The Effect of Mycorrhizal Fungi and PGPR on Tree Nutritional Status and Growth in Organic Apple Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Mycorrhizal Parameters
3.2. Nutritional Status of Trees
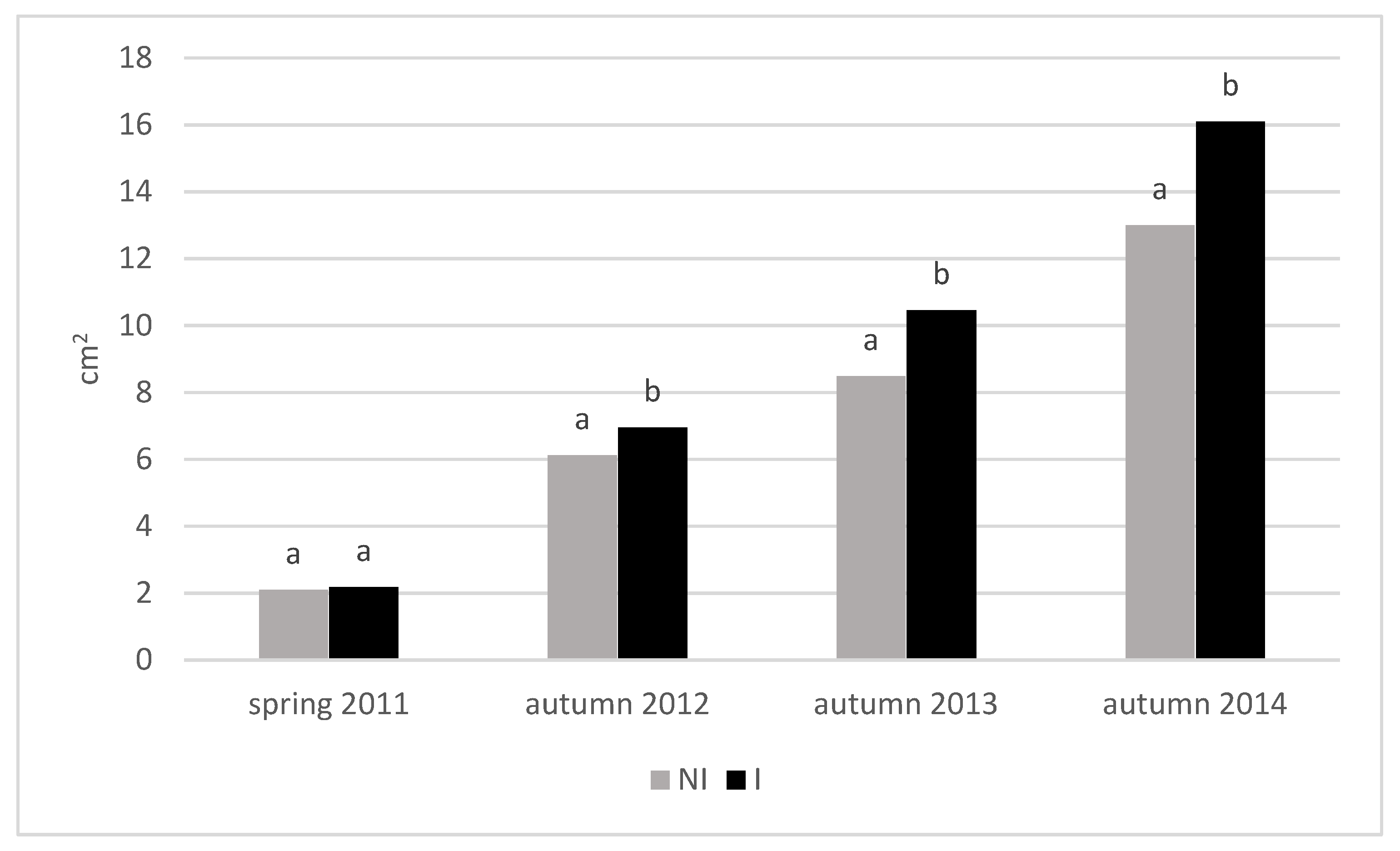
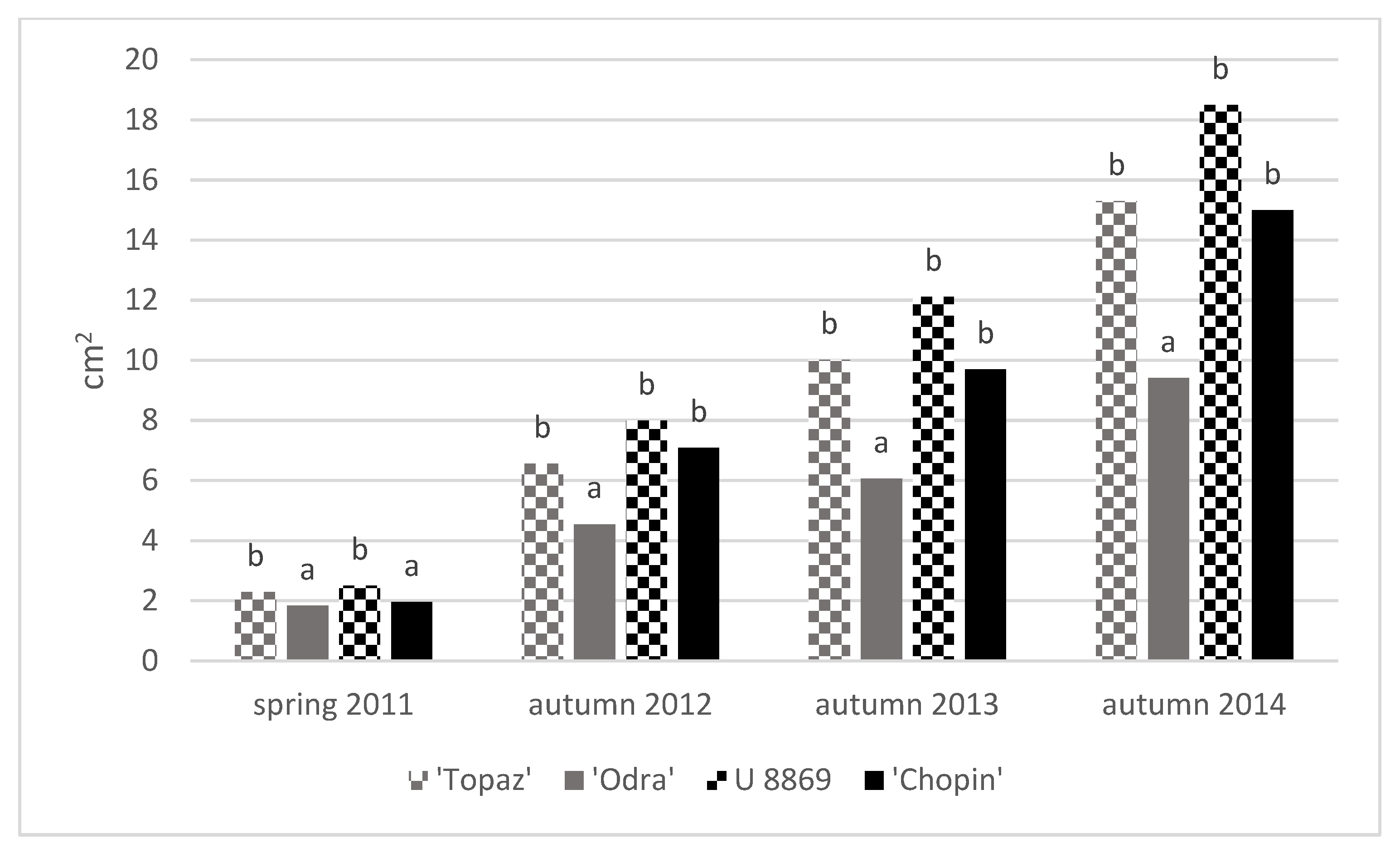
3.3. Growth of Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barański, M.; Rempelos, L.; Iversen, P.O.; Leifert, C. Effects of organic food consumption on human health; the jury is still out! Food Nutr. Res. 2017, 61, 1287333. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Content of minerals in soil, apple tree leaves and fruits depending on nitrogen fertilization. J. Elem. 2012, 22, 67–77. [Google Scholar] [CrossRef]
- Sas-Paszt, L.; Głuszek, S. Rola korzeni oraz ryzosfery we wzroście i plonowaniu roślin sadowniczych. Postęp. Nauk Roln. 2007, 6, 27–39. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Schüβler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Derkowska, E.; Paszt, L.S.; Dyki, B.; Sumorok, B. Assessment of Mycorrhizal Frequency in the Roots of Fruit Plants Using Different Dyes. Adv. Microbiol. 2015, 5, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal Fungi Can Dominate Phosphate Supply to Plants Irrespective of Growth Responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, I.; Trappe, J.M. Translocation of 14C from Festuca Plants to their Endomycorrhizal Fungi. Nat. New Biol. 1973, 244, 30–31. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Douds, D.D.; Bécard, G.; Shachar-Hill, Y. Carbon Uptake and the Metabolism and Transport of Lipids in an Arbuscular Mycorrhiza1. Plant Physiol. 1999, 120, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, I.; Rosendahl, L. Carbon Flow into Soil and External Hyphae of Vesicular–Arbuscular Mycorrhizae in Carbon and Mineral Nutrition. In Mycorrhizal Ecology; van der Heijden, G.A., Sanders, I.R., Eds.; Springer: Berlin, Germany, 1990; pp. 75–92. [Google Scholar]
- Bryla, D.R.; Eissenstat, D.M. Respiratory Costs of Mycorrhizal Associations. In Plant Respiration, 1st ed.; Lambers, H., Ribas Carbo, M., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 18, pp. 207–224. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, UK, 2007. [Google Scholar]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [Green Version]
- Drew, E.; Murray, R.; Smith, S.; Jakobsen, I. Beyond the rhizosphere: Growth and function of arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant Soil 2003, 251, 105–114. [Google Scholar] [CrossRef]
- Schnepf, A.; Jones, D.; Roose, T. Modelling Nutrient Uptake by Individual Hyphae of Arbuscular Mycorrhizal Fungi: Temporal and Spatial Scales for an Experimental Design. Bull. Math. Biol. 2011, 73, 2175–2200. [Google Scholar] [CrossRef] [Green Version]
- López-Pedrosa, A.; Gonzalez-Guerrero, M.; Valderas, A.; Azcon, C.; Ferrol, N. GintAMT1 encodes a functional high-affinity ammonium transporter that is expressed in the extraradical mycelium of Glomus intraradices. Fungal Genet. Biol. 2006, 43, 102–110. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef] [Green Version]
- Olsson, P.A.; Hammer, E.C.; Pallon, J.; van Aarle, I.M.; Wallander, H. Elemental composition in vesicles of an arbuscular mycorrhizal fungus, as revealed by PIXE analysis. Fungal Biol. 2011, 115, 643–648. [Google Scholar] [CrossRef]
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallon, J.; Wallander, H.; Hammer, E.; Arteaga Marrero, N.; Auzelyte, V.; Elfman, M. Symbiotic fungi that are essential for plant nutrient uptake investigated with NMP. Nucl. Instrum. Methods Phys. Res. B 2007, 260, 149–152. [Google Scholar] [CrossRef]
- Olsson, P.A.; Hammer, E.C.; Wallander, H.; Pallon, J. Phosphorus Availability Influences Elemental Uptake in the Mycorrhizal Fungus Glomus intraradices, as Revealed by Particle-Induced X-Ray Emission Analysis. Appl. Environ. Microbiol. 2008, 74, 4144–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondratowicz-Maciejewska, K.; Kobierski, M. Content of available magnesium, phosphorus and potassium forms in soil exposed to varied crop rotation and fertilisation. J. Elem. 2011, 16, 543–553. [Google Scholar] [CrossRef]
- Stafecka, A.; Komosa, A. The effect of soil orchard samples drying on the phosphorus, potassium, and magnesium contents determined by the universal, Egner-Riehm and Schachtschabel methods. Rocz. Akad. Rol. Pozn. CCCLVI 2004, 199–208. [Google Scholar]
- Łądkiewicz, K.; Wszędyrówny-Nast, M.; Jaśkowiak, K. Comparison of different methods for determination of organic matter content. Sci. Rev. Eng. Sci. 2017, 26, 99–107. [Google Scholar] [CrossRef]
- Sadowski, A.; Nurzyński, J.; Pacholak, E.; Smolarz, K. Określanie Potrzeb Nawożenia Roślin Sadowniczych. Zasady, Liczby Graniczne i Dawki Nawożenia, 1st ed.; SGGW-AR: Warszawa, Poland, 1990. [Google Scholar]
- Bilbao, B.; Giraldo, D.; Hevia, P. Quantitative determination of nitrogen content in plant tissue by a colorimetric method. Commun. Soil Sci. Plant Anal. 1999, 30, 1997–2005. [Google Scholar] [CrossRef]
- Galas, W.; Kita, A. Application of the ICP-AES method to the multielemental analysis in the reference samples of tobacco leaves. Rocz. Państw. Zakł. Hig. 1995, 46, 53–57. [Google Scholar]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du Taux de Mycorhization VA d’un Systemeradiculaire. Recherche de Methods D’estimation Avant une Signification Fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae, 1st ed.; Gia-ninazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Katsoulas, N.; Løes, A.-K.; Andrivon, D.; Cirvilleri, G.; De Cara, M.; Kir, A.; Knebl, L.; Malińska, K.; Oudshoorn, F.W.; Willer, H.; et al. Current use of copper, mineral oils and sulphur for plant protection in organic horticultural crops across 10 European countries. Org. Agric. 2020, 10, 159–171. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F.; Trinchera, A. An Overview on Agroecology and Organic Agriculture Strategies for Sustainable Crop Production. Agronomy 2021, 11, 223. [Google Scholar] [CrossRef]
- Gąstoł, M.; Domagała-Świątkiewicz, I. Mycorrhizal inoculation of apple in replant soils–enhanced tree growth and mineral status. Acta Sci. Pol. Hortorum Cultus 2015, 14, 17–37. Available online: https://czasopisma.up.lublin.pl/index.php/asphc/article/view/2589 (accessed on 14 May 2021).
- Derkowska, E.; Paszt, L.S.; Sumorok, B.; Dyki, B. Colonisation of apple and blackcurrant roots by arbuscular mycorrhizal fungi following mycorrhisation and the use of organic mulches. Folia Hortic. 2013, 25, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Berdeni, D.; Cotton, T.E.A.; Daniell, T.J.; Bidartondo, M.I.; Cameron, D.; Evans, K.L. The Effects of Arbuscular Mycorrhizal Fungal Colonisation on Nutrient Status, Growth, Productivity, and Canker Resistance of Apple (Malus pumila). Front. Microbiol. 2018, 9, 1461. [Google Scholar] [CrossRef] [PubMed]
- Gnekow, M.A.; Marschner, H. Role of VA-mycorrhiza in growth and mineral nutrition of apple (Malus pumila var. domestica) rootstock cuttings. Plant Soil 1989, 119, 285–293. [Google Scholar] [CrossRef]
- Derkowska, E.; Sas-Paszt, L.; Szwonek, E.; Głuszek, S. The influence of mycorrhization and mulches on myccorhizal frequency in apple and strawberry roots. J. Fruit Ornam. Plant Res. 2008, 16, 227–242. [Google Scholar]
- Sumorok, B.; Sas-Paszt, L.; Głuszek, S.; Derkowska, A.; Żurawicz, E. The effect of mycorrhizal and mulching of apple trees ‘Gold Milenium’ and blackcurrant bushes ‘Tiben’ on the occurrence of arbuscular mycorrhizal fungi. J. Fruit Ornam. Plant Res. 2011, 19, 35–49. [Google Scholar]
- Miller, R.L.; Jackson, L.E. Survey of vesicular–arbuscular mycorrhizae in lettuce production in relation to management and soil factors. J. Agric. Sci. 1998, 130, 173–182. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Ketoja, E.; Vestberg, M. Promotion of utilization of arbuscular mycorrhiza through reduced P fertilization 1. Bioassays in a growth chamber. Plant Soil 2000, 227, 191–206. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Ketiga, E.; Vestberg, M. Promotion of utilization of arbuscular mycorrhiza through reduced P fertilization. 2. Field studies. Plant Soil. 2001, 231, 65–79. [Google Scholar] [CrossRef]
- Świerczyński, S.; Stachowiak, A. The influence of mycorrhizal fungi on the growth and yielding of plum and sour cherry trees. J. Fruit Ornam. Plant Res. 2010, 18, 71–77. [Google Scholar]
- Granger, R.L.; Plenchette, C.; Fortin, J.A. Effect of A Vesicular Arbuscular (Va) Endomycorrhizal Fungus (Glomus Epigaeum) on The Growth and Leaf Mineral Content of Two Apple Clones Propagated In Vitro. Can. J. Plant Sci. 1983, 63, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Pinochet, J.; Fernández, C.; Jaizme, M.D.C.; Tenoury, P. Micropropagated Banana Infected with Meloidogyne javanica Responds to Glomus intraradices and Phosphorus. HortScience 1997, 32, 101–103. [Google Scholar] [CrossRef] [Green Version]
- Syvertsen, J.P.; Graham, J.H. Phosphorus supply and arbuscular mycorrhizas increase growth and net gas exchange responses of two Citrus spp. grown at elevated [CO2]. Plant Soil 1999, 208, 209–219. [Google Scholar] [CrossRef]
- Kothari, S.K.; Marschner, H.; Römheld, V. Direct and indirect effects of VA mycorrhizal fungi and rhizosphere microorganisms on acquisition of mineral nutrients by maize (Zea mays L.) in a calcareous soil. New Phytol. 1990, 116, 637–645. [Google Scholar] [CrossRef]
- Pietranek, A.; Jadczuk, E. Mineral status of ‘Katja’ apple trees depending on irrigation, fertilisation and rootstock. Acta Sci. Pol. Hortorum Cultus 2005, 4, 69–76. [Google Scholar]
- Wrona, D.; Sadowski, A. Effect of nitrogen and soil management on soil mineral nitrogen in the apple orchard. J. Fruit Ornam. Plant Res. 2004, 12, 191–199. [Google Scholar]
- Allen, M.F. The Ecology of Micorrhizae, 1st ed.; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Latef, A.A.H.A.; He, C. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Xiao, J.X.; Hu, C.Y.; Chen, Y.Y.; Yang, B.; Hua, J. Effects of low magnesium and an arbuscular mycorrhizal fungus on the growth, magnesium distribution and photosynthesis of two citrus cultivars. Sci. Hortic. 2014, 177, 14–20. [Google Scholar] [CrossRef]
- Taylor, J.; Harrier, L.A. A comparison of development and mineral nutrition of micropropagated Fragaria×ananassa cv. Elvira (strawberry) when colonised by nine species of arbuscular mycorrhizal fungi. Appl. Soil Ecol. 2001, 18, 205–215. [Google Scholar] [CrossRef]
- Xia, G.; Cheng, L.; Lakso, A.; Goffinet, M. Effects of Nitrogen Supply on Source-sink Balance and Fruit Size of ‘Gala’ Apple Trees. J. Am. Soc. Hortic. Sci. 2009, 134, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; De-Bashan, L.E. Bacteria/Plant Growth Promotion. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 103–115. [Google Scholar]
- Estiken, A.; Karlidag, S.; Ercisli, M.T.; Sahin, F. The effect of spraying a growth promoting bacterium on the yield, growth and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu). Aust. J. Agric. Res. 2003, 54, 377–380. [Google Scholar] [CrossRef]
- Tenuta, M. Plant Growth Promoting Rhizobacteria: Prospects for Increasing Nutrient Acquisition and Disease Control. 2003. Available online: http://www.umanitoba.ca/faculties/afs/MAC_proceedings/2003/pdf/tenuta_rhizobacteria.pdf (accessed on 24 June 2021).
- Plenchette, C.; Fortin, J.A.; Furlan, V. Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility. Plant Soil 1983, 70, 199–209. [Google Scholar] [CrossRef]
- Saif, S.R. Growth responses of tropical forage plant species to vesicular-arbuscular mycorrhizae. Plant Soil 1987, 97, 25–35. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Saggin-Júnior, O.J. Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza 2001, 11, 245–255. [Google Scholar] [CrossRef]
- Tawaraya, K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci. Plant Nutr. 2003, 49, 655–668. [Google Scholar] [CrossRef]




| Depth | pHKCl | Available Macroelements in Soil mg⋅100 g−1 | K/Mg Ratio | Organic Matter % | Share of Soil Particles with Diameter < 0.02 | ||
|---|---|---|---|---|---|---|---|
| P | K | Mg | |||||
| 0–20 | 6.2 | 1.7 | 5.8 | 17.0 | 0.34 | 2.52 | 37.6 |
| 21–40 | 7.3 | 1.3 | 2.9 | 11.4 | 0.25 | 0.99 | 25.1 |
| 41–60 | 7.5 | 1.0 | 2.5 | 10.5 | 0.24 | 0.50 | 17.9 |
| Year | Treatment | Cultivar | Mycorrhizal Parameters (%) | ||
|---|---|---|---|---|---|
| Mycorrhizal Frequency | Absolute Mycorrhizal Intensity | Relative Mycorrhizal Intensity | |||
| 2012 | NI | Topaz | A 20.7 ± 4.73 ab | A 1.33 ± 0.59 a | A 7.25 ± 4.17 a |
| Odra | A 24.8 ± 6.38 ab | A 0.66 ± 0.45 a | A 2.98 ± 2.50 a | ||
| U 8869 | A 16.2 ± 7.20 a | A 1.36 ± 1.60 a | A 7.34 ± 6.47 a | ||
| Chopin | A 37.3 ± 11.01 b | A 2.32 ± 1.54 a | A 6.05 ± 3.54 a | ||
| I | Topaz | B 72.9 ± 9.57 a | B 19.15 ± 3.86 a | B 26.43 ± 4.37 a | |
| Odra | B 91.3 ± 4.19 b | B 36.41 ± 9.76 b | B 40.23 ± 11.29 b | ||
| U 8869 | B 69.6 ± 11.35 a | B 39.77 ± 4.81 b | B 57.85 ± 2.98 c | ||
| Chopin | B 83.0 ± 7.39 ab | B 39.22 ± 4.58 b | B 47.60 ± 4.85 bc | ||
| 2013 | NI | Topaz | A 32.1 ± 11.01 ab | A 3.20 ± 1.76 a | A 9.84 ± 3.96 a |
| Odra | A 28.8 ± 8.77 ab | A 1.60 ± 1.61 a | A 5.68 ± 5.16 a | ||
| U 8869 | A 24.6 ± 8.82 a | A 2.31 ± 1.16 a | A 9.13 ± 3.26 a | ||
| Chopin | A 44.2 ± 4.19 b | A 6.28 ± 3.09 a | A 13.97 ± 5.74 a | ||
| I | Topaz | B 72.6 ± 4.20 a | B 47.58 ± 9.65 ab | B 65.32 ± 11.12 a | |
| Odra | B 67.7 ± 7.39 a | B 52.45 ± 12.44 b | B 77.08 ± 12.63 a | ||
| U 8869 | B 55.9 ± 5.69 a | B 38.08 ± 6.35 a | B 68.17 ± 8.90 a | ||
| Chopin | B 62.6 ± 16.68 a | B 56.87 ± 10.85 ab | B 65.22 ± 11.30 a | ||
| 2014 | NI | Topaz | A 37.3 ± 11.34 a | A 10.56 ± 2.34 a | A 23.73 ± 8.67 a |
| Odra | A 33.9 ± 9.95 a | A 5.03 ± 1.82 a | A 16.67 ± 9.31 a | ||
| U 8869 | A 39.6 ± 15.64 a | A 9.63 ± 5.58 a | A 22.89 ± 6.02 a | ||
| Chopin | A 31.0 ± 5.67 a | A 6.20 ± 3.77 a | A 19.32 ± 9.26 a | ||
| I | Topaz | B 80.3 ± 12.15 b | B 50.68 ± 7.46 a | B 64.15 ± 5.74 a | |
| Odra | B 73.8 ± 9.81 ab | B 47.65 ± 3.42 a | B 65.50 ± 6.21 a | ||
| U 8869 | B 60.1 ± 9.18 a | B 38.45 ± 12.11 a | B 63.53 ± 13.13 a | ||
| Chopin | B 61.8 ± 9.62 a | B 40.85 ± 1.83 a | B 67.16 ± 8.04 a | ||
| Year | 0.0882 | <0.0001 | <0.0001 | ||
| Treatment | <0.0001 | <0.0001 | <0.0001 | ||
| Cultivar | 0.0062 | 0.1259 | 0.0982 | ||
| Year × Treatment | <0.0001 | 0.0001 | <0.0001 | ||
| Year × Cultivar | 0.0061 | 0.0001 | 0.0444 | ||
| Treatment × Cultivar | 0.0015 | 0.0204 | 0.0130 | ||
| Year × Treatment × Cultivar | 0.1927 | 0.0056 | 0.0239 | ||
| Year | Treatment | Cultivar | Macronutrient (% d.m.) | |||
|---|---|---|---|---|---|---|
| N | P | K | Mg | |||
| 2012 | NI | Topaz | A 1.98 ± 0.02 c | A 0.32 ± 0.04 a | B 1.13 ± 0.09 b | A 0.24 ± 0.02 a |
| Odra | A 1.78 ± 0.05 b | B 0.42 ± 0.08 b | B 1.18 ± 0.14 b | A 0.18 ± 0.01 a | ||
| U 8869 | A 1.65 ± 0.11 a | B 0.34 ± 0.03 a | B 1.12 ± 0.24 b | A 0.17 ± 0.04 a | ||
| Chopin | A 1.95 ± 0.08 c | B 0.48 ± 0.06 b | B 0.75 ± 0.12 a | A 0.21 ± 0.13 a | ||
| I | Topaz | A 1.97 ± 0.04 a | A 0.36 ± 0.01 c | A 0.74 ± 0.04 ab | B 0.34 ± 0.03 ab | |
| Odra | B 1.94 ± 0.05 a | A 0.25 ± 0.04 b | A 0.50 ± 0.05 a | B 0.39 ± 0.05 ab | ||
| U 8869 | B 1.88 ± 0.06 a | A 0.15 ± 0.01 a | A 0.79 ± 0.05 b | B 0.30 ± 0.01 a | ||
| Chopin | B 2.11 ± 0.09 b | A 0.21 ± 0.03 ab | A 0.37 ± 0.04 a | B 0.45 ± 0.01 b | ||
| 2013 | NI | Topaz | A 2.00 ± 0.02 b | B 0.47 ± 0.03 b | B 1.22 ± 0.12 b | A 0.31 ± 0.04 a |
| Odra | A 1.84 ± 0.05 a | B 0.54 ± 0.03 b | B 1.03 ± 0.06 ab | A 0.28 ± 0.01 a | ||
| U 8869 | A 1.72 ± 0.08 a | B 0.32 ± 0.05 a | B 1.01 ± 0.24 ab | A 0.29 ± 0.03 a | ||
| Chopin | A 1.99 ± 0.07 b | B 0.39 ± 0.08 ab | B 0.80 ± 0.17 a | A 0.32 ± 0.02 a | ||
| I | Topaz | A 2.07 ± 0.06 a | A 0.40 ± 0.07 c | A 0.71 ± 0.31 b | A 0.32 ± 0.17 a | |
| Odra | B 2.09 ± 0.09 a | A 0.31 ± 0.06 b | A 0.45 ± 0.07 ab | B 0.47 ± 0.04 b | ||
| U 8869 | B 2.17 ± 0.08 a | A 0.19 ± 0.02 a | A 0.35 ± 0.03 a | B 0.43 ± 0.03 ab | ||
| Chopin | B 2.14 ± 0.07 a | A 0.21 ± 0.02 a | A 0.32 ± 0.02 a | B 0.57 ± 0.05 b | ||
| 2014 | NI | Topaz | A 2.10 ± 0.07 a | A 0.27 ± 0.06 a | B 1.27 ± 0.07 b | A 0.26 ± 0.16 a |
| Odra | A 2.04 ± 0.07 a | B 0.37 ± 0.05 ab | B 0.90 ± 0.02 a | A 0.25 ± 0.02 a | ||
| U 8869 | A 1.98 ± 0.08 a | B 0.30 ± 0.02 a | B 1.01 ± 0.13 ab | A 0.22 ± 0.04 a | ||
| Chopin | A 2.11 ± 0.05 a | B 0.42 ± 0.11 b | B 0.81 ± 0.12 a | A 0.30 ± 0.02 a | ||
| I | Topaz | A 2.07 ± 0.07 a | A 0.30 ± 0.04 a | A 0.80 ± 0.11 b | B 0.36 ± 0.03 a | |
| Odra | A 2.04 ± 0.03 a | A 0.27 ± 0.04 a | A 0.52 ± 0.13 a | B 0.39 ± 0.01 a | ||
| U 8869 | A 2.01 ± 0.11 a | A 0.27 ± 0.02 a | A 0.57 ± 0.11 ab | B 0.40 ± 0.08 a | ||
| Chopin | A 2.09 ± 0.07 a | A 0.30 ± 0.04 b | A 0.56 ± 0.22 ab | B 0.47 ± 0.05 b | ||
| Year | <0.0001 | <0.0001 | 0.0246 | <0.0001 | ||
| Treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Cultivar | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Year × Treatment | <0.0001 | 0.0107 | 0.0289 | 0.6460 | ||
| Year × Cultivar | 0.0312 | <0.0001 | 0.0451 | 0.2806 | ||
| Treatment × Cultivar | <0.0001 | 0.0002 | 0.2699 | 0.0006 | ||
| Year × Treatment × Cultivar | 0.0358 | <0.0001 | 0.4473 | 0.3523 | ||
| Treatment | Cultivar | TCSA (cm2) | TCSA Increment 2011–2014 (cm) | Leaf Area (cm2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2012 | 2013 | 2014 | |||
| NI | Topaz | 2.10 ± 0.08 | 3.46 ± 0.38 | 9.06 ± 1.41 | 13.9 ± 1.5 | 11.8 ± 1.5 | 20.4 ± 2.1 | 20.5 ± 3.1 | 19.6 ± 1.2 |
| Odra | 1.79 ± 0.25 | 2.39 ± 0.31 | 5.39 ± 0.62 | 9.50 ± 1.2 | 7.80 ± 1.1 | 25.0 ± 3.7 | 25.5 ± 3.0 | 31.6 ± 3.1 | |
| U 8869 | 2.64 ± 0.21 | 4.37 ± 0.43 | 11.2 ± 0.72 | 17.5 ± 1.7 | 14.8 ± 1.6 | 31.7 ± 2.7 | 32.1 ± 3.6 | 27.3 ± 0.6 | |
| Chopin | 1.85 ± 0.09 | 3.43 ± 0.60 | 7.99 ± 1.68 | 13.7 ± 2.4 | 11.9 ± 2.5 | 33.7 ± 5.1 | 30.7 ± 2.3 | 30.7 ± 1.5 | |
| Mean value for NI | 6.89 ± 5.0 | 11.6 ± 3.0 | 27.4 ± 5.6 | ||||||
| I | Topaz | 2.46 ± 0.15 | 4.11 ± 0.35 | 10.9 ± 2.03 | 16.4 ± 1.4 | 14.0 ± 1.4 | 25.2 ± 5.1 | 25.4 ± 5.2 | 25.1 ± 0.9 |
| Odra | 1.87 ± 0.49 | 2.83 ± 0.68 | 6.62 ± 0.97 | 11.3 ± 2.7 | 9.50 ± 2.3 | 30.3 ± 1.2 | 31.1 ± 1.7 | 35.0 ± 3.1 | |
| U 8869 | 2.37 ± 0.32 | 5.02 ± 0.08 | 12.9 ± 1.47 | 20.1 ± 0.3 | 17.7 ± 0.5 | 32.9 ± 6.1 | 31.7 ± 4.7 | 28.4 ± 0.6 | |
| Chopin | 2.05 ± 0.18 | 4.51 ± 0.96 | 11.2 ± 4.25 | 18.1 ± 3.9 | 16.0 ± 3.8 | 36.4 ± 4.7 | 38.9 ± 2.2 | 33.0 ± 1.0 | |
| Mean value for I | 8.29 ± 6.2 | 14.3 ± 3.8 | 31.1 ± 5.4 | ||||||
| Year | <0.0001 | - | 0.9337 | ||||||
| Treatment | <0.0001 | 0.0009 | <0.0001 | ||||||
| Cultivar | <0.0001 | <0.0001 | <0.0001 | ||||||
| Year × Treatment | 0.0162 | - | 0.9559 | ||||||
| Year × Cultivar | <0.0001 | - | 0.0020 | ||||||
| Treatment × Cultivar | 0.1282 | 0.6907 | 0.0904 | ||||||
| Year × Treatment × Cultivar | 0.9439 | - | 0.9759 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybyłko, S.; Kowalczyk, W.; Wrona, D. The Effect of Mycorrhizal Fungi and PGPR on Tree Nutritional Status and Growth in Organic Apple Production. Agronomy 2021, 11, 1402. https://doi.org/10.3390/agronomy11071402
Przybyłko S, Kowalczyk W, Wrona D. The Effect of Mycorrhizal Fungi and PGPR on Tree Nutritional Status and Growth in Organic Apple Production. Agronomy. 2021; 11(7):1402. https://doi.org/10.3390/agronomy11071402
Chicago/Turabian StylePrzybyłko, Sebastian, Wojciech Kowalczyk, and Dariusz Wrona. 2021. "The Effect of Mycorrhizal Fungi and PGPR on Tree Nutritional Status and Growth in Organic Apple Production" Agronomy 11, no. 7: 1402. https://doi.org/10.3390/agronomy11071402
APA StylePrzybyłko, S., Kowalczyk, W., & Wrona, D. (2021). The Effect of Mycorrhizal Fungi and PGPR on Tree Nutritional Status and Growth in Organic Apple Production. Agronomy, 11(7), 1402. https://doi.org/10.3390/agronomy11071402






