Decreased Water Use in a Super-Intensive Olive Orchard Mediates Arthropod Populations and Pest Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling Procedure
2.3. Data Analysis
3. Results
3.1. Vacuum Sampling
3.2. Visual Sampling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | Phase | Irrigation 1 | Threshold I | Threshold II | Threshold III |
|---|---|---|---|---|---|
| 1—Control | 100% Etc | ||||
| 2—Confederation RDI | I | 780 m3·ha−1 | ψ = −1.2 MPa | ψ = −1.4 MPa | |
| (blossoming) | |||||
| II | 130 m3·ha−1 | ψ = −2.0 MPa | ψ = −2.0 MPa | ψ = −4.0 MPa | |
| (pit hardening) | |||||
| III | 390 m3·ha−1 | ψ = −1.4 MPa | ψ = −3.0 MPa | ψ = −3.0 MPa | |
| (pre-harvest rehydration) |
| Treatment | Year | Total | Phase II | Phase III |
|---|---|---|---|---|
| (Pit Hardening) | (Pre-Harvest Rehydration) | |||
| 1—Control | 2017 | 56.1 ± 1.0 | 53.4 ± 9.1 | 0.05 ± 35.5 |
| 2018 | 19.3 ± 8.7 | 18.2 ± 8.1 | 0.0 ± 0.0 | |
| 2019 | 0.68 ± 0.47 | 0.34 ± 20.4 | 0.34 ± 0.20 | |
| 2—Confederation RDI | 2017 | 182.9 ± 39.9 | 140.4 ± 25.3 | 41.7 ± 24.2 |
| 2018 | 142.8 ± 16.9 | 103.9 ± 16.9 | 30.0 ± 18.7 | |
| 2019 | 218.8 ± 58.6 | 150.7 ± 30.2 | 66.0 ± 19.4 |
Appendix B
| VACUUM | 2017 | 2018 | 2019 | 2017 to 2019 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tr | Tr × SD | Tr | Tr × SD | Tr | Tr × SD | Tr | Y | Tr × Y | |
| Araneae | 0.40 | 1.47 (4.5, 27.2) | 1.0 | 1.70 (4.0, 24.1) | 12.03 | 1.0 (4.4, 26.4) | 6.31 | 2.90 | 1.03 |
| Acari | 0.48 | 0.52 (3.2, 19.2) | 0.01 | 3.12 (2.4, 14.2) | 1.03 | 2.2 (3.0, 17.9) | 0.04 | 30.6 | 0.81 |
| Collembola | 0.33 | 3.70 (2.7, 16.2) | <0.01 | 0.80 (2.9, 17.4) | 0.87 | 3.1 (3.8, 22.6) | 0.35 | 7.11 | 0.04 |
| Coleoptera | 3.64 | 1.31 (4.0, 24.0) | 2.20 | 0.83 (4.5, 26.7) | 1.66 | 1.06 (3.3, 19.8) | 6.51 | 6.19 | 0.01 |
| Nematocera | 1.91 | 0.75 (4.2,25.3) | 21.52 | 0.70 (3.7, 22.2) | 5.02 | 2.69 (2.6, 15.6) | 16.2 | 40.0 | 0.35 |
| Brachycera | 0.95 | 0.66 (3.4, 20.6) | 0.04 | 1.35 (4.2, 25.2) | 3.05 | 2.91 (3.0, 18.2) | 0.22 | 10.3 | 2.07 |
| Heteroptera | 1.17 | 0.91 (3.9, 23.4) | 0.04 | 2.24 (4.1, 24.5) | 2.93 | 2.47 (3.2, 19.5) | 3.26 | 2.71 | 1.67 |
| Cicadomorpha | 0.05 | 1.46 (3.2, 19.2) | <0.01 | 2.24 (3.1, 18.7) | 1.19 | 1.05 (3.0, 17.8) | 0.07 | 1.58 | 0.52 |
| Fulgoromorpha | 1.45 | 0.94 (1.4, 8.2) | 3.0 | <0.01 (1.0, 6.0) | --(2) | 2.48 | 10.7 | 0.44 | |
| Sternorrhyncha | 0.81 | 0.78 (4.1, 24.6) | 1.48 | 0.99 (3.4, 20.3) | 7.43 | 2.18 (4.5, 27.2) | 1.11 | 0.11 | 2.39 |
| Chalcidoidea | 0.49 | 2.39 (3.7, 22.4) | 0.37 | 0.49 (3.9, 23.4) | --(2) | 4.30 | 56.0 | 1.05 | |
| Ichneumonoidea | 0.001 | 4.25 (3.7, 22.0) | 0.80 | 0.88 (2.4, 14.1) | 6.59 | 6.87 (3.3, 19.7) | 4.02 | 88.2 | 3.41 |
| Formicidae | 0.13 | 0.64 (4.2, 25.1) | 0.06 | 4.31 (2.9, 17.4) | 1.20 | 3.06 (3.2, 19.0) | 0.03 | 2.99 | 0.19 |
| Other Hymenoptera | --(2) | 0.16 | 2.26 (2.0, 12.1) | --(1) | |||||
| Neuroptera | 0.54 | 0.55 (3.9, 23.2) | 3.01 | 1.92 (3.8, 22.6) | <0.01 | 1.90 (4.3, 25.6) | 0.81 | 3.70 | 1.41 |
| Psocoptera | --(2) | 2.13 | 2.10 (3.1, 18.4) | 0.01 | 1.08 (2.9, 17.5) | 0.06 | 3.22 | 0.16 | |
| Thysanoptera | --(2) | 1.54 | 0.77 (2.3, 13.9) | --(2) | |||||
| Total arthropods | 0.24 | 0.46 (3.8, 23.1) | 10.95 | 0.39 (3.4, 20.5) | 8.70 | 3.94 (3.9, 23.4) | 15.6 | 49.5 | 3.42 |
| VISUAL | |||||||||
| Euphyllura (fl+sh) | 2.39 | 1.30 (3.1, 24.7) | |||||||
| Euphyllura (fl) | 0.37 | 0.04 (1, 8) | --(2) | 0.14 | 0.33 (1.0, 3.0) | 1.85 | 24.1 | 1.90 | |
| Euphyllura (sh) | --(1) | --(2) | <0.01 | <0.01 (1.7, 13.8) | |||||
| Lepidoptera | 2.13 | 0.85 (3.4, 27) | 16.7 | 1.58 (4.8, 38.2) | 2.27 | 2.48 (2.9, 22.8) | 14.0 | 61.3 | 11.9 |
| Feeding spots (a) | 1.50 | 0.57 (4.2, 34) | 0.22 | 1.19 (4.2, 25.4) | 0.14 | 0.89 (4.1, 24.4) | 1.26 | 15.6 | 0.74 |
| Feeding spots (b) | --(1) | 9.91 | 0.92 (5.0, 40.1) | 0.03 | 2.11 (3.9, 31.3) | 4.56 | 49.8 | 4.06 | |
| Heteroptera | --(2) | 0.29 | 1.49 (3.6, 29.1) | 4.68 | 0.78 (2.4, 19.1) | 2.28 | 4.46 | 1.82 | |
| Neuroptera | --(2) | 0.07 | 0.74 (3.6, 28.8) | 0.67 | 1.32 (3.4, 27.2) | 0.81 | 9.20 | 0.03 | |
| Eriophyidae | 0,16 | 0.63 (5, 38.4) | 26.44 | 2.8 (4.7, 37.6) | 17.20 | 2.22 (5.1, 41.1) | 5.31 | 9.77 | 1.51 |
| Phytoseiidae | --(2) | 0.38 | 1.58 (3.6, 28.8) | --(2) | |||||
| Other arthropods | 1.05 | 0.51 (4.6, 36.6) | --(2) | --(2) | |||||
| Venturia | 0.07 | 1.19 (4.8, 38) | 0.12 | 1.06 (1.9, 15.1) | 0.06 | 1.99 (3.0, 23.8) | 0.01 | 43.1 | 0.07 |
References
- Junta de Andalucia Análisis de la Densidad en las Plantaciones de Olivar en Andalucia. Available online: https://www.juntadeandalucia.es/organismos/agriculturaganaderiapescaydesarrollosostenible/servicios/estadistica-cartografia/estudios-informes/detalle/184193.html (accessed on 27 April 2020).
- Tribaldos Campos, J.; Tribaldos Campos, H. El Olivar en España: Tradicional, Intensivo y Superintensivo. Available online: https://www.agro.basf.es/es/Camposcopio/Secciones/Protección-y-sanidad/olivar-en-espana/ (accessed on 5 May 2021).
- Moriana, A.; Pérez-López, D.; Gómez-Rico, A.; Salvador, M.; de los Desampadoros Salvador, M.; Olmedilla, N.; Ribas, F.; Fregapane, G. Irrigation scheduling for traditional, low-density olive orchards: Water relations and influence on oil characteristics. Agric. Water Manag. 2007, 87, 171–179. [Google Scholar] [CrossRef]
- Fernández, J.E.; Perez-Martin, A.; Torres-Ruiz, J.M.; Cuevas, M.V.; Rodriguez-Dominguez, C.M.; Elsayed-Farag, S.; Morales-Sillero, A.; García, J.M.; Hernandez-Santana, V.; Diaz-Espejo, A. A regulated deficit irrigation strategy for hedgerow olive orchards with high plant density. Plant Soil 2013, 372, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Moriana, A.; Pérez-López, D.; Prieto, M.H.; Ramírez-Santa-Pau, M.; Pérez-Rodriguez, J.M. Midday stem water potential as a useful tool for estimating irrigation requirements in olive trees. Agric. Water Manag. 2012, 112, 43–54. [Google Scholar] [CrossRef]
- Corell, M.; Martín-Palomo, M.J.; Pérez-López, D.; Centeno, A.; Girón, I.; Moreno, F.; Torrecillas, A.; Moriana, A. Approach for using trunk growth rate (TGR) in the irrigation scheduling of table olive orchards. Agric. Water Manag. 2017, 192, 12–20. [Google Scholar] [CrossRef]
- Morales, F. Sustainable water use: Irrigation strategies in traditional Mediterranean and emerging crops. Agric. Water Manag. 2019, 217, 57–59. [Google Scholar] [CrossRef]
- Galindo, A.; Collado-González, J.; Griñán, I.; Corell, M.; Centeno, A.; Martín-Palomo, M.J.; Girón, I.F.; Rodríguez, P.; Cruz, Z.N.; Memmi, H.; et al. Deficit irrigation and emerging fruit crops as a strategy to save water in Mediterranean semiarid agrosystems. Agric. Water Manag. 2018, 202, 311–324. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Hodson, A.K.; Lampinen, B.D. Effects of cultivar and leaf traits on the abundance of Pacific spider mites in almond orchards. Arthropod-Plant. Interact. 2018, 13, 453–463. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M.; Salinas, M. Regulated deficit irrigation in almonds: Effects of variations in applied water and stress timing on yield and yield components. Irrig. Sci. 2006, 24, 101–114. [Google Scholar] [CrossRef]
- English-Loeb, G.M. Plant drought stress and outbreaks of spider mites: A field test. Ecology 1990, 71, 1401–1411. [Google Scholar] [CrossRef]
- Sconiers, W.B.; Eubanks, M.D. Not all droughts are created equal? The effects of stress severity on insect herbivore abundance. Arthropod-Plant. Interact. 2017, 11, 45–60. [Google Scholar] [CrossRef]
- Frampton, G.K.; Van Den Brink, P.J.; Gould, P.J.L. Effects of spring drought and irrigation on farmland arthropods in southern Britain. J. Appl. Ecol. 2000, 37, 865–883. [Google Scholar] [CrossRef]
- Lensing, J.R.; Todd, S.; Wise, D.H. The impact of altered precipitation on spatial stratification and activity-densities of springtails (Collembola) and spiders (Araneae). Ecol. Entomol. 2005, 30, 194–200. [Google Scholar] [CrossRef]
- Lindberg, N.; Engtsson, J.B.; Persson, T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J. Appl. Ecol. 2002, 39, 924–936. [Google Scholar] [CrossRef]
- Gutbrodt, B.; Dorn, S.; Mody, K. Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod-Plant. Interact. 2012, 6, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Mody, K.; Eichenberger, D.; Dorn, S. Stress magnitude matters: Different intensities of pulsed water stress produce non-monotonic resistance responses of host plants to insect herbivores. Ecol. Entomol. 2009, 34, 133–143. [Google Scholar] [CrossRef]
- Weldegergis, B.T.; Zhu, F.; Poelman, E.H.; Dicke, M. Drought stress affects plant metabolites and herbivore preference but not host location by its parasitoids. Oecologia 2015, 177, 701–713. [Google Scholar] [CrossRef]
- Tariq, M.; Wright, D.J.; Rossiter, J.T.; Staley, J.T. Aphids in a changing world: Testing the plant stress, plant vigour and pulsed stress hypotheses. Agric. For. Entomol. 2012, 14, 177–185. [Google Scholar] [CrossRef]
- Gely, C.; Laurance, S.G.W.; Stork, N.E. How do herbivorous insects respond to drought stress in trees? Biol. Rev. 2020, 95, 434–448. [Google Scholar] [CrossRef]
- Ângelo Rodrigues, M.; Coelho, V.; Arrobas, M.; Gouveia, E.; Raimundo, S.; Correia, C.M.; Bento, A. The effect of nitrogen fertilization on the incidence of olive fruit fly, olive leaf spot and olive anthracnose in two olive cultivars grown in rainfed conditions. Sci. Hortic. 2019, 256, 108658. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.; Santos, S.A.P.; Sousa, J.P.; Ferreira, A.; da Silva, P.M.; Patanita, I.; Ortega, M.; Pascual, S.; Pereira, J.A. Landscape composition and configuration affect the abundance of the olive moth (Prays oleae, Bernard) in olive groves. Agric. Ecosyst. Environ. 2020, 294, 106854. [Google Scholar] [CrossRef]
- Porcel, M.; Ruano, F.; Cotes, B.; Pena, A.; Campos, M. Agricultural Management Systems Affect the Green Lacewing Community (Neuroptera: Chrysopidae) in Olive Orchards in Southern Spain. Environ. Entomol. 2013, 42, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Cardenas, M.; Pascual, F.; Campos, M.; Pekar, S. The Spider Assemblage of Olive Groves Under Three Management Systems. Environ. Entomol. 2015, 44, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Paredes, D.; Cayuela, L.; Gurr, G.M.; Campos, M. Is ground cover vegetation an effective biological control enhancement strategy against Olive Pests? PLoS ONE 2015, 10, e0117265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Gil, A.; Ruiz-Torres, M. Guía de Gestión Integrada de Plagas-Olivar; Ministerio de Agricultura, Pesca y Alimentacion: Madrid, Spain, 2014; ISBN 9788449113871. [Google Scholar]
- Rius, X.; Lacarte, J.M. Enfermedades y Plagas. In La Revolución del Olivar: El Cultivo en Seto; Rius, X., Lacarte, J.M., Eds.; Ediciones Paraninfo: Barcelona, Spain, 2015; pp. 383–405. ISBN 9780646938646. [Google Scholar]
- Agustí-Brisach, C.; Jiménez-Urbano, J.P.; del Carmen Raya, M.; López-Moral, A.; Trapero, A. Vascular fungi associated with branch dieback of olive in super-high-density systems in Southern Spain. Plant Dis. 2021, 105, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, J.A. Bases Para un Curso Práctico de Etomología; Asociación Española de Entomología: Salamanca, Spain, 1988; ISBN 84-404-2417-5. [Google Scholar]
- Chinery, M. Guía de Campo de Los Insectos de España y de Europa; Omega: Barcelona, Spain, 2005; ISBN 84-282-0469-1. [Google Scholar]
- Van Den Brink, P.J.; Ter Braak, C.J.F.T. Principal response curves: Analysis of time-dependent multivariate responses of biological community to stress. Environ. Toxicol. Chem. 1999, 18, 138–148. [Google Scholar] [CrossRef]
- Dively, G.P. Impact of Transgenic VIP3A × Cry1Ab Lepidopteran-resistant Field Corn on the Nontarget Arthropod Community. Environ. Entomol. 2005, 34, 1267–1291. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Hellmich, R.L.; Dively, G.P.; Lewis, L.C. Assessing the Effects of Pest Management on Nontarget Arthropods: The Influence of Plot Size and Isolation. Environ. Entomol. 2005, 34, 1181–1192. [Google Scholar] [CrossRef]
- Whitehouse, M.E.A.; Wilson, L.J.; Fitt, G.P. A comparison of arthropod communities in transgenic Bt and conventional cotton in Australia. Environ. Entomol. 2005, 34, 1224–1241. [Google Scholar] [CrossRef]
- Naranjo, S.E. Long-term assessment of the effects of transgenic Bt cotton on the abundance of nontarget arthropod natural enemies. Environ. Entomol. 2005, 34, 1193–1210. [Google Scholar] [CrossRef] [Green Version]
- Auber, A.; Travers-Trolet, M.; Villanueva, M.C.; Ernande, B. A new application of principal response curves for summarizing abrupt and cyclic shifts of communities over space. Ecosphere 2017, 8, e02023. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.N. Statistical aspects of field experiments. In Methods in Ecological and Agricultural Entomology; Dent, D.R., Walton, M.P., Eds.; CAB International: Oxford, UK, 1997; pp. 171–202. ISBN 0851991327. [Google Scholar]
- González, M.I.; Alvarado, M.; Durán, J.M.; De la Rosa, A.; Serrano, A. Los eriófidos (Acarina, Eriophidae) del olivar de la provincia de Sevilla. Problemática y control. Boletín Sanid. Veg. 2000, 26, 203–214. [Google Scholar]
- Quesada-Moraga, E.; Santiago-Álvarez, C.; Cubero-González, S.; Casado-Mármol, G.; Ariza-Fernández, A.; Yousef, M. Field evaluation of the susceptibility of mill and table olive varieties to egg-laying of olive fly. J. Appl. Entomol. 2018, 142, 765–774. [Google Scholar] [CrossRef] [Green Version]
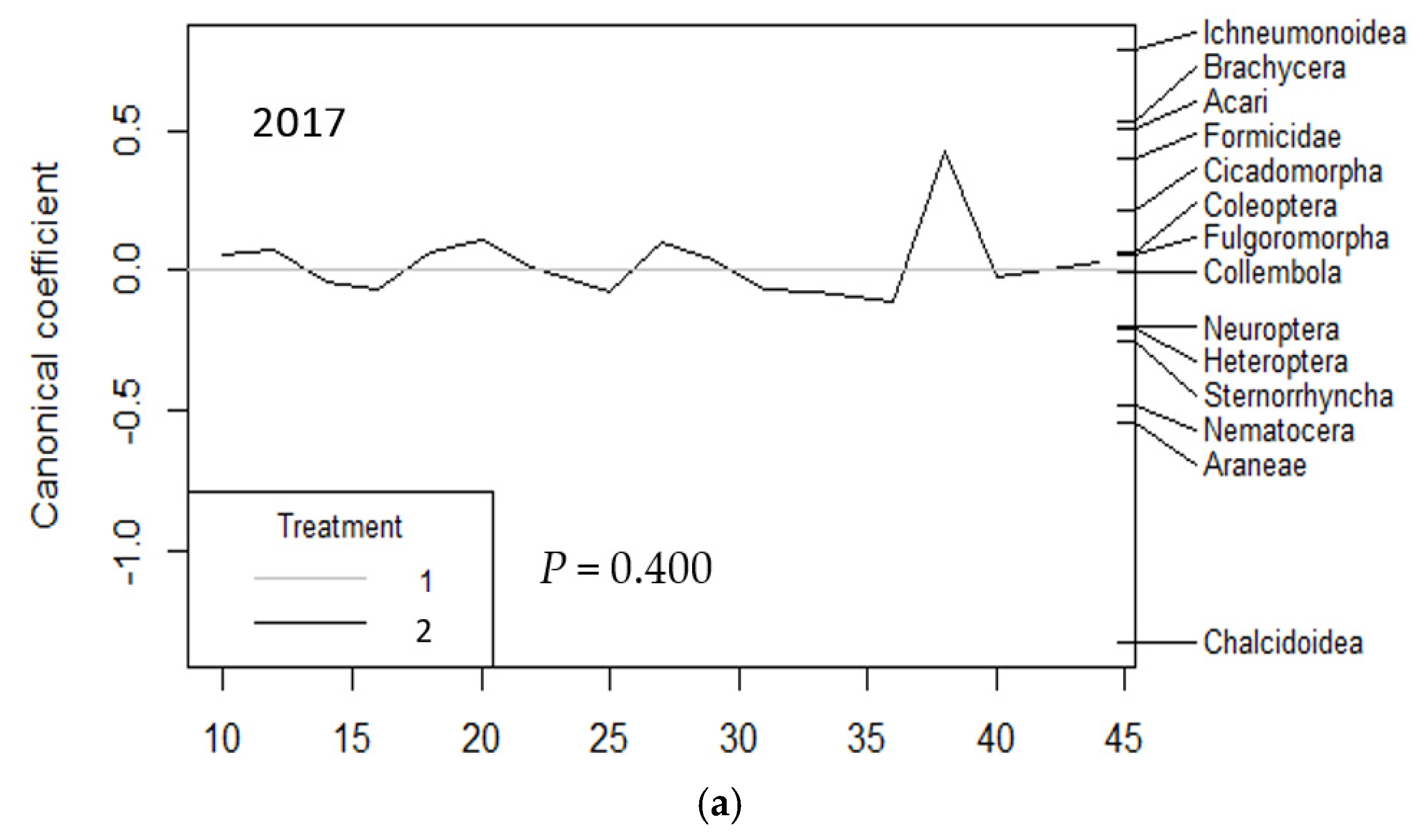
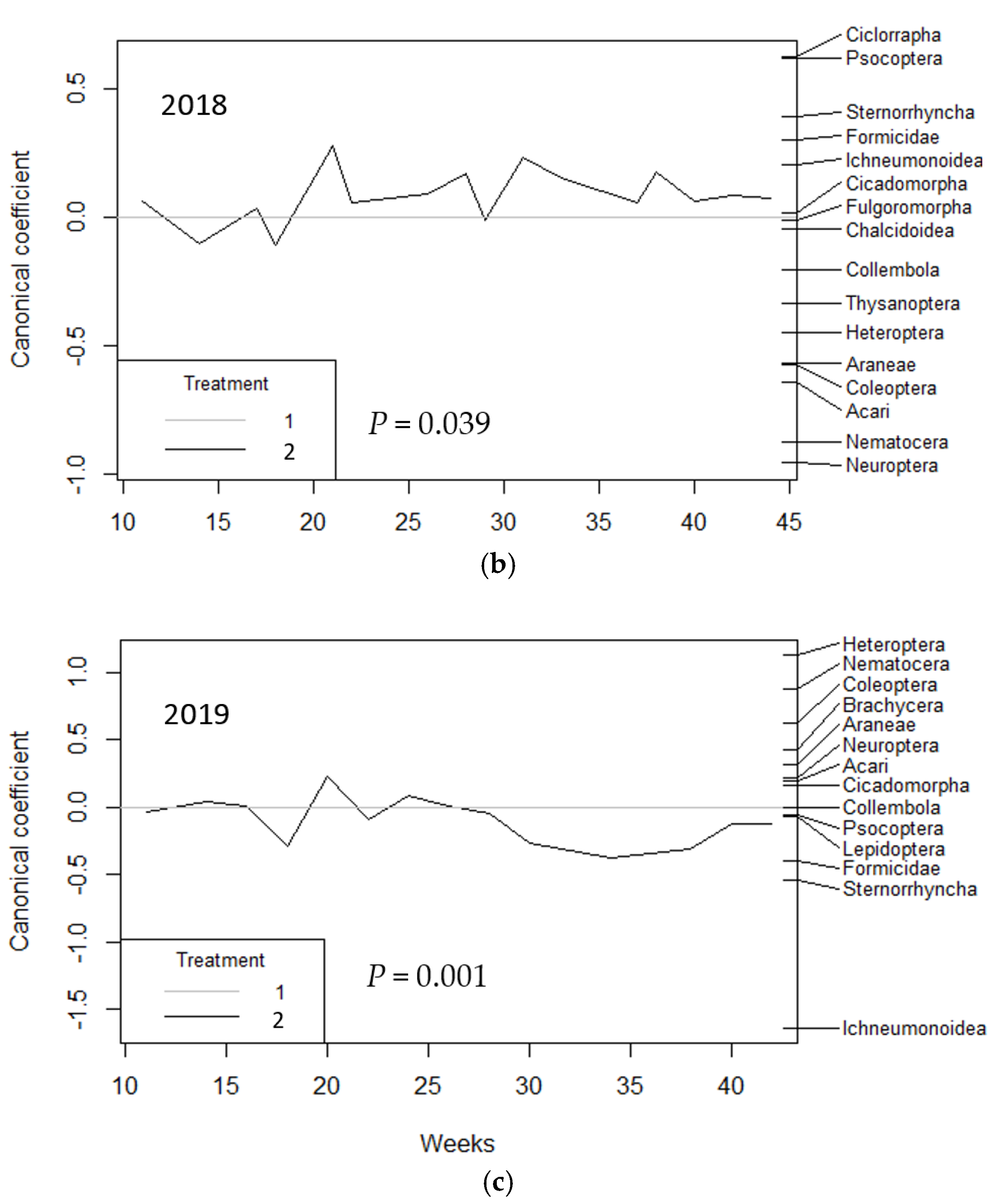
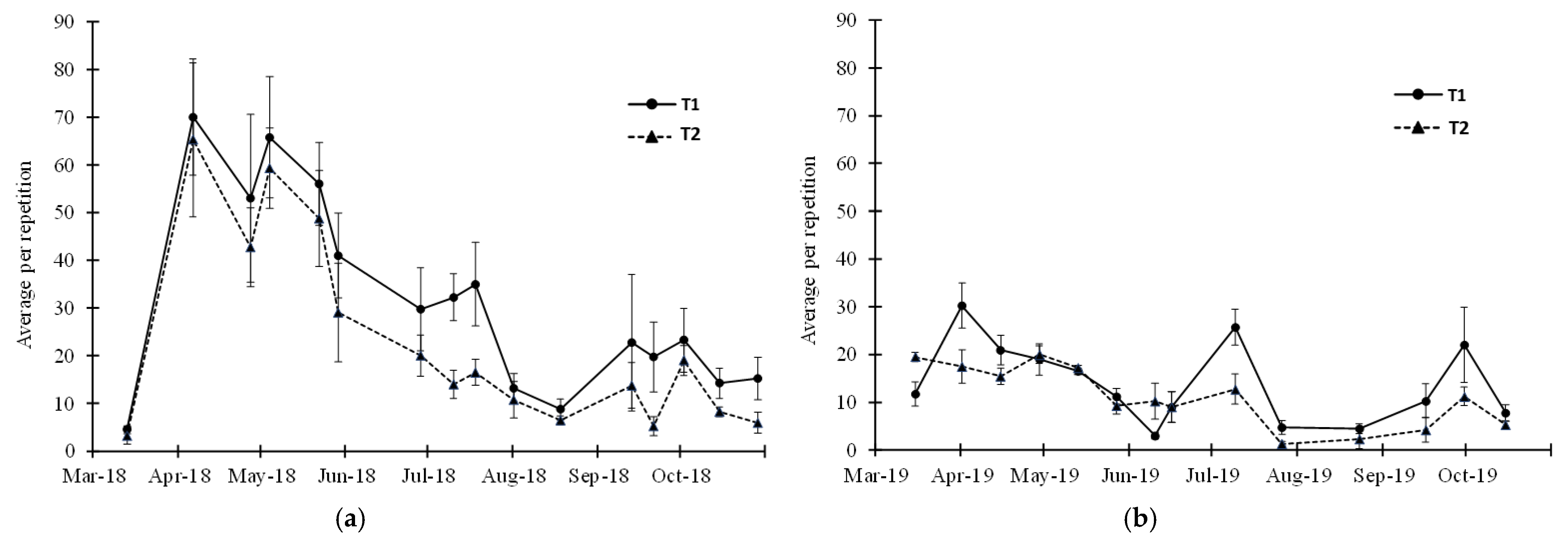

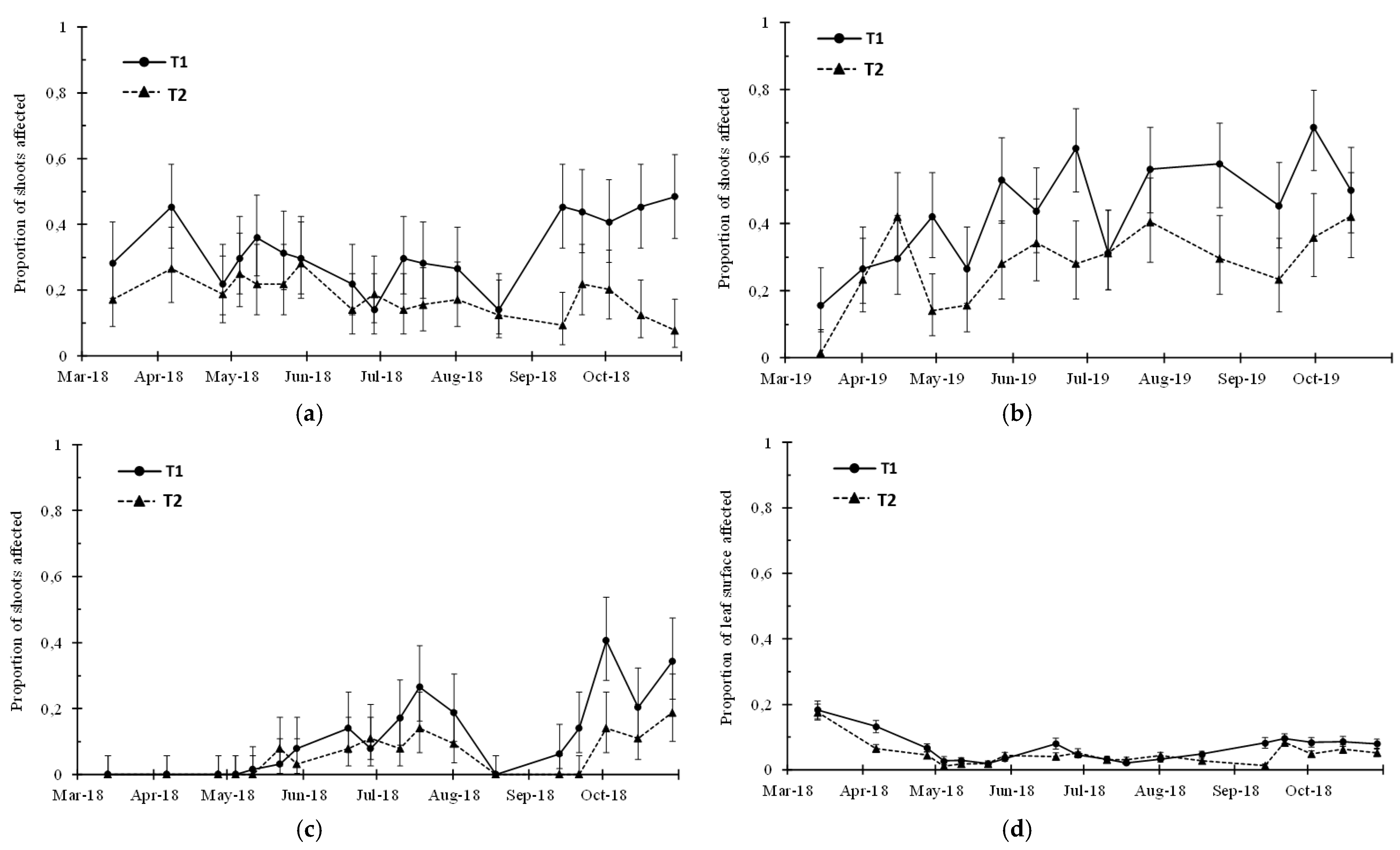
| VACUUM | 2017 | 2018 | 2019 | 2017 to 2019 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Tr × SD | Treatment | Tr × SD | Treatment | Tr × SD | Tr | Y | Tr × Y | |
| Araneae | 0.551 | 0.236 | 0.355 | 0.183 | 0.013 (T1 > T2) | 0.432 | 0.022 (T1 > T2) | 0.081 | 0.378 |
| Acari | 0.514 | 0.685 | 0.943 | 0.069 | 0.350 | 0.123 | 0.850 | 0.001 | 0.460 |
| Collembola | 0.585 | 0.037 (*) | 0.987 | 0.509 | 0.387 | 0.038 (*) | 0.563 | 0.005 | 0.963 |
| Coleoptera | 0.105 | 0.296 | 0.189 | 0.527 | 0.245 | 0.392 | 0.020 (T1 > T2) | 0.009 | 0.994 |
| Nematocera | 0.216 | 0.572 | 0.004 (T1 > T2) | 0.591 | 0.066 (·) | 0.088 (·) | 0.001 (T1 > T2) | 0.001 | 0.708 |
| Brachycera | 0.367 | 0.603 | 0.841 | 0.278 | 0.131 | 0.062 (·) | 0.644 | 0.001 | 0.155 |
| Heteroptera | 0.322 | 0.474 | 0.850 | 0.093 (·) | 0.138 | 0.089 (·) | 0.088 (·) | 0.094 | 0.217 |
| Cicadomorpha | 0.824 | 0.256 | 0.977 | 0.115 | 0.317 | 0.393 | 0.796 | 0.233 | 0.601 |
| Fulgoromorpha | 0.274 | 0.392 | 0.134 | 1.0 | --(2) | 0.133 | 0.001 | 0.649 | |
| Sternorrhyncha | 0.403 | 0.554 | 0.270 | 0.423 | 0.034 (T2 > T1) | 0.091 (·) | 0.305 | 0.899 | 0.120 |
| Chalcidoidea | 0.509 | 0.085 (·) | 0.566 | 0.740 | --(2) | 0.060 (·) | 0.001 | 0.326 | |
| Ichneumonoidea | 0.977 | 0.012 (*) | 0.405 | 0.453 | 0.043 (T2 > T1) | 0.002 (**) | 0.060 (·) | 0.001 | 0.055 |
| Formicidae | 0.732 | 0.644 | 0.823 | 0.020 (*) | 0.316 | 0.051 (·) | 0.872 | 0.109 | 0.674 |
| Other Hymenoptera | --(2) | 0.700 | 0.146 | --(1) | |||||
| Neuroptera | 0.491 | 0.695 | 0.145 | 0.145 | 0.985 | 0.139 | 0.381 | 0.045 | 0.270 |
| Psocoptera | --(2) | 0.195 | 0.133 | 0.914 | 0.383 | 0.806 | 0.098 | 0.696 | |
| Thysanoptera | --(2) | 0.261 | 0.497 | --(2) | |||||
| Total arthropods | 0.640 | 0.759 | 0.016 (T1 > T2) | 0.780 | 0.026 (T1 > T2) | 0.014 (*) | 0.001 (T1 > T2) | 0.001 | 0.055 |
| VISUAL | |||||||||
| Euphyllura (fl+sh) | 0.161 | 0.297 | |||||||
| Euphyllura (fl) | 0.853 | 0.853 | --(2) | 0.715 | 0.580 | 0.181 | 0.001 | 0.162 | |
| Euphyllura (sh) | --(1) | --(2) | 1.0 | 1.0 | |||||
| Lepidoptera | 0.183 | 0.493 | 0.004 (T1 > T2) | 0.192 | 0.170 | 0.089 (·) | 0.001 (T1 > T2) | 0.001 | 0.001 |
| Feeding spots (a) | 0.256 | 0.565 | 0.217 | 0.339 | 0.725 | 0.486 | 0.276 | 0.001 | 0.491 |
| Feeding spots (b) | --(1) | 0.014 (T1 > T2) | 0.478 | 0.878 | 0.104 | 0.042 (T1 > T2) | 0.001 | 0.054 | |
| Heteroptera | --(2) | 0.604 | 0.233 | 0.062 (·) | 0.494 | 0.143 | 0.044 | 0.188 | |
| Neuroptera | --(2) | 0.779 | 0.558 | 0.438 | 0.288 | 0.374 | 0.005 | 0.858 | |
| Eriophyidae | 0.695 | 0.675 | 0.001 (T1 > T2) | 0.033 (*) | 0.003 (T1 > T2) | 0.068 (·) | 0.026 (T1 > T2) | 0.001 | 0.234 |
| Phytoseiidae | --(2) | 0.557 | 0.209 | --(2) | |||||
| Other arthropods | 0.335 | 0.753 | --(2) | --(2) | |||||
| Venturia | 0.802 | 0.330 | 0.739 | 0.368 | 0.807 | 0.143 | 0.948 | 0.001 | 0.930 |
| 2017 | 2018 | 2019 | Total 1 | ||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| Arachnida | 3.44 ± 0.58 | 4.14 ± 0.92 | 2.25 ± 0.37 | 1.80 ± 0.33 | 1.48 ± 0.31 | 1.00 ± 0.23 | 883 |
| Araneae | 1.14 ± 0.17 | 1.02 ± 0.17 | 1.52 ± 0.38 | 1.16 ± 0.25 | 0.91 * ± 0.16 | 0.61 * ± 0.18 | 394 |
| Acari | 2.30 ± 0.49 | 3.13 ± 0.83 | 0.73 ± 0.30 | 0.64 ± 0.31 | 0.57 ± 0.22 | 0.39 ± 0.20 | 489 |
| Collembola | 0.86 ± 0.41 | 0.59 ± 0.23 | 1.64 ± 0.71 | 1.58 ± 0.73 | 0.16 ± 0.09 | 0.27 ± 0.14 | 323 |
| Coleoptera | 0.44 ± 0.09 | 0.20 ± 0.06 | 0.81 ± 0.16 | 0.59 ± 0.12 | 0.88 ± 0.21 | 0.68 ± 0.20 | 218 |
| Diptera | 18.56 ± 2.27 | 16.66 ± 1.75 | 33.16 ± 3.27 | 24.36 ± 2.94 | 15.50 ± 1.45 | 12.05 ± 1.09 | 7491 |
| Nematocera | 15.91 ± 1.78 | 13.42 ± 1.50 | 31.53 * ± 3.17 | 23.02 * ± 2.85 | 14.05 ± 1.35 | 11.09 ± 0.97 | 6794 |
| Brachycera | 2.66 ± 0.72 | 3.23 ± 0.78 | 1.16 ± 0.18 | 1.27 ± 0.23 | 1.45 ± 0.2 | 0.96 ± 0.23 | 697 |
| Hemiptera | 8.25 ± 1.1 | 7.20 ± 0.9 | 8.08 ± 2.0 | 7.81 ± 1.7 | 6.34 ± 1.2 | 4.14 ± 1.02 | 2591 |
| Heteroptera | 6.41 ± 0.9 | 5.81 ± 0.8 | 7.09 ± 2.0 | 6.44 ± 1.7 | 5.36 ± 1.1 | 2.98 ± 0.89 | 2126 |
| Cicadomorph | 0.19 ± 0.0 | 0.19 ± 0.0 | 0.33 ± 0.1 | 0.28 ± 0.0 | 0.25 ± 0.0 | 0.14 ± 0.05 | 88 |
| Fulgoromorpha | 0.44 ± 0.30 | 0.13 ± 0.07 | 0.03 ± 0.02 | 0.00 ± 0.00 | 0.02 ± 0.02 | 0.00 ± 0.00 | 42 |
| Sternorryncha | 1.09 ± 0.24 | 0.83 ± 0.16 | 0.63 ± 0.12 | 1.09 ± 0.21 | 0.68 * ± 0.20 | 1.02 * ± 0.23 | 335 |
| Hymenoptera | 4.38 ± 0.42 | 4.28 ± 0.48 | 4.34 ± 0.74 | 3.95 ± 0.67 | 2.68 ± 0.54 | 2.93 ± 0.41 | 1399 |
| Chalcidoidea | 2.70 ± 0.39 | 2.63 ± 0.43 | 1.64 ± 0.42 | 0.66 ± 0.13 | 0.0 ± 0.0 | 0.07 ± 0.03 | 492 |
| Ichneumonoidea | 0.52 ± 0.10 | 0.63 ± 0.17 | 0.30 ± 0.11 | 0.42 ± 0.13 | 1.89 * ± 0.39 | 2.30 * ± 0.36 | 354 |
| Formicidae | 1.13 ± 0.21 | 0.94 ± 0.17 | 0.77 ± 0.26 | 0.75 ± 0.22 | 0.79 ± 0.22 | 0.55 ± 0.17 | 304 |
| Other | 0.02 ± 0.02 | 0.09 ± 0.04 | 1.64 ± 0.63 | 2.13 ± 0.64 | 249 | ||
| Lepidoptera | 0.22 ± 0.09 | 0.06 ± 0.03 | 0.25 ± 0.07 | 0.20 ± 0.08 | 0.18 ± 0.08 | 0.23 ± 0.11 | 70 |
| Neuroptera | 0.63 ± 0.15 | 0.69 ± 0.13 | 1.61 ± 0.54 | 1.00 ± 0.35 | 0.55 ± 0.16 | 0.57 ± 0.18 | 313 |
| Psocoptera | 0.17 ± 0.08 | 0.17 ± 0.06 | 0.72 ± 0.37 | 0.89 ± 0.38 | 0.52 ± 0.30 | 0.41 ± 0.19 | 177 |
| Thysanoptera | 0.13 ± 0.05 | 0.09 ± 0.04 | 1.06 ± 0.73 | 0.59 ± 0.43 | 0.02 ± 0.02 | 0.02 ± 0.02 | 122 |
| Total | 37.00 ± 3.33 | 33.98 ± 2.24 | 53.92 * ± 4.06 | 42.78 * ± 3.83 | 28.30 * ± 1.76 | 22.34 * ± 1.45 | 13587 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Zamora, J.E.; Alonso-López, M.T.; Gómez-Regife, Y.; Ruiz-Muñoz, S. Decreased Water Use in a Super-Intensive Olive Orchard Mediates Arthropod Populations and Pest Damage. Agronomy 2021, 11, 1337. https://doi.org/10.3390/agronomy11071337
González-Zamora JE, Alonso-López MT, Gómez-Regife Y, Ruiz-Muñoz S. Decreased Water Use in a Super-Intensive Olive Orchard Mediates Arthropod Populations and Pest Damage. Agronomy. 2021; 11(7):1337. https://doi.org/10.3390/agronomy11071337
Chicago/Turabian StyleGonzález-Zamora, José Enrique, Maria Teresa Alonso-López, Yolanda Gómez-Regife, and Sara Ruiz-Muñoz. 2021. "Decreased Water Use in a Super-Intensive Olive Orchard Mediates Arthropod Populations and Pest Damage" Agronomy 11, no. 7: 1337. https://doi.org/10.3390/agronomy11071337
APA StyleGonzález-Zamora, J. E., Alonso-López, M. T., Gómez-Regife, Y., & Ruiz-Muñoz, S. (2021). Decreased Water Use in a Super-Intensive Olive Orchard Mediates Arthropod Populations and Pest Damage. Agronomy, 11(7), 1337. https://doi.org/10.3390/agronomy11071337





