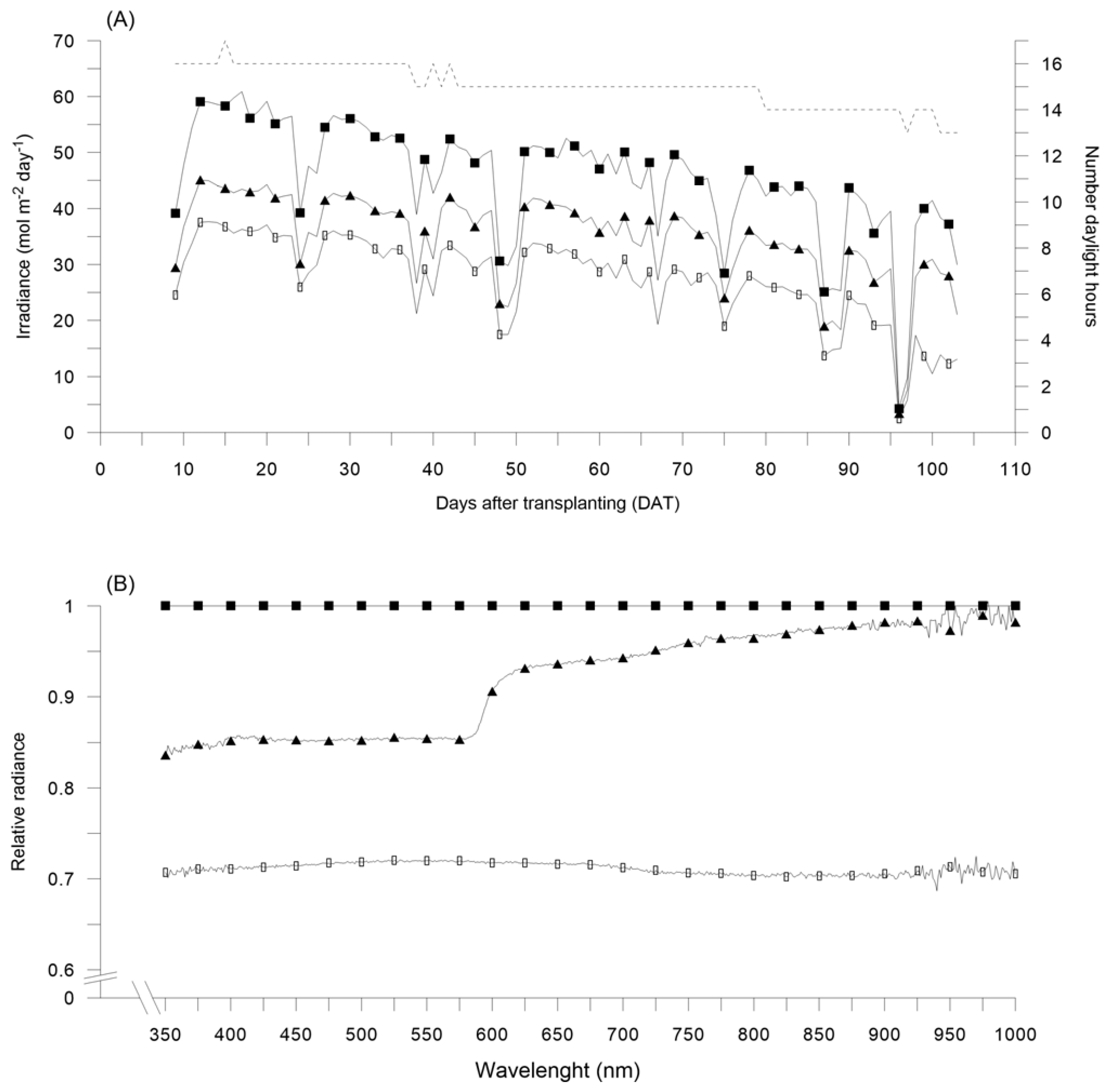
Figure 1.
(A) Irradiance daily quantum input (mol m−2 day−1) as observed under red (RN) and black (BN) photo-selective nets with respect to unshaded control (Control), starting form 9 days after bell pepper transplanting (DAT), in 2017; dashed line indicates the number of daylight hours during pepper growing cycle. (B) Relative radiance as observed under RN and BN with respect to Control, in 2017. Control: black squares; RN: black triangles; BN: black circles.
Figure 1.
(A) Irradiance daily quantum input (mol m−2 day−1) as observed under red (RN) and black (BN) photo-selective nets with respect to unshaded control (Control), starting form 9 days after bell pepper transplanting (DAT), in 2017; dashed line indicates the number of daylight hours during pepper growing cycle. (B) Relative radiance as observed under RN and BN with respect to Control, in 2017. Control: black squares; RN: black triangles; BN: black circles.
Figure 2.
Biomass allocation among the structural aerial fractions of pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017. Light-gray chart: leaf mass ratio (LMR); white chart: stem mass ratio (SMR); deep-gray chart: reproductive organs (i.e., flowers and/or fruits) mass ratio (ReMR). Data are means ± standard errors, over Exp_1 and Exp_2 (n = 6). In box, p-values from the analysis of variance (ANOVA) at 5% level of probability: N fertilization rates (N_rates); Sh levels (Sh_lev). * p < 0.05; ** p < 0.01; n.s. = not significant.
Figure 2.
Biomass allocation among the structural aerial fractions of pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017. Light-gray chart: leaf mass ratio (LMR); white chart: stem mass ratio (SMR); deep-gray chart: reproductive organs (i.e., flowers and/or fruits) mass ratio (ReMR). Data are means ± standard errors, over Exp_1 and Exp_2 (n = 6). In box, p-values from the analysis of variance (ANOVA) at 5% level of probability: N fertilization rates (N_rates); Sh levels (Sh_lev). * p < 0.05; ** p < 0.01; n.s. = not significant.
Figure 3.
Time course of leaf+stem N content per shoot (NLw, g plant−1), total N content per shoot (NTw, g plant−1)—left y-axis—and leaf+stem N content per unit leaf area (NLa, g cm−2)—right y-axis—from transplanting (0 days after transplanting, DAT) till 83 DAT. ♦ represents the NLw value recorded at 0 DAT, before the treatments application; ∆ represents the NLa value recorded at 0 DAT, before the treatments application. Data are averaged over Exp_1 and Exp_2, for n = 6. Aggregated mean values of the standard errors are: NLw, ±0.032 g plant−1 for 0_N, ±0.051 g plant−1 for 100_N, ±0.044 g plant−1 for 200_N; NTw, ±0.247 g plant−1 for 0_N, ±0.240 g plant−1 for 100_N, ±0.288 g plant−1 for 200_N; NLa, ±0.041 g cm−2 for 0_N, ±0.048 g cm−2 for 100_N, ±0.051 g cm−2 for 200_N.
Figure 3.
Time course of leaf+stem N content per shoot (NLw, g plant−1), total N content per shoot (NTw, g plant−1)—left y-axis—and leaf+stem N content per unit leaf area (NLa, g cm−2)—right y-axis—from transplanting (0 days after transplanting, DAT) till 83 DAT. ♦ represents the NLw value recorded at 0 DAT, before the treatments application; ∆ represents the NLa value recorded at 0 DAT, before the treatments application. Data are averaged over Exp_1 and Exp_2, for n = 6. Aggregated mean values of the standard errors are: NLw, ±0.032 g plant−1 for 0_N, ±0.051 g plant−1 for 100_N, ±0.044 g plant−1 for 200_N; NTw, ±0.247 g plant−1 for 0_N, ±0.240 g plant−1 for 100_N, ±0.288 g plant−1 for 200_N; NLa, ±0.041 g cm−2 for 0_N, ±0.048 g cm−2 for 100_N, ±0.051 g cm−2 for 200_N.
Figure 4.
N concentration in the structural aerial fractions (A), leaves + stems; (B), green fruits; (C) ripening fruits; (D), mature fruits) of pepper plants subjected to different N fertilization rates 0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 83 days after transplanting (DAT) in 2017. Data are means, over Exp_1 and Exp_2, for n = 6. In boxes, significance from the analysis of variance (ANOVA) at 5% level of probability: N fertilization rates (N_rates); Sh levels (Sh_lev). Vertical bars represent the standard errors of the difference between means (s.e.d.) for N_rates (s.e.d. 1), Sh_lev (s.e.d. 2) and N_rates × Sh_lev (s.e.d. 3). * p < 0.05; ** p < 0.01; n.s. = not significant.
Figure 4.
N concentration in the structural aerial fractions (A), leaves + stems; (B), green fruits; (C) ripening fruits; (D), mature fruits) of pepper plants subjected to different N fertilization rates 0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 83 days after transplanting (DAT) in 2017. Data are means, over Exp_1 and Exp_2, for n = 6. In boxes, significance from the analysis of variance (ANOVA) at 5% level of probability: N fertilization rates (N_rates); Sh levels (Sh_lev). Vertical bars represent the standard errors of the difference between means (s.e.d.) for N_rates (s.e.d. 1), Sh_lev (s.e.d. 2) and N_rates × Sh_lev (s.e.d. 3). * p < 0.05; ** p < 0.01; n.s. = not significant.
Figure 5.
Dynamic of SPAD as recorded during the growth cycle of pepper plants subjected to different N fertilization rates 0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN), in 2017. Control: black squares; RN: black triangles; BN: black circles. Data are means, over Exp_1 and Exp_2, for n = 6. In boxes, significance from the analysis of variance (ANOVA) at 5% level of probability: N fertilization rates (N_rates); Sh levels (Sh_lev). At each sampling date, vertical bars represent the standard errors of the difference between means (s.e.d.) for N_rates × Sh_lev. * p < 0.05; ** p < 0.01; n.s. = not significant.
Figure 5.
Dynamic of SPAD as recorded during the growth cycle of pepper plants subjected to different N fertilization rates 0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN), in 2017. Control: black squares; RN: black triangles; BN: black circles. Data are means, over Exp_1 and Exp_2, for n = 6. In boxes, significance from the analysis of variance (ANOVA) at 5% level of probability: N fertilization rates (N_rates); Sh levels (Sh_lev). At each sampling date, vertical bars represent the standard errors of the difference between means (s.e.d.) for N_rates × Sh_lev. * p < 0.05; ** p < 0.01; n.s. = not significant.
Figure 6.
Dynamic of leaf temperature (TIR, °C) as recorded during the growth cycle of pepper plants subjected to different N fertilization rates 0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN), in 2017. Control: black squares; RN: black triangles; BN: black circles. Data are means, over Exp_1 and Exp_2, for n = 6. In boxes, significance from the analysis of variance (ANOVA) at 5% level of probability: N rates (Fact_1); Sh levels (Fact_2). At each sampling date, vertical bars represent the standard errors of the difference between means (s.e.d.) for Fact_1 × Fact_2. * p < 0.05; ** p < 0.01; n.s. = not significant.
Figure 6.
Dynamic of leaf temperature (TIR, °C) as recorded during the growth cycle of pepper plants subjected to different N fertilization rates 0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN), in 2017. Control: black squares; RN: black triangles; BN: black circles. Data are means, over Exp_1 and Exp_2, for n = 6. In boxes, significance from the analysis of variance (ANOVA) at 5% level of probability: N rates (Fact_1); Sh levels (Fact_2). At each sampling date, vertical bars represent the standard errors of the difference between means (s.e.d.) for Fact_1 × Fact_2. * p < 0.05; ** p < 0.01; n.s. = not significant.
Table 1.
Leaves dry weight (DW, g plant−1), stems DW (g plant−1), reproductive organs DW (g plant−1) and total DW (g plant−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
Table 1.
Leaves dry weight (DW, g plant−1), stems DW (g plant−1), reproductive organs DW (g plant−1) and total DW (g plant−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
| Effects | Leaves DW | Stems DW | Reproductive Organs DW | Total DW |
|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. |
|---|
| 21 DAT | | | | | | | | | | | | | | | | |
| 0_N | 0.85 | 0.82 | 0.73 | 0.80 A | 0.27 | 0.29 | 0.27 | 0.28 A | 0.05 a | 0.06 ab | 0.06 ab | 0.06 | 1.18 | 1.17 | 1.06 | 1.13 A |
| 100_N | 1.38 | 1.19 | 1.16 | 1.24 B | 0.42 | 0.39 | 0.40 | 0.40 B | 0.12 d | 0.08 bc | 0.08 bc | 0.10 | 1.92 | 1.66 | 1.64 | 1.74 B |
| 200_N | 1.03 | 1.23 | 1.10 | 1.12 B | 0.33 | 0.39 | 0.42 | 0.38 B | 0.09 bc | 0.11 cd | 0.10 cd | 0.10 | 1.45 | 1.73 | 1.62 | 1.60 B |
| o.m. | 1.09 | 1.08 | 0.99 | | 0.34 | 0.36 | 0.36 | | 0.09 | 0.08 | 0.08 | | 1.52 | 1.52 | 1.44 | |
| N_rates | < 0.01 ** | < 0.01 ** | < 0.01 ** | < 0.01 ** |
| Sh_lev | 0.39 n.s. | 0.69 n.s. | 0.77 n.s. | 0.65n.s. |
| N_rates × Sh_lev | 0.32 n.s. | 0.31 n.s. | 0.02 * | 0.25 n.s. |
| 42 DAT | | | | | | | | | | | | | | | | |
| 0_N | 6.8 | 7.3 | 6.5 | 6.9 A | 4.5 | 4.6 | 4.5 | 4.5 A | 4.1 | 3.7 | 3.3 | 3.7 A | 15.4 | 15.6 | 14.0 | 15.0 A |
| 100_N | 7.8 | 10.6 | 8.7 | 9.0 B | 5.3 | 7.1 | 5.7 | 6.0 B | 5.7 | 6.3 | 3.9 | 5.3 B | 18.8 | 24.0 | 18.3 | 20.4 B |
| 200_N | 8.4 | 10.7 | 7.8 | 9.0 B | 5.8 | 7.7 | 5.2 | 6.2 B | 5.8 | 6.7 | 4.4 | 5.6 B | 20.0 | 25.1 | 17.4 | 20.9 B |
| o.m. | 7.7 A | 9.5 B | 7.7 A | | 5.2 A | 6.5 B | 5.1 A | | 5.2 B | 5.6 B | 3.9 A | | 18.1 A | 21.6 B | 16.6 A | |
| N_rates | < 0.01 ** | < 0.01 ** | < 0.01 ** | < 0.01 ** |
| Sh_lev | 0.02 * | 0.04 * | < 0.01 ** | < 0.01 ** |
| N_rates × Sh_lev | 0.61 n.s. | 0.47 n.s. | 0.27 n.s. | 0.43 n.s. |
| 83 DAT | | | | | | | | | | | | | | | | |
| 0_N | 24.8 | 22.8 | 33.6 | 27.1 A | 6.9 | 7.4 | 7.5 | 7.3 A | 84.0 | 78.9 | 86.3 | 83.0 A | 115.7 | 109.0 | 127.4 | 117.4 A |
| 100_N | 37.8 | 38.3 | 45.6 | 40.6 B | 9.1 | 10.1 | 8.4 | 9.2 B | 103.8 | 109.0 | 84.4 | 99.1 B | 150.7 | 157.5 | 138.3 | 148.8 B |
| 200_N | 41.8 | 45.1 | 46.0 | 44.3 B | 11.2 | 10.2 | 11.6 | 11.0 C | 119.9 | 107.7 | 91.3 | 106.3 B | 172.9 | 163.1 | 149.0 | 161.7 B |
| o.m. | 34.8 A | 35.4 A | 41.7 B | | 9.1 | 9.2 | 9.2 | | 102.6 | 98.5 | 87.3 | | 146.4 | 143.2 | 138.2 | |
| N_rates | < 0.01 ** | < 0.01 ** | < 0.01 ** | < 0.01 ** |
| Sh_lev | < 0.01 ** | 0.98 n.s. | 0.07 n.s. | 0.54 n.s. |
| N_rates × Sh_lev | 0.31 n.s. | 0.62 n.s. | 0.21 n.s. | 0.19 n.s. |
Table 2.
Number of leaves (NumLf, num plant−1), leaf area (LA, cm2 plant−1), single LA (cm2 leaf−1) and specific LA (SLA, cm2 g−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
Table 2.
Number of leaves (NumLf, num plant−1), leaf area (LA, cm2 plant−1), single LA (cm2 leaf−1) and specific LA (SLA, cm2 g−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
| Effects | NumLf | LA | Single LA | SLA |
|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. |
|---|
| 21 DAT | | | | | | | | | | | | | | | | |
| 0_N | 28 | 30 | 28 | 29 A | 63.9 | 60.6 | 58.2 | 60.9 A | 2.3 a | 2.1 a | 2.1 a | 2.2 | 75.9 | 76.0 | 81.2 | 77.7 |
| 100_N | 43 | 39 | 35 | 39 B | 98.8 | 95.1 | 105.2 | 99.7 C | 2.3 a | 2.5 a | 3.0 b | 2.6 | 75.3 | 82.4 | 91.9 | 83.2 |
| 200_N | 37 | 39 | 36 | 37 B | 80.6 | 90.8 | 79.1 | 83.5 B | 2.2 a | 2.3 a | 2.2 a | 2.3 | 80.2 | 74.3 | 72.4 | 75.6 |
| o.m. | 36 | 36 | 33 | | 81.1 | 82.2 | 80.8 | | 2.3 | 2.3 | 2.5 | | 77.1 | 77.6 | 81.8 | |
| N_rates | <0.01 ** | <0.01 ** | <0.01 ** | 0.10 n.s. |
| Sh_lev | 0.25 n.s. | 0.93 n.s. | 0.24 n.s. | 0.42 n.s. |
| N_rates × Sh_lev | 0.39 n.s. | 0.16 n.s. | 0.03 * | 0.20 n.s. |
| 42 DAT | | |
| 0_N | 164 | 151 | 133 | 149 A | 445 a | 615 b | 623 b | 561 | 2.7 a | 4.1 cd | 4.7 d | 3.8 | 72.5 ab | 84.7 c | 95.8 de | 84.3 |
| 100_N | 166 | 188 | 143 | 166 B | 692 bc | 657 bc | 878 d | 742 | 4.2 d | 3.5 bc | 6.2 e | 4.6 | 89.9 cd | 63.3 a | 103.1 e | 85.5 |
| 200_N | 188 | 170 | 133 | 164 B | 621 b | 923 d | 759 c | 768 | 3.3 ab | 5.5 e | 5.8 e | 4.9 | 73.8 b | 88.6 cd | 97.8 de | 86.7 |
| o.m. | 173 B | 170 B | 136 A | | 586 | 731 | 753 | | 3.4 | 4.4 | 5.5 | | 78.7 | 78.9 | 98.9 | |
| N_rates | <0.01 ** | <0.01 ** | <0.01 ** | 0.60 n.s. |
| Sh_lev | <0.01 ** | <0.01 ** | <0.01 ** | <0.01 ** |
| N_rates × Sh_lev | 0.21 n.s. | <0.01 ** | <0.01 ** | <0.01 ** |
| 83 DAT | | | | | | | | | | | | | | | | |
| 0_N | 232 bcd | 181 a | 199 ab | 204 | 1085 | 1054 | 1545 | 1228 A | 4.7 | 5.9 | 7.9 | 6.2 A | 47.1 | 48.0 | 45.6 | 46.9 |
| 100_N | 249 cde | 217 bc | 257 de | 241 | 1357 | 1629 | 1813 | 1599 B | 5.5 | 7.5 | 7.1 | 6.7 A | 38.0 | 42.7 | 40.9 | 40.5 |
| 200_N | 274 e | 283 e | 234 bcd | 264 | 1718 | 2097 | 2240 | 2018 C | 6.3 | 7.4 | 9.6 | 7.8 B | 42.2 | 46.8 | 49.0 | 46.0 |
| o.m. | 252 | 227 | 230 | | 1387 A | 1593 AB | 1866 B | | 5.5 A | 6.9 B | 8.2 C | | 42.4 | 45.8 | 45.2 | |
| N_rates | <0.01 ** | <0.01 ** | <0.01 ** | 0.37 n.s. |
| Sh_lev | 0.03 * | 0.01 * | <0.01 ** | 0.63 n.s. |
| N_rates × Sh_lev | <0.01 ** | 0.79 n.s. | 0.45 n.s. | 0.92 n.s. |
Table 3.
Stem length (L, cm plant−1) and stem diameter (D, mm plant−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
Table 3.
Stem length (L, cm plant−1) and stem diameter (D, mm plant−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
| Effects | L | D |
|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. |
|---|
| 21 DAT | | | | | | | | |
| 0_N | 14.8 | 15.2 | 15.0 | 15.0 | 3.41 | 3.65 | 3.71 | 3.59 A |
| 100_N | 14.3 | 15.7 | 16.6 | 15.5 | 4.54 | 4.39 | 4.33 | 4.42 B |
| 200_N | 15.0 | 15.6 | 15.7 | 15.4 | 4.17 | 4.73 | 4.56 | 4.48 B |
| o.m. | 14.7 A | 15.5 B | 15.8 B | | 4.04 | 4.26 | 4.20 | |
| N_rates | 0.36 n.s. | <0.01 ** |
| Sh_lev | 0.01 * | 0.50 n.s. |
| N_rates × Sh_lev | 0.14 n.s. | 0.56 n.s. |
| 42 DAT | |
| 0_N | 31.28 | 34.73 | 38.23 | 34.74 A | 7.49 a | 7.95 abcd | 8.36 cde | 7.93 |
| 100_N | 34.18 | 40.50 | 41.68 | 38.78 B | 7.88 abc | 8.37 de | 8.73 ef | 8.32 |
| 200_N | 33.88 | 38.66 | 40.38 | 37.64 B | 7.71 ab | 9.05 f | 8.12 bcd | 8.29 |
| o.m. | 33.11 A | 37.96 B | 40.09 B | | 7.69 | 8.46 | 8.40 | |
| N_rates | <0.01 ** | 0.15 n.s. |
| Sh_lev | <0.01 ** | <0.01 ** |
| N_rates × Sh_lev | 0.92 n.s. | <0.01 ** |
| 83 DAT | |
| 0_N | 35.0 | 37.0 | 37.4 | 36.5 | 10.3 b | 9.8 a | 10.8 bc | 10.3 |
| 100_N | 36.3 | 36.2 | 38.8 | 37.1 | 11.4 de | 11.2 cde | 11.5 e | 11.3 |
| 200_N | 37.5 | 38.7 | 38.0 | 38.1 | 10.9 cd | 11.4 de | 12.0 f | 11.4 |
| o.m. | 36.3 | 37.3 | 38.1 | | 10.9 | 10.8 B | 11.4 | |
| N_rates | 0.09 n.s. | <0.01 ** |
| Sh_lev | 0.18 n.s. | < 0.01 ** |
| N_rates × Sh_lev | 0.66 n.s. | 0.02 * |
Table 4.
Relative growth rate (RGR, g g−1 DAT—days after transplanting), net assimilation rate (NAR, g cm−2 DAT−1) and leaf area ratio (cm2 g−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 42 and 83 DAT in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
Table 4.
Relative growth rate (RGR, g g−1 DAT—days after transplanting), net assimilation rate (NAR, g cm−2 DAT−1) and leaf area ratio (cm2 g−1) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 42 and 83 DAT in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
| Effects | RGR | NAR | LAR |
|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. |
|---|
| 42 DAT | |
| 0_N | 0.120 | 0.123 | 0.124 | 0.123 | 0.0033 def | 0.0029 bcd | 0.0027 bc | 0.0029 | 42.7 | 46.6 | 49.8 | 46.4 AB |
| 100_N | 0.110 | 0.127 | 0.115 | 0.117 | 0.0026 abc | 0.0037 f | 0.0022 a | 0.0028 | 45.0 | 43.5 | 56.9 | 48.5 B |
| 200_N | 0.127 | 0.127 | 0.113 | 0.122 | 0.0034 ef | 0.0031 cde | 0.0025 ab | 0.0030 | 44.6 | 45.1 | 46.6 | 45.4 A |
| o.m. | 0.119 | 0.126 | 0.117 | | 0.0031 | 0.0032 | 0.0024 | | 44.1 A | 45.1 A | 51.1 B | |
| N_rates | 0.22 n.s. | 0.24 n.s. | 0.04 * |
| Sh_lev | 0.05 n.s. | <0.01 ** | <0.01 ** |
| N_rates × Sh_lev | 0.07 n.s. | <0.01 ** | 0.06 n.s. |
| 83 DAT | |
| 0_N | 0.050 | 0.048 | 0.054 | 0.051 | 0.0035 | 0.0028 | 0.0028 | 0.0030 | 20.1 a | 24.9 cd | 27.7 de | 24.2 |
| 100_N | 0.051 | 0.046 | 0.050 | 0.049 | 0.0033 | 0.0031 | 0.0023 | 0.0029 | 23.1 bc | 19.1 a | 30.9 f | 24.4 |
| 200_N | 0.053 | 0.046 | 0.053 | 0.050 | 0.0036 | 0.0024 | 0.0024 | 0.0028 | 20.6 ab | 25.1 cd | 29.4 ef | 25.0 |
| o.m. | 0.051 | 0.046 | 0.052 | | 0.0035 B | 0.0028 A | 0.0025 A | | 21.3 | 23.0 | 29.3 | |
| N_rates | 0.59 n.s. | 0.44 n.s. | 0.64 n.s. |
| Sh_lev | 0.06 n.s. | <0.01 ** | <0.01 ** |
| N_rates × Sh_lev | 0.93 n.s. | 0.33 n.s. | <0.01 ** |
Table 5.
Chlorophyll a (Chla, µg g−1 FW), Chlb (µg g−1 FW), and carotenoid (Car, µg g−1 FW) concentration as determined in leaves of pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21 and 42 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
Table 5.
Chlorophyll a (Chla, µg g−1 FW), Chlb (µg g−1 FW), and carotenoid (Car, µg g−1 FW) concentration as determined in leaves of pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21 and 42 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
| Effects | 21 DAT | 42 DAT |
|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. |
|---|
| Chla | | | | | | | | |
| 0_N | 1237.3 | 1256.9 | 1534.8 | 1343.0 A | 1533.6 | 1522.3 | 1688.4 | 1581.4 |
| 100_N | 1367.4 | 1552.6 | 1556.8 | 1492.3 B | 1688.5 | 1702.8 | 1631.8 | 1674.4 |
| 200_N | 1382.8 | 1440.3 | 1618.6 | 1480.6 AB | 1874.0 | 1751.3 | 1801.5 | 1808.9 |
| o.m. | 1329.2 A | 1416.6 AB | 1570.1 B | | 1698.7 | 1658.8 | 1707.2 | |
| N_rates | <0.01 ** | 0.14 n.s. |
| Sh_lev | <0.01 ** | 0.91 n.s. |
| N_rates × Sh_lev | 0.55 n.s. | 0.89 n.s. |
| Chlb | |
| 0_N | 523.6 | 527.8 | 658.4 | 569.9 A | 660.1 | 656.8 | 692.0 | 669.7 |
| 100_N | 586.6 | 713.2 | 707.5 | 669.1 B | 705.8 | 703.4 | 693.6 | 700.9 |
| 200_N | 593.1 | 577.5 | 682.8 | 617.8 AB | 805.6 | 736.1 | 774.7 | 772.1 |
| o.m. | 567.8 A | 606.2 AB | 682.9 B | | 723.9 | 698.8 | 720.1 | |
| N_rates | 0.03 * | 0.13 n.s. |
| Sh_lev | 0.02 * | 0.87 n.s. |
| N_rates × Sh_lev | 0.46 n.s. | 0.97 n.s. |
| Car | |
| 0_N | 412.4 | 418.8 | 438.6 | 423.2 A | 464.6 | 426.4 | 452.6 | 447.9 |
| 100_N | 430.0 | 461.5 | 486.2 | 459.2 AB | 526.1 | 474.8 | 464.5 | 488.5 |
| 200_N | 475.6 | 459.7 | 481.1 | 472.1 B | 567.2 | 500.2 | 505.7 | 524.4 |
| o.m. | 439.3 | 446.7 | 468.6 | | 519.3 | 467.1 | 474.3 | |
| N_rates | <0.01 ** | 0.14 n.s. |
| Sh_lev | 0.19 n.s. | 0.27 n.s. |
| N_rates × Sh_lev | 0.72 n.s. | 0.97 n.s. |
Table 6.
Normalized difference vegetation index (NDVI); green normalized difference vegetation index (GNDVI); modified chlorophyll absorption ratio index (MCARI); optimized soil-adjusted vegetation index (OSAVI); and water index (WI), as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 15, 41 and 91 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
Table 6.
Normalized difference vegetation index (NDVI); green normalized difference vegetation index (GNDVI); modified chlorophyll absorption ratio index (MCARI); optimized soil-adjusted vegetation index (OSAVI); and water index (WI), as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 15, 41 and 91 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
| Effects | 15 DAT | 41 DAT | 91 DAT |
|---|
| Control | RN | BN | o.m. | Control | RN | BN | o.m. | Control | RN | BN | o.m. |
|---|
| NDVI | |
| 0_N | 0.785 | 0.802 | 0.809 | 0.799 | 0.926 | 0.926 | 0.901 | 0.918 | 0.872 | 0.858 | 0.866 | 0.865 |
| 100_N | 0.839 | 0.816 | 0.822 | 0.826 | 0.940 | 0.922 | 0.895 | 0.919 | 0.869 | 0.864 | 0.860 | 0.864 |
| 200_N | 0.825 | 0.837 | 0.766 | 0.809 | 0.928 | 0.915 | 0.892 | 0.912 | 0.892 | 0.867 | 0.869 | 0.876 |
| o.m. | 0.816 | 0.819 | 0.799 | | 0.931 B | 0.921 B | 0.896 A | | 0.878 | 0.863 | 0.865 | |
| N_rates | 0.25 n.s. | 0.23 n.s. | 0.19 n.s. |
| Sh_lev | 0.61 n.s. | <0.01 ** | 0.11 n.s. |
| N_rates × Sh_lev | 0.40 n.s. | 0.32 n.s. | 0.80 n.s. |
| GNDVI | |
| 0_N | 0.571 | 0.584 | 0.578 | 0.577 A | 0.694 | 0.734 | 0.715 | 0.714 A | 0.762 | 0.763 | 0.769 | 0.765 |
| 100_N | 0.598 | 0.651 | 0.600 | 0.617 B | 0.740 | 0.769 | 0.762 | 0.757 B | 0.762 | 0.756 | 0.760 | 0.759 |
| 200_N | 0.628 | 0.608 | 0.605 | 0.614 B | 0.723 | 0.776 | 0.767 | 0.756 B | 0.768 | 0.764 | 0.774 | 0.769 |
| o.m. | 0.599 | 0.614 | 0.594 | | 0.719 A | 0.760 B | 0.748 B | | 0.764 | 0.761 | 0.768 | |
| N_rates | <0.01 ** | <0.01 ** | 0.59 n.s. |
| Sh_lev | 0.17 n.s. | <0.01 ** | 0.70 n.s. |
| N_rates × Sh_lev | 0.09 n.s. | 0.81 n.s. | 0.98 n.s. |
| MCARI | |
| 0_N | 0.049 | 0.052 | 0.057 | 0.053 | 0.059 | 0.046 | 0.048 | 0.051 B | 0.051 | 0.043 | 0.034 | 0.043 |
| 100_N | 0.044 | 0.054 | 0.057 | 0.052 | 0.050 | 0.047 | 0.046 | 0.048 AB | 0.047 | 0.042 | 0.039 | 0.042 |
| 200_N | 0.051 | 0.060 | 0.048 | 0.053 | 0.047 | 0.040 | 0.037 | 0.041 A | 0.043 | 0.044 | 0.043 | 0.043 |
| o.m. | 0.048 | 0.055 | 0.054 | | 0.052 B | 0.044 A | 0.044 A | | 0.047 B | 0.043 AB | 0.039 A | |
| N_rates | 0.67 n.s. | 0.03 * | 0.91 n.s. |
| Sh_lev | 0.22 n.s. | 0.02 * | <0.01 ** |
| N_rates × Sh_lev | 0.42 n.s. | 0.81 n.s. | 0.07 n.s. |
| OSAVI | | | | | | | | | | | | |
| 0_N | 0.633 | 0.654 | 0.644 | 0.644 | 0.880 | 0.886 | 0.844 | 0.870 | 0.837 | 0.820 | 0.827 | 0.828 |
| 100_N | 0.698 | 0.649 | 0.611 | 0.653 | 0.888 | 0.883 | 0.852 | 0.874 | 0.851 | 0.831 | 0.837 | 0.840 |
| 200_N | 0.712 | 0.667 | 0.674 | 0.684 | 0.894 | 0.886 | 0.863 | 0.881 | 0.834 | 0.846 | 0.857 | 0.845 |
| o.m. | 0.681 | 0.656 | 0.643 | | 0.888 B | 0.885 B | 0.853 A | | 0.841 | 0.832 | 0.840 | |
| N_rates | 0.21 n.s. | 0.16 n.s. | 0.11 n.s. |
| Sh_lev | 0.27 n.s. | <0.01 ** | 0.53 n.s. |
| N_rates × Sh_lev | 0.50 n.s. | 0.77 n.s. | 0.37 n.s. |
| WI | | | | | | | | | | | | |
| 0_N | 1.047 | 1.059 | 1.057 | 1.054 | 1.101 | 1.099 | 1.062 | 1.088 | 1.140 | 1.078 | 1.036 | 1.085 |
| 100_N | 1.060 | 1.076 | 1.051 | 1.062 | 1.130 | 1.089 | 1.045 | 1.088 | 1.146 | 1.061 | 1.025 | 1.077 |
| 200_N | 1.047 | 1.034 | 1.065 | 1.049 | 1.081 | 1.077 | 1.051 | 1.070 | 1.164 | 1.059 | 1.037 | 1.087 |
| o.m. | 1.051 | 1.057 | 1.058 | | 1.104 B | 1.088 B | 1.053 A | | 1.150 B | 1.066 A | 1.033 A | |
| N_rates | 0.44 n.s. | 0.11 n.s. | 0.58 n.s. |
| Sh_lev | 0.63 n.s. | <0.01 ** | <0.01 ** |
| N_rates × Sh_lev | 0.06 n.s. | 0.06 n.s. | 0.32 n.s. |
Table 7.
Cuticular waxes (µg cm−2) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
Table 7.
Cuticular waxes (µg cm−2) as recorded in pepper plants subjected to different N fertilization rates (0_N: 0 kg N ha−1; 100_N: 100 kg N ha−1; 200_N: 200 kg N ha−1) and shading (Sh) levels (accomplished using: no cover, Control; red photoselective net, RN; black photoselective net, BN) at 21, 42 and 83 days after transplanting (DAT) in 2017 a; data are means over Exp_1 and Exp_2. Means followed by different letters (upper case letters: main effects; lower case letters: effects of interaction) significantly differ (Fisher’s LSD, p < 0.05).
| Effects | Cuticolar Waxes |
|---|
| Control | RN | BN | o.m. |
|---|
| 21 DAT | | | | |
| 0_N | 4.574 | 4.280 | 4.630 | 4.495 |
| 100_N | 3.888 | 3.802 | 3.170 | 3.620 |
| 200_N | 4.822 | 3.253 | 2.935 | 3.670 |
| o.m. | 4.428 | 3.778 | 3.579 | |
| N_rates | 0.17 n.s. |
| Sh_lev | 0.16 n.s. |
| N_rates × Sh_lev | 0.40 n.s. |
| 42 DAT | | | | |
| 0_N | 4.650 | 3.868 | 3.544 | 4.021 B |
| 100_N | 4.373 | 3.514 | 3.428 | 3.772 AB |
| 200_N | 3.668 | 3.371 | 2.676 | 3.238 A |
| o.m. | 4.230 B | 3.584 AB | 3.216 A | |
| N_rates | 0.03 * |
| Sh_lev | <0.01 ** |
| N_rates × Sh_lev | 0.87 n.s. |