ± 1.00 cA | 306.5 ± 1.15 cB | 312.4 ± 2.01 bA |
| NPK | 357.0 ± 1.77 aA | 358.9 ± 2,06 aA | 340.8 ± 5.30 bC | 351.0 ± 2.26 aB |
| NPKFe1 | 348.4 ± 1.51 aB | 359.1 ± 7.19 aA | 343.5 ± 3.29 aB | 347.1 ± 2.40 aB |
| NPKMo1 | 352.4 ± 0.94 aA | 353.1 ± 1.23 bA | 347.7 ± 2.36 aB | 353.3 ± 5.77 aA |
| NPKFe1Mo1 | 353.7 ± 3.10 aA | 353.4 ± 1.92 bA | 347.7 ± 2.18 aB | 351.2 ± 1.08 aA |
| NPKFe2 | 351.9 ± 1.81 aA | 351.3 ± 0.46 bA | 347.5 ± 0.93 aA | 349.6 ± 0.36 aA |
| NPKMo2 | 352.6 ± 1.11 aA | 351.8 ± 1.60 bA | 348.7 ± 0.51 aA | 351.5 ± 1.32 aA |
| NPKFe2Mo2 | 356.6 ± 0.71 aA | 354.1 ± 0.40 bA | 350.2 ± 1.01 aB | 353.7 ± 0.95 aA |
| Fertilizer Treatment | Time of Soil Sampling | |||
|---|---|---|---|---|
| August 2012 | September 2012 | June 2013 | July 2014 | |
| Control * | 23.2 ± 1.48 eA | 21.0 ± 0.46 dA | 20.9 ± 0.25 eB | 21.2 ± 0.79 eA |
| NPK | 26.5 ± 1.07 dA | 24.2 ± 0.30 cA | 23.6 ± 1.10 dB | 25.5 ± 1.07 dA |
| NPKFe1 | 33.4 ± 1.03 bA | 32.5 ± 1.19 bA | 29.0 ± 0.60 bA | 30.8 ± 0.38 cA |
| NPKMo1 | 33.6 ± 2.74 bA | 32.7 ± 0.85 bA | 27.1 ± 0.40 cB | 29.2 ± 0.70 cB |
| NPKFe1Mo1 | 38.3 ± 1.11 aA | 35.9 ± 0.89 aB | 35.6 ± 1.06 aB | 38.2 ± 1.37 aA |
| NPKFe2 | 32.4 ± 0.87 bA | 30.8 ± 0.56 bA | 27.5 ± 0.86 cB | 29.8 ± 1.04 cA |
| NPKMo2 | 29.1 ± 0.40 cA | 28.2 ± 0.42 bA | 26.5 ± 0.98 cA | 28.3 ± 0.92 cA |
| NPKFe2Mo2 | 35.1 ± 2.33 bA | 31.7 ± 1.04 bB | 30.2 ± 0.76 bB | 33.4 ± 2.10 bA |
| Fertilizer Treatment | Time of Soil Sampling | |||
|---|---|---|---|---|
| August 2012 | September 2012 | June 2013 | July 2014 | |
| Control * | 0.67 ± 0.02 aB | 0.69 ± 0.01 bA | 0.67 ± 0.03 bB | 0.63 ± 0.02 cC |
| NPK | 0.60 ± 0.01 bB | 0.66 ± 0.02 bA | 0.52 ± 0.01 cD | 0.55 ± 0.02 cC |
| NPKFe1 | 0.76 ± 0.04 aA | 0.71 ± 0.01 bB | 0.64 ± 0.03 bD | 0.69 ± 0.01 bC |
| NPKMo1 | 0.69 ± 0.01 aB | 0.72 ± 0.01 bA | 0.61 ± 0.04 bD | 0.63 ± 0.02 cC |
| NPKFe1Mo1 | 0.80 ± 0.01 aB | 0.82 ± 0.02 aA | 0.77 ± 0.02 aC | 0.78 ± 0.01 aC |
| NPKFe2 | 0.60 ± 0.01 bB | 0.63 ± 0.02 bA | 0.52 ± 0.02 cD | 0.56 ± 0.01 dC |
| NPKMo2 | 0.72 ± 0.02 aB | 0.74 ± 0.04 bA | 0.66 ± 0.03 bC | 0.71 ± 0.02 bB |
| NPKFe2Mo2 | 0.71 ± 0.01 aB | 0.74 ± 0.03 bA | 0.67 ± 0.02 bC | 0.70 ± 0.01 bB |
| Fertilizer Treatment | Time of Soil Sampling | |||
|---|---|---|---|---|
| August 2012 | September 2012 | June 2013 | July 2014 | |
| Control * | 0.54 ± 0.02 aA | 0.51 ± 0.01 aB | 0.49 ± 0.03 bC | 0.49 ± 0.01 bC |
| NPK | 0.47 ± 0.01 cA | 0.44 ± 0.02 bB | 0.40 ± 0.01 dD | 0.41 ± 0.01 cC |
| NPKFe1 | 0.52 ± 0.01 bB | 0.53 ± 0.01 aA | 0.48 ± 0.02 bC | 0.52 ± 0.01 bB |
| NPKMo1 | 0.52 ± 0.02 bB | 0.53 ± 0.01 aA | 0.53 ± 0.02 aA | 0.53 ± 0.03 aA |
| NPKFe1Mo1 | 0.57 ± 0.01 aA | 0.56 ± 0.02 aB | 0.56 ± 0.02 aB | 0.56 ± 0.02 aB |
| NPKFe2 | 0.43 ± 0.01 dB | 0.44 ± 0.01 bA | 0.40 ± 0.02 dD | 0.41 ± 0.01 cC |
| NPKMo2 | 0.45 ± 0.01 cB | 0.43 ± 0.01 bD | 0.46 ± 0.01 cA | 0.44 ± 0.02 cC |
| NPKFe2Mo2 | 0.51 ± 0.01 bA | 0.49 ± 0.01 aC | 0.50 ± 0.01 bB | 0.51 ± 0.01 bA |
| Fertilizer Treatment | Time of Soil Sampling | Mean | |||
|---|---|---|---|---|---|
| August 2012 | September 2012 | June 2013 | July 2014 | ||
| Control * | 3.47 ± 0.31 aA | 3.43 ± 0.21 aA | 3.34 ± 0.25 bA | 3.50 ± 0.23 aA | 3.44 a |
| NPK | 3.45 ± 0.18 aA | 3.24 ± 0.23 aB | 3.06 ± 0.23 cB | 3.20 ± 0.11 cB | 3.24 a |
| NPKFe1 | 3.30 ± 0.22 bB | 3.26 ± 0.24 aB | 3.40 ± 0.19 bA | 3.07 ± 0.21 cB | 3.24 a |
| NPKMo1 | 3.22 ± 0.13 bC | 3.42 ± 0.20 aB | 3.92 ± 0.17 aA | 3.13 ± 0.21 bC | 3.42 a |
| NPKFe1Mo1 | 3.43 ± 0.19 aA | 3.36 ± 0.14 aA | 3.10 ± 0.15 cB | 3.54 ± 0.11 aA | 3.36 a |
| NPKFe2 | 3.14 ± 0.11 bC | 3.34 ± 0.15 aB | 3.72 ± 0.19 aA | 3.15 ± 0.13 cC | 3.34 a |
| NPKMo2 | 3.22 ± 0.13 bA | 2.91 ± 0.11 bA | 2.48 ± 0.11 aB | 3.02 ± 0.12 cA | 2.91 a |
| NPKFe2Mo2 | 3.38 ± 0.15 aA | 3.08 ± 0.12 bB | 2.52 ± 0.14 dC | 3.34 ± 0.13 bA | 3.08 a |
| Mean | 3.32 A | 3.25 A | 3.32 A | 3.19 A | 3.08 a |
| Fertilizer Treatment | Time of Soil Sampling | Mean | |||
|---|---|---|---|---|---|
| August 2012 | September 2012 | June 2013 | July 2014 | ||
| Control * | 197.4 ± 4.2 fA | 189.5 ± 2.5 dA | 180.9 ± 5.1 cB | 191.1 ± 3.8 eA | 189.9 d |
| NPK | 222.0 ± 3.1 eA | 202.3 ± 3.3 dB | 186.8 ± 4.3 cC | 199.9 ± 4.3 eB | 202.6 d |
| NPKFe1 | 235.6 ± 2.9 cA | 232.6 ± 2.7 bA | 233.0 ± 2.8 bA | 206.6 ± 2.1 dB | 225.3 c |
| NPKMo1 | 232.7 ± 3.1 cC | 243.5 ± 1.9 aB | 258.7 ± 1.6 aA | 209.3 ± 1.8 dD | 236.4 b |
| NPKFe1Mo1 | 271.7 ± 2.7 aA | 249.6 ± 2.3 aB | 230.9 ± 3.7 bC | 267.3 ± 3.1 aA | 254.9 a |
| NPKFe2 | 223.6 ± 2.5 eC | 232.1 ± 1.8 bB | 248.6 ± 4.2 aA | 213.2 ± 2.4 cD | 229.7 c |
| NPKMo2 | 227.6 ± 3.7 dA | 199.3 ± 2.1 dB | 164.9 ± 5.3 dC | 197.5 ± 2.1 eB | 197.1 d |
| NPKFe2Mo2 | 247.2 ± 3.2 bA | 218 ± 2.0 cC | 172.2 ± 7.2 dD | 239.4 ± 3.3 bB | 218.9 c |
| Mean | 231.9 A | 220.6 B | 209.6 C | 215.1 C | 219.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symanowicz, B.; Skorupka, W.; Becher, M.; Jaremko, D.; Krasuski, S. The Effect of Alfalfa Mineral Fertilization and Times of Soil Sampling on Enzymatic Activity. Agronomy 2021, 11, 1335. https://doi.org/10.3390/agronomy11071335
Symanowicz B, Skorupka W, Becher M, Jaremko D, Krasuski S. The Effect of Alfalfa Mineral Fertilization and Times of Soil Sampling on Enzymatic Activity. Agronomy. 2021; 11(7):1335. https://doi.org/10.3390/agronomy11071335
Chicago/Turabian StyleSymanowicz, Barbara, Wojciech Skorupka, Marcin Becher, Dawid Jaremko, and Sebastian Krasuski. 2021. "The Effect of Alfalfa Mineral Fertilization and Times of Soil Sampling on Enzymatic Activity" Agronomy 11, no. 7: 1335. https://doi.org/10.3390/agronomy11071335
APA StyleSymanowicz, B., Skorupka, W., Becher, M., Jaremko, D., & Krasuski, S. (2021). The Effect of Alfalfa Mineral Fertilization and Times of Soil Sampling on Enzymatic Activity. Agronomy, 11(7), 1335. https://doi.org/10.3390/agronomy11071335






