Combining Ability and Gene Action Controlling Grain Yield and Its Related Traits in Bread Wheat under Heat Stress and Normal Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parental Genotypes and Crossing
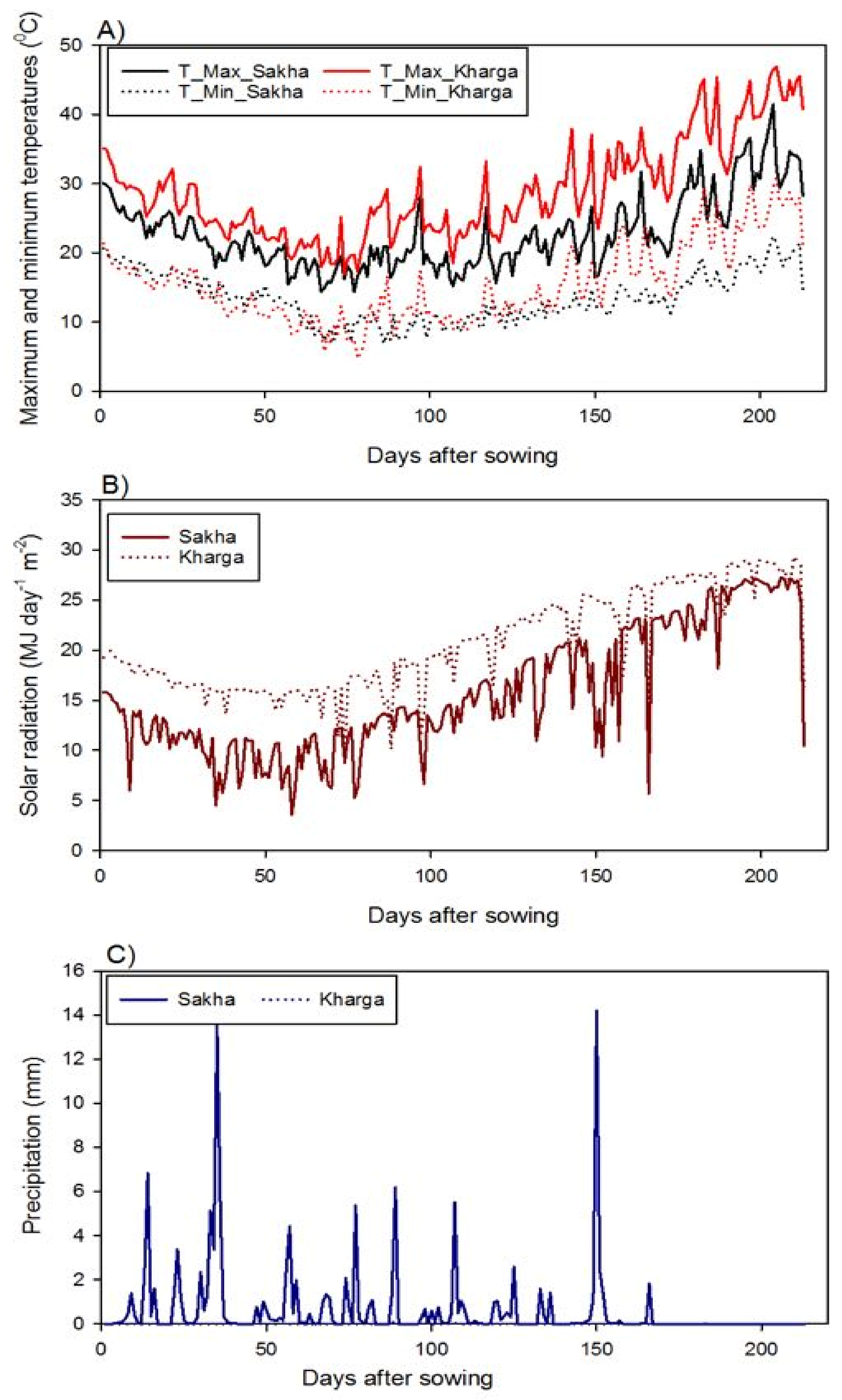
2.2. Experimental Sites and Agronomic Practices
2.3. Measured Traits
2.4. Molecular Analysis
2.4.1. PCR Amplification and SNP Assay of Dehydrin Gene
- Dehydrin-1 (forward): GGTGGGTTTACTGGTGAAGCCGGCAGACAA
- Dehydrin-2 (reverse): CTAGTGTCCAGTACATCCTCCAGTACCAGG
2.4.2. Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Analysis of Variance
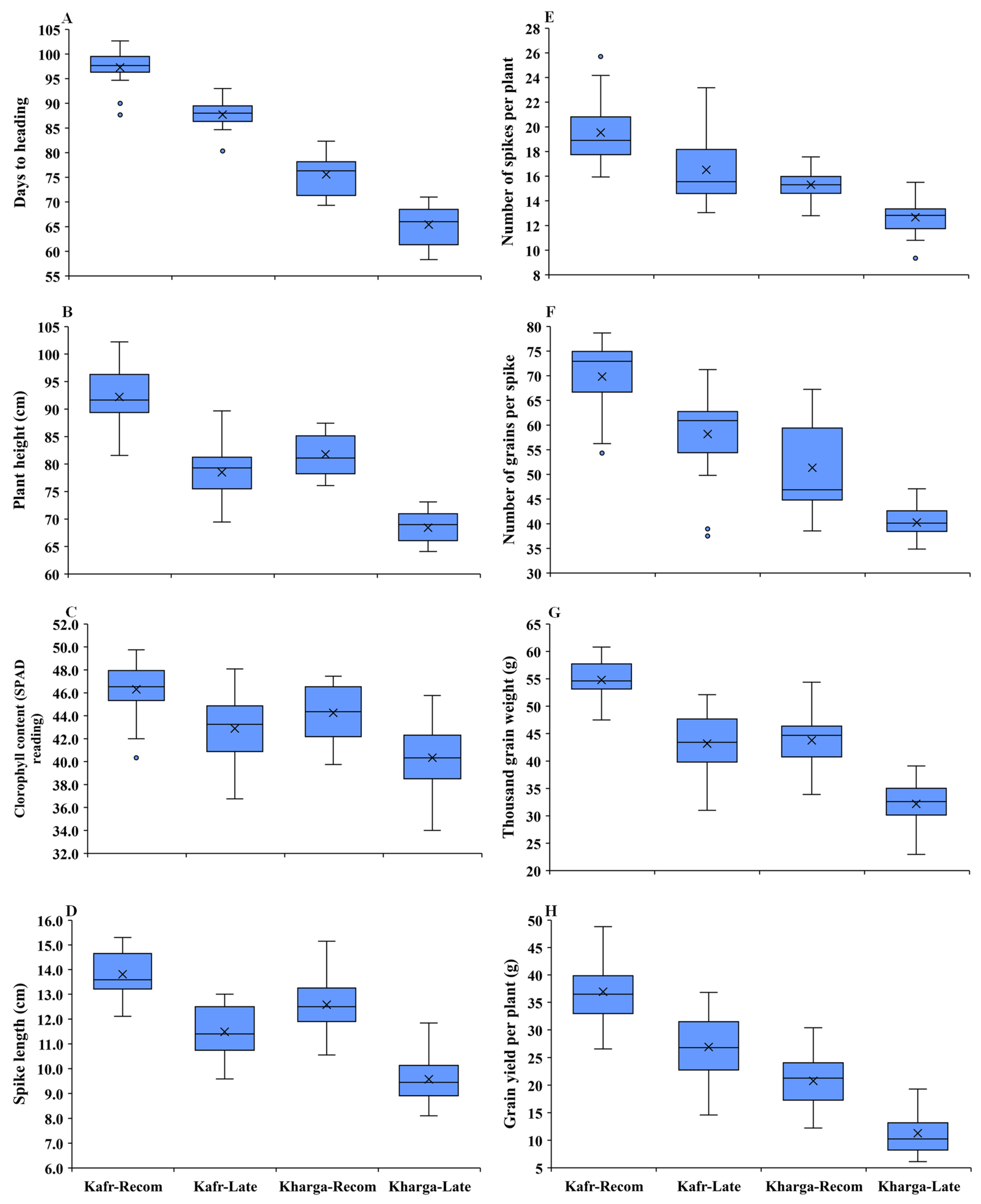
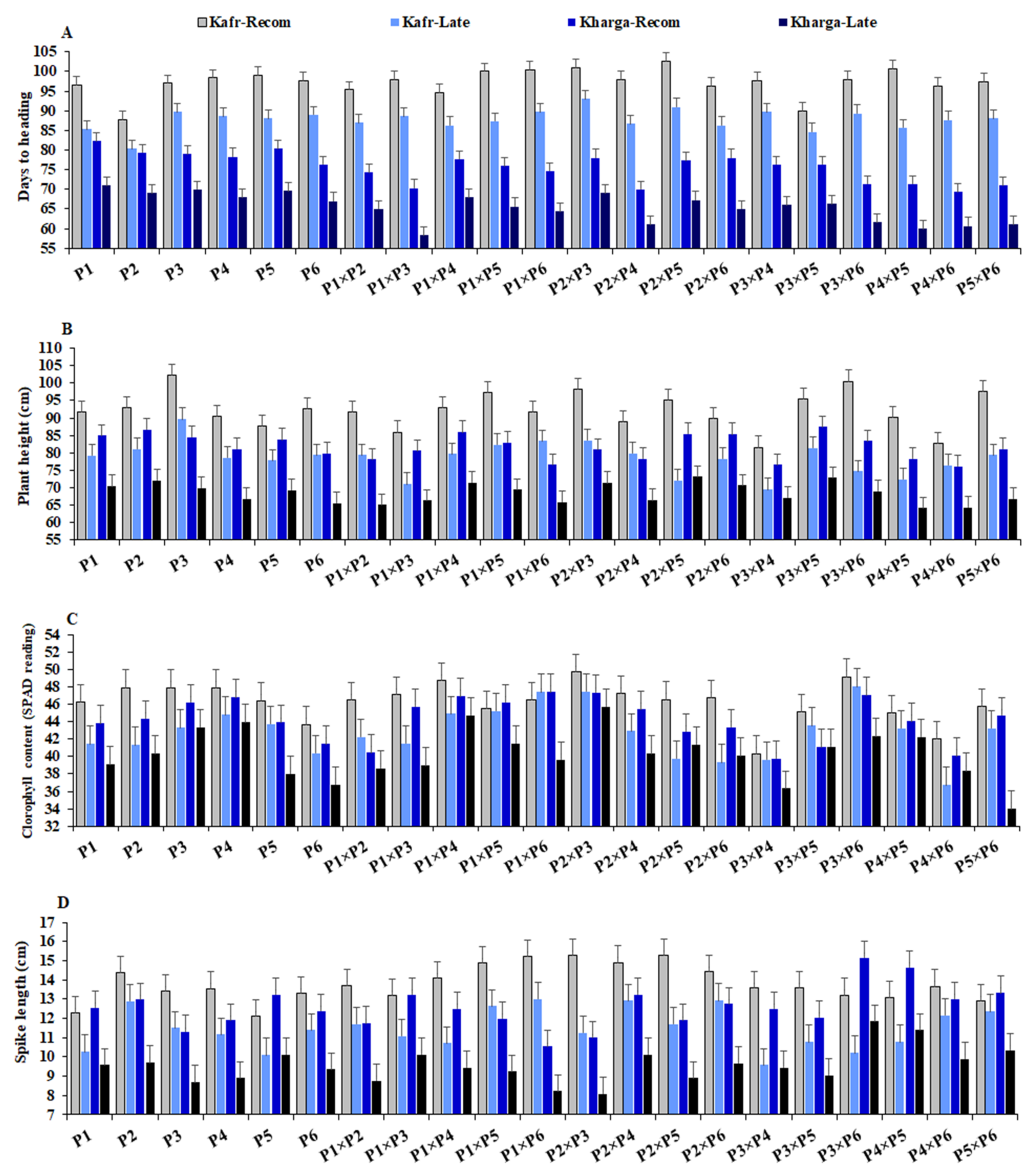
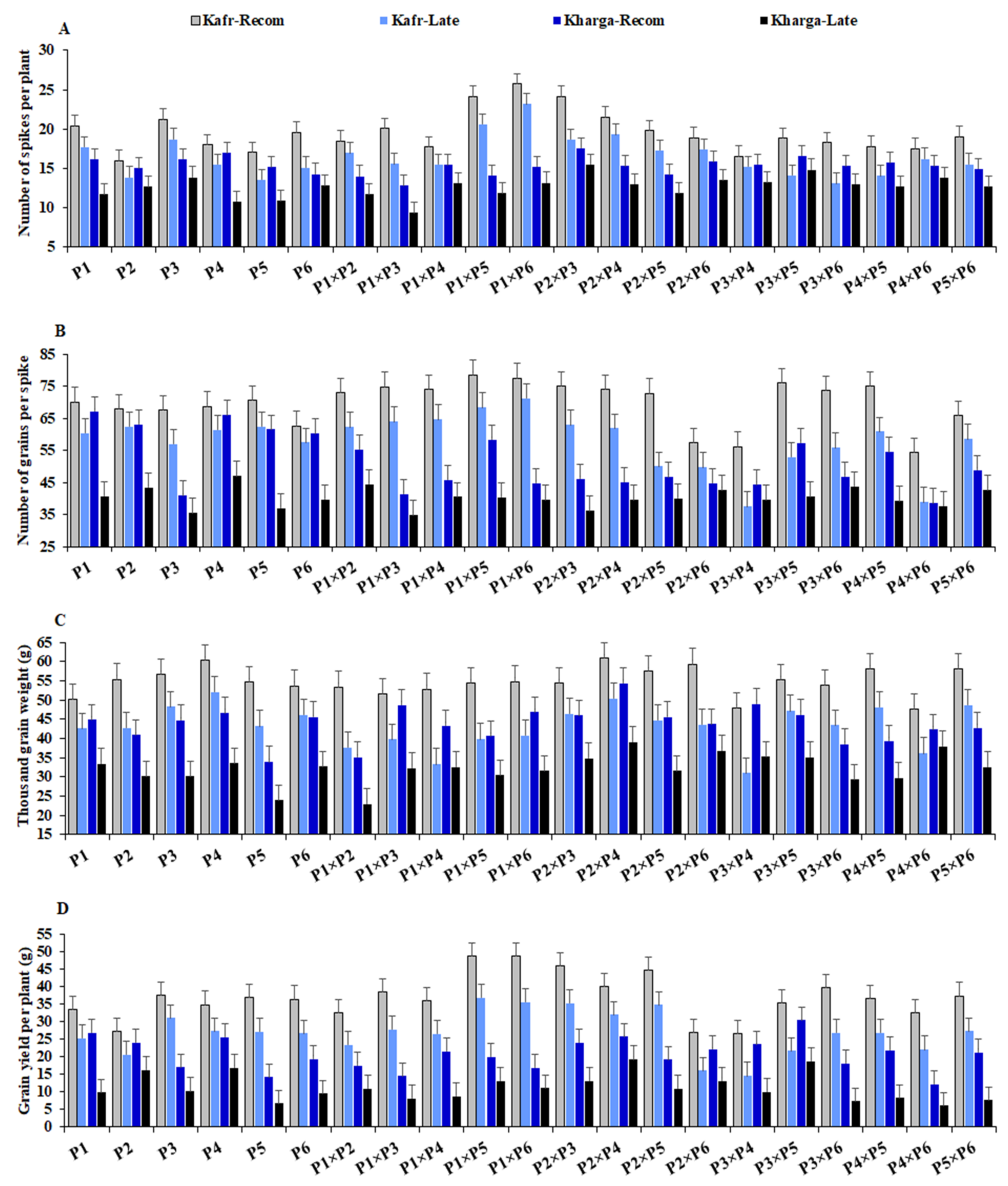
3.2. Mean Performance of Used Parents and Their F1 Hybrids
3.3. Genotypic Classification According to Heat Tolerance Indices
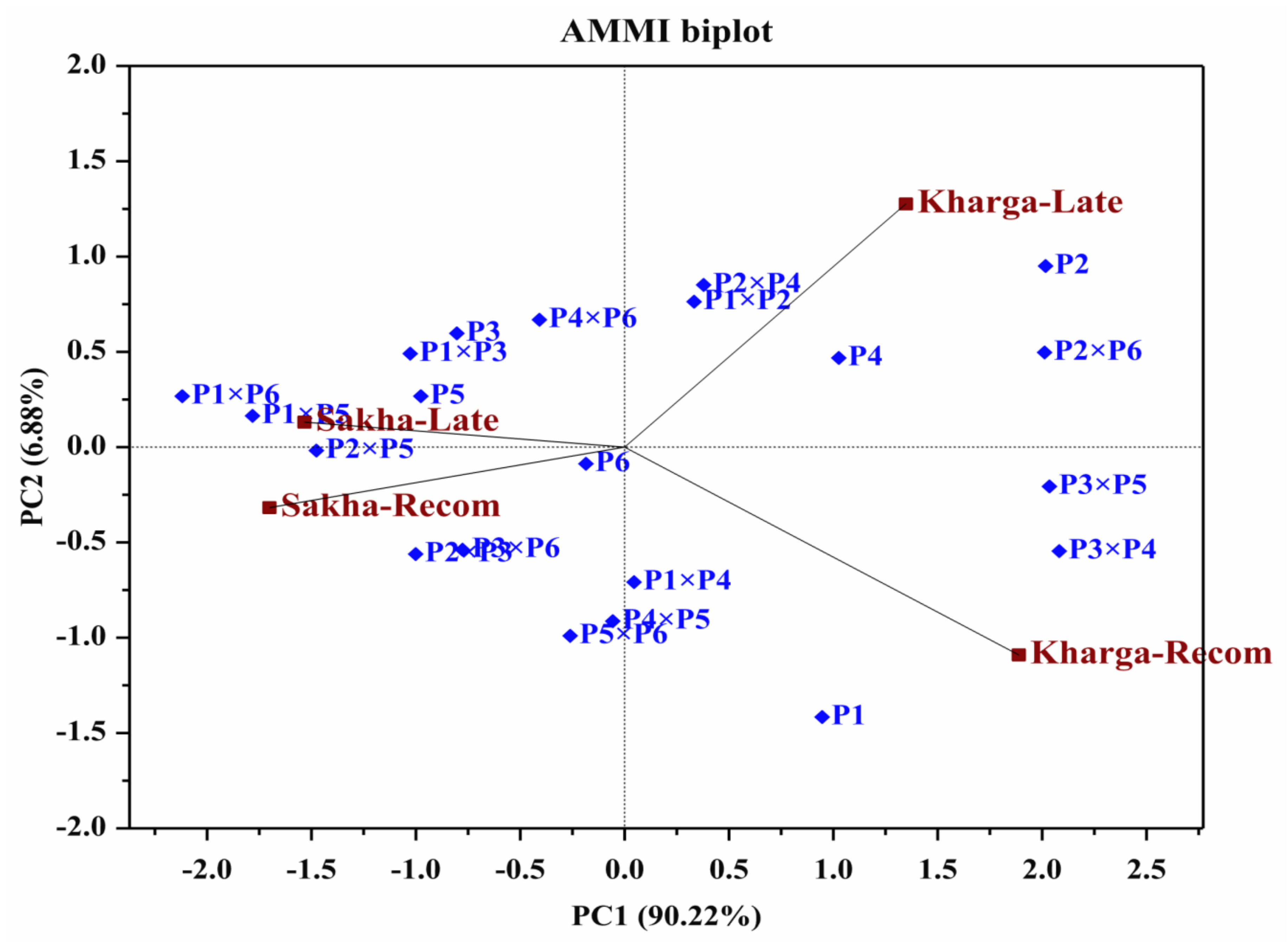
3.4. Additive Main Effect and Multiplicative Interaction Model (AMMI)
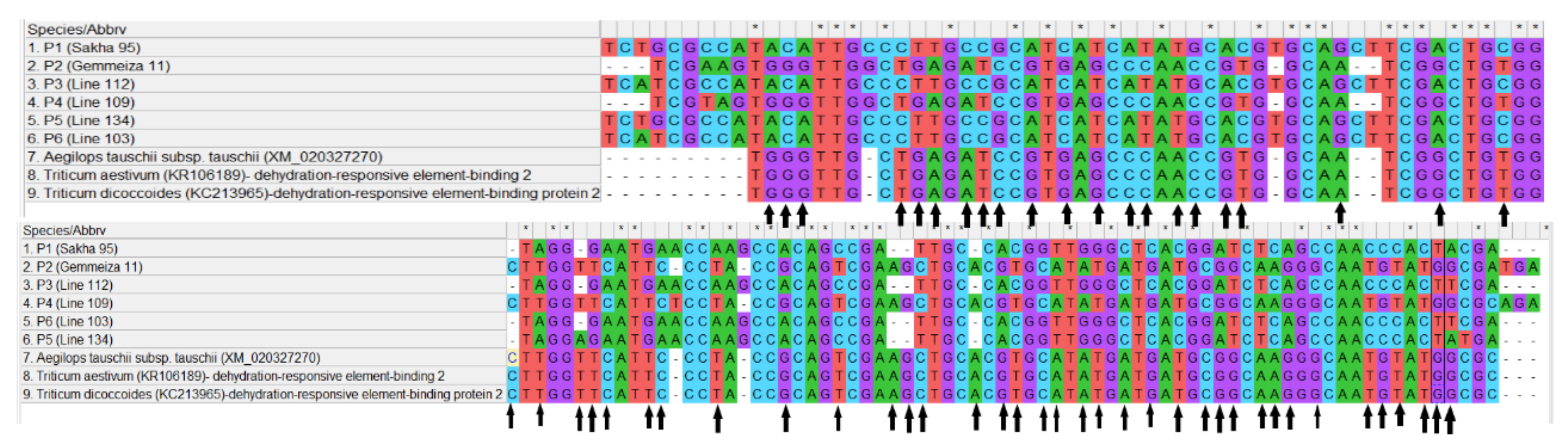
3.5. Phylogenetic Tree, SNP Analysis and Protein Sequence of Dehydrin Gene
3.6. General Combining Ability (GCA) Effects
3.7. Specific Combining Ability (SCA) Effects
3.8. Heterosis Relative to Mid-Parent (MP) and Better-Parent (BP)
3.9. Components of Genetic Variation and Heritability
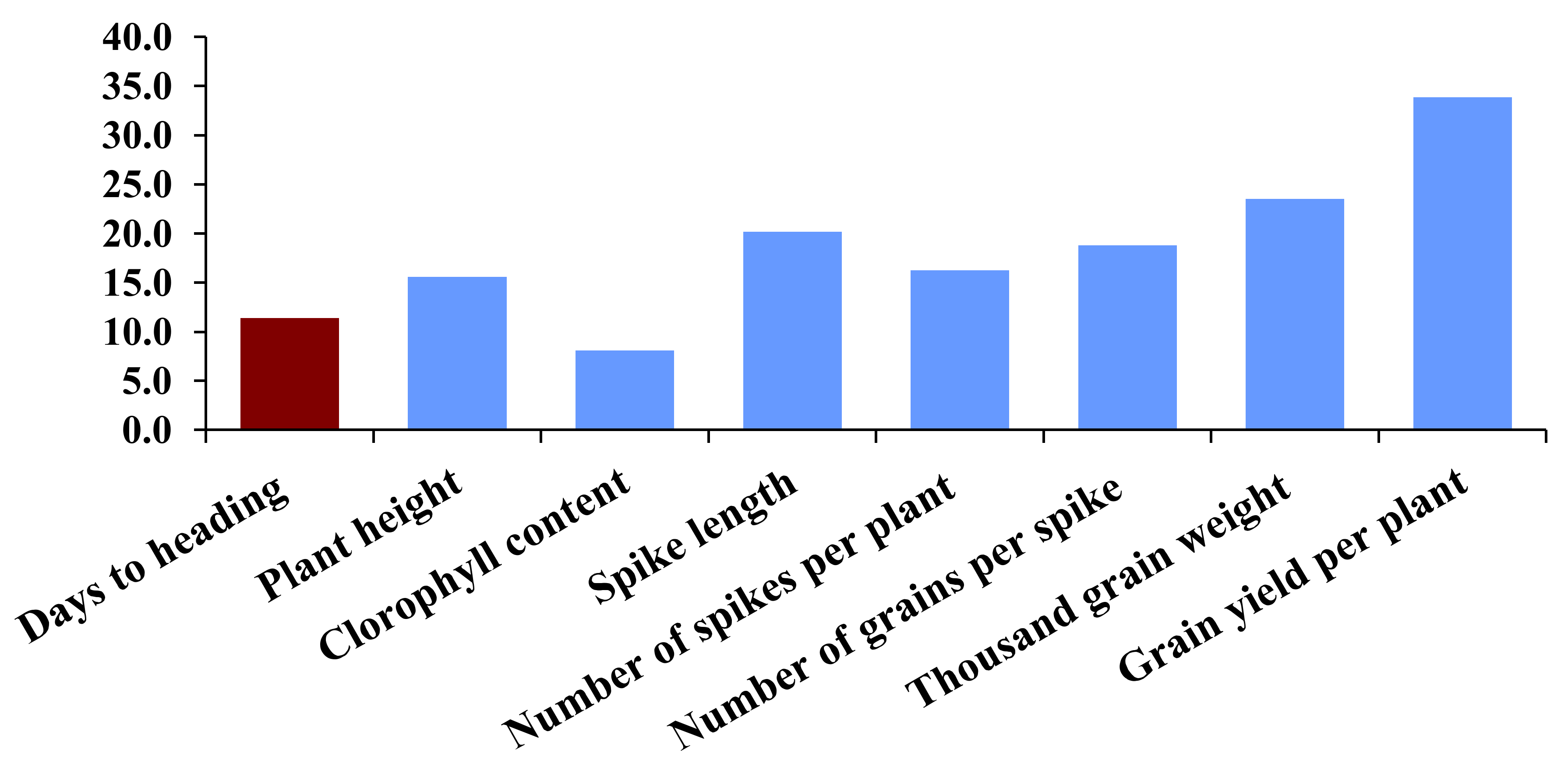
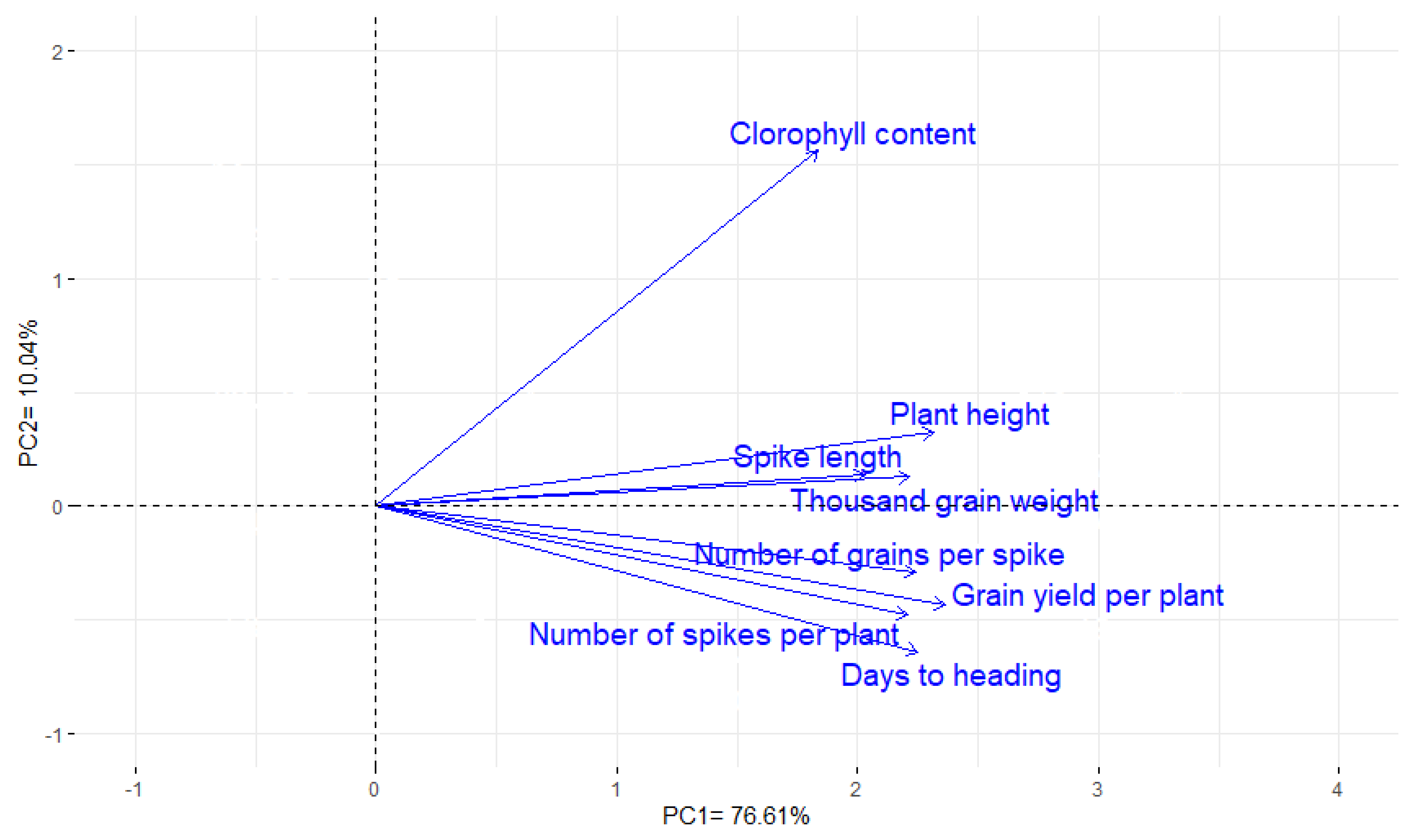
3.10. Interrelationship among Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Statistical Database. Available online: http://www.fao.org/home/en/ (accessed on 13 May 2021).
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar]
- Asseng, S.; Kheir, A.M.; Kassie, B.T.; Hoogenboom, G.; Abdelaal, A.I.; Haman, D.Z.; Ruane, A.C. Can Egypt become self-sufficient in wheat? Environ. Res. Lett. 2018, 13, 094012. [Google Scholar] [CrossRef] [Green Version]
- Attia, A.; El-Hendawy, S.; Al-Suhaibani, N.; Tahir, M.U.; Mubushar, M.; dos Santos Vianna, M.; Ullah, H.; Mansour, E.; Datta, A. Sensitivity of the DSSAT model in simulating maize yield and soil carbon dynamics in arid Mediterranean climate: Effect of soil, genotype and crop management. Field Crops Res. 2021, 260, 107981. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Z.; Sun, L.; Chen, B.; Tubiello, F.N.; Xu, Y. The impacts of increased heat stress events on wheat yield under climate change in China. Clim. Chang. 2017, 140, 605–620. [Google Scholar] [CrossRef]
- Mansour, E.; Merwad, A.; Yasin, M.; Abdul-Hamid, M.; El-Sobky, E.; Oraby, H. Nitrogen use efficiency in spring wheat: Genotypic variation and grain yield response under sandy soil conditions. J. Agric. Sci. 2017, 155, 1407–1423. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of IPCC the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1535. [Google Scholar]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.; White, J.W. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020, 20, 268. [Google Scholar] [CrossRef]
- Prasad, P.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, T.; Wardlaw, I. The response to high temperature shock and humidity changes prior to and during the early stages of grain development in wheat. Funct. Plant Biol. 1990, 17, 551–561. [Google Scholar] [CrossRef]
- Moustafa, E.S.; El-Sobky, E.-S.E.; Farag, H.I.; Yasin, M.A.; Attia, A.; Rady, M.O.; Awad, M.F.; Mansour, E. Sowing date and genotype influence on yield and quality of dual-purpose barley in a salt-affected arid region. Agronomy 2021, 11, 717. [Google Scholar] [CrossRef]
- Yang, J.; Sears, R.; Gill, B.; Paulsen, G. Growth and senescence characteristics associated with tolerance of wheat-alien amphiploids to high temperature under controlled conditions. Euphytica 2002, 126, 185–193. [Google Scholar] [CrossRef]
- Wardlaw, I.; Wrigley, C. Heat tolerance in temperate cereals: An overview. Funct. Plant Biol. 1994, 21, 695–703. [Google Scholar] [CrossRef]
- Wiegand, C.; Cuellar, J. Duration of grain filling and kernel weight of wheat as affected by temparature. Crop Sci. 1981, 21, 95–101. [Google Scholar] [CrossRef]
- Al-Khatib, K.; Paulsen, G.M. Mode of high temperature injury to wheat during grain development. Physiol. Plant. 1984, 61, 363–368. [Google Scholar] [CrossRef]
- Elbasyoni, I.S. Performance and stability of commercial wheat cultivars under terminal heat stress. Agronomy 2018, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.W.; Yang, L.; Yousaf, M.I.; Sami, A.; Mei, X.D.; Shah, L.; Rehman, S.; Xue, L.; Si, H.; Ma, C. Effects of heat stress on growth, physiology of plants, yield and grain quality of different spring wheat (Triticum aestivum L.) genotypes. Sustainability 2021, 13, 2972. [Google Scholar] [CrossRef]
- Tariq, A.; Ashraf, I.; Ahmed, M.; Muscolo, A.; Basra, S.; Reynolds, M. Evaluation of physiological and morphological traits for improving spring wheat adaptation to terminal heat stress. Plants 2021, 10, 455. [Google Scholar]
- Farooq, J.; Khaliq, I.; Ali, M.A.; Kashif, M.; Rehman, A.U.; Naveed, M.; Ali, Q.; Nazeer, W.; Farooq, A. Inheritance pattern of yield attributes in spring wheat at grain filling stage under different temperature regimes. Aust. J. Crop Sci. 2011, 5, 1745–1753. [Google Scholar]
- Abaza, G.M.S.M.; Awaad, H.A.; Attia, Z.M.; Abdel-lateif, K.S.; Gomaa, M.A.; Abaza, S.M.S.M.; Mansour, E. Inducing potential mutants in bread wheat using different doses of certain physical and chemical mutagens. Plant Breed. Biotech. 2020, 8, 252–264. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.; Qabil, N.; Abdelsalam, A.; Wafa, H.A.; El Kenawy, A.; Casas, A.M.; Igartua, E. Assessing different barley growth habits under Egyptian conditions for enhancing resilience to climate change. Field Crops Res. 2018, 224, 67–75. [Google Scholar] [CrossRef]
- Moustafa, E.S.; Ali, M.; Kamara, M.M.; Awad, M.F.; Hassanin, A.A.; Mansour, E. Field screening of wheat advanced lines for salinity tolerance. Agronomy 2021, 11, 281. [Google Scholar] [CrossRef]
- Salem, T.; Rabie, H.; Mowafy, S.; Eissa, A.; Mansour, E. Combining ability and genetic components of egyptian cotton for earliness, yield, and fiber quality traits. SABRAO J. Breed. Genet. 2020, 52, 369–389. [Google Scholar]
- Kamara, M.M.; Ghazy, N.A.; Mansour, E.; Elsharkawy, M.M.; Kheir, A.; Ibrahim, K.M. Molecular genetic diversity and line × tester analysis for resistance to late wilt disease and grain yield in maize. Agronomy 2021, 11, 898. [Google Scholar] [CrossRef]
- Celliers, P.; Labuschagne, M.; Van Deventer, C. Combining ability effects of some spring wheat cultivars at two different temperature levels. S. Afr. J. Plant Soil 1999, 16, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Menon, U.; Sain, R. Combining ability for physiological traits in spring wheat over environments. Acta Agron. Hung. 2004, 52, 63–68. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alotaibi, M.; Refay, Y.; Ghazy, A.; Zakri, A.; Al-Doss, A. Selection criteria for high-yielding and early-flowering bread wheat hybrids under heat stress. PLoS ONE 2020, 15, e0236351. [Google Scholar] [CrossRef]
- Adel, M.; Ali, E. Gene action and combining ability in a six parent diallel cross of wheat. Asian J. Crop Sci. 2013, 5, 14–23. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, V.; Vyas, R.; Singh, V. Heterosis and combining ability analysis in bread wheat (Triticum aestivum L.). J. Plant Breed. Crop Sci. 2011, 3, 209–217. [Google Scholar]
- Borghi, B.; Perenzin, M. Diallel analysis to predict heterosis and combining ability for grain yield, yield components and bread-making quality in bread wheat (T. aestivum). Theor. Appl. Genet. 1994, 89, 975–981. [Google Scholar] [CrossRef]
- Hei, N.; Hussein, S.; Laing, M. Heterosis and combining ability analysis of slow rusting stem rust resistance and yield and related traits in bread wheat. Euphytica 2016, 207, 501–514. [Google Scholar] [CrossRef]
- Sharma, D.; Jaiswal, J.P.; Gahtyari, N.C.; Chauhan, A.; Singh, N.K. Genetic dissection of physiological traits over trait based breeding in bread wheat conferring terminal heat tolerance. Cereal Res. Commun. 2021, 1–9. [Google Scholar] [CrossRef]
- Garg, B.; Lata, C.; Prasad, M. A study of the role of gene TaMYB2 and an associated SNP in dehydration tolerance in common wheat. Mol. Biol. Rep. 2012, 39, 10865–10871. [Google Scholar] [CrossRef]
- Zotova, L.; Kurishbayev, A.; Jatayev, S.; Khassanova, G.; Zhubatkanov, A.; Serikbay, D.; Sereda, S.; Sereda, T.; Shvidchenko, V.; Lopato, S. Genes encoding transcription factors TaDREB5 and TaNFYC-A7 are differentially expressed in leaves of bread wheat in response to drought, dehydration and ABA. Front. Plant Sci. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Lai, K.; Duran, C.; Berkman, P.J.; Lorenc, M.T.; Stiller, J.; Manoli, S.; Hayden, M.J.; Forrest, K.L.; Fleury, D.; Baumann, U. Single nucleotide polymorphism discovery from wheat next-generation sequence data. Plant Biotechnol. J. 2012, 10, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Edae, E.A.; Byrne, P.F.; Manmathan, H.; Haley, S.D.; Moragues, M.; Lopes, M.S.; Reynolds, M.P. Association mapping and nucleotide sequence variation in five drought tolerance candidate genes in spring wheat. Plant Genome 2013, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rustamova, S.; Shrestha, A.; Naz, A.A.; Huseynova, I. Expression profiling of DREB1 and evaluation of vegetation indices in contrasting wheat genotypes exposed to drought stress. Plant Gene 2021, 25, 100266. [Google Scholar] [CrossRef]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, S.; Bahieldin, A.; Abdelgawad, B.; Badr, A. Construction of a dehydrin gene cassette for drought tolerance from wild origin for wheat transformation. Int. J. Bot. 2005, 1, 175–182. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhu, H.; Tao, Y.; Zhang, G.; Zhang, L.; Zhang, C.; Zhang, Z.; Ma, Z. Classification and expression diversification of wheat dehydrin genes. Plant Sci. 2014, 214, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures For Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Hayman, B. The analysis of variance of diallel tables. Biometrics 1954, 10, 235–244. [Google Scholar] [CrossRef]
- Mather, K.; Jinks, J.L. Components of Means: Additive and Dominance Effects. In Biometrical Genetics; Springer: Berlin/Heidelberg, Germany, 1971; pp. 65–82. [Google Scholar]
- Fonsecca, S.; Patterson, F. Hybrid vigour in seven-parent diallel cross in common wheat (T. aestivum L.). Crop Sci. 1968, 2, 85–88. [Google Scholar] [CrossRef]
- Rosielle, A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment 1. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campanile, R.; Ricciardi, G.; Borghi, B. Evaluation of field and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–531. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Gharib, M.A.A.H.; Qabil, N.; Salem, A.H.; Ali, M.M.A.; Awaad, H.A.; Mansour, E. Characterization of wheat landraces and commercial cultivars based on morpho-phenological and agronomic traits. Cereal Res. Commun. 2021, 49, 149–159. [Google Scholar] [CrossRef]
- Shaukat, S.; Khan, A.S.; Hussain, M.; Kashif, M.; Ahmed, N. Selection of superior parents and cross combinations for quality traits in bread wheat (Triticum aestivum L.) under normal and heat stressed conditions. Pak. J. Agric. Sci. 2018, 55, 801–807. [Google Scholar]
- Fleitas, M.C.; Mondal, S.; Gerard, G.S.; Hernández-Espinosa, N.; Singh, R.P.; Crossa, J.; Guzmán, C. Identification of CIMMYT spring bread wheat germplasm maintaining superior grain yield and quality under heat-stress. J. Cereal Sci. 2020, 93, 102981. [Google Scholar] [CrossRef]
- Aziz, A.; Mahmood, T.; Mahmood, Z.; Shazadi, K.; Mujeeb-Kazi, A.; Rasheed, A. Genotypic variation and genotype × environment interaction for yield-related traits in synthetic hexaploid wheats under a range of optimal and heat-stressed environments. Crop Sci. 2018, 58, 295–303. [Google Scholar] [CrossRef]
- Tadesse, W.; Suleiman, S.; Tahir, I.; Sanchez-Garcia, M.; Jighly, A.; Hagras, A.; Thabet, S.; Baum, M. Heat-tolerant QTLs associated with grain yield and its components in spring bread wheat under heat-stressed environments of Sudan and Egypt. Crop Sci. 2019, 59, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Sharma, D.K.; Ottosen, C.-O. Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum L.) cultivars acclimated to different growth temperatures. Environ. Exp. Bot. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Singh, J.P.; Shambhoo, P.; Singh, K.; Randhir, S. Screening of heat tolerant wheat varieties by membrane thermostability index in relation to yield and yield attributing traits. Int. J. Plant Sci. 2007, 2, 159–165. [Google Scholar]
- Schittenhelm, S.; Langkamp-Wedde, T.; Kraft, M.; Kottmann, L.; Matschiner, K. Effect of two-week heat stress during grain filling on stem reserves, senescence, and grain yield of European winter wheat cultivars. J. Agron. Crop Sci. 2020, 206, 722–733. [Google Scholar] [CrossRef] [Green Version]
- Talukder, A.; McDonald, G.K.; Gill, G.S. Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat. Field Crops Res. 2014, 160, 54–63. [Google Scholar] [CrossRef]
- Akter, N.; Islam, M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Khan, M.I.; Amin, M.; Shah, S.T. Agronomic evaluation of different bread wheat (Triticum aestivum L.) genotypes for terminal heat stress. Pak. J. Bot. 2007, 39, 2415–2425. [Google Scholar]
- Fasahat, P.; Rajabi, A.; Rad, J.; Derera, J. Principles and utilization of combining ability in plant breeding. Biom. Biostat. Int. J. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Gracia, M.; Mansour, E.; Casas, A.; Lasa, J.; Medina, B.; Cano, J.L.M.; Moralejo, M.; López, A.; Fuster, P.L.; Escribano, J. Progress in the Spanish National Barley Breeding Program. Span. J. Agric. Res. 2012, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Desoky, E.-S.M.; Mansour, E.; Yasin, M.A.; El Sobky, E.-S.E.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2020, 18, 16. [Google Scholar] [CrossRef]
- Joshi, R.K.; Nayak, S. Gene pyramiding-A broad spectrum technique for developing durable stress resistance in crops. Biotechnol. Mol. Biol. Rev. 2010, 5, 51–60. [Google Scholar]
- Mwadzingeni, L.; Shimelis, H.; Tsilo, T.J. Combining ability and gene action controlling yield and yield components in bread wheat (Triticum aestivum L.) under drought-stressed and nonstressed conditions. Plant Breed. 2018, 137, 502–513. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, S.; Liu, R.; Lu, J.; Lu, L.; Zhang, C.; Liu, Z.; Luo, C.; Zhang, L.; Yant, L. Genome-wide identification, phylogenetic and expression analysis of the heat shock transcription factor family in bread wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 505. [Google Scholar] [CrossRef]
- Barakat, M.N.; Al-Ashkar, I.M.; Al-Doss, A.A.; Moustafa, K.A.; Motawei, M.I.; Alamri, M.S.; Mergoum, M.; Sallam, M.S. Identification of genotyping-by-sequencing tags associated with bread-making quality traits in spring wheat under heat stress. Cereal Res. Commun. 2020, 48, 347–353. [Google Scholar] [CrossRef]
- Baloch, M.J.; Dunwell, J.; Khan, N.U.; Khakwani, A.A.; Dennett, M.; Jatoi, W.A. Profiling dehydrin gene sequence and physiological parameters in drought tolerant and susceptible spring wheat cultivars. Pak. J. Bot. 2012, 44, 801–806. [Google Scholar]
- Chen, Z.-X.; Deng, M.; Wang, J.-R. SNP Mining in functional genes from nonmodel species by next-generation sequencing: A case of flowering, pre-harvest sprouting, and dehydration resistant genes in wheat. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Longmei, N.; Gill, G.K.; Zaidi, P.H.; Kumar, R.; Nair, S.K.; Hindu, V.; Vinayan, M.T.; Vikal, Y. Genome wide association mapping for heat tolerance in sub-tropical maize. BMC Genom. 2021, 22, 154. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, P.; Tyagi, B.; Singh, G. Heterosis for yield component traits and protein content in bread wheat under normal and heat-stress environment. Cereal Res. Commun. 2014, 42, 151–162. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, P. Relationship of heterosis for protein content with yield component trait (s) under normal/heat-stress environment in spring wheat. Crop Improv. 2012, 39, 8–17. [Google Scholar]
- Farooq, J.; Khaliq, I.; Akbar, M.; Kashif, M.; Mahpara, S. Hybrid vigor studies for different yield contributing traits in wheat under normal and heat stress conditions. Comun. Sci. 2013, 4, 139–152. [Google Scholar]
- EL-Hendawy, S.; Hu, Y.; Sakagami, J.-I.; Schmidhalter, U. Screening Egyptian wheat genotypes for salt tolerance at early growth stages. Int. J. Plant Prod. 2011, 5, 283–298. [Google Scholar]
- Mansour, E.; Moustafa, E.S.; Desoky, E.-S.M.; Ali, M.; Yasin, M.A.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S. Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.; El-Naggar, N.Z.; Abdelsalam, A.; Igartua, E. Grain yield stability of high-yielding barley genotypes under Egyptian conditions for enhancing resilience to climate change. Crop Pasture Sci. 2018, 69, 681–690. [Google Scholar] [CrossRef]
- Mansour, E.; Desoky, E.-S.M.; Ali, M.M.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Sareen, S.; Bhusal, N.; Singh, G.; Tyagi, B.; Tiwari, V.; Singh, G.; Sarial, A. Genetics of grain yield and its components in wheat under heat stress. Cereal Res. Commun. 2018, 46, 448–459. [Google Scholar] [CrossRef]
- Modarresi, M.; Mohammadi, V.; Zali, A.; Mardi, M. Response of wheat yield and yield related traits to high temperature. Cereal Res. Commun. 2010, 38, 23–31. [Google Scholar] [CrossRef]
- Pandey, G.C.; Mamrutha, H.; Tiwari, R.; Sareen, S.; Bhatia, S.; Siwach, P.; Tiwari, V.; Sharma, I. Physiological traits associated with heat tolerance in bread wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2015, 21, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Yıldırım, M.; Koç, M.; Akıncı, C.; Barutçular, C. Variations in morphological and physiological traits of bread wheat diallel crosses under timely and late sowing conditions. Field Crops Res. 2013, 140, 9–17. [Google Scholar] [CrossRef]


| Source of Variance | DF | DTH | PH | CHLC | SL | NSPP | NGPS | TGW | GYPP |
|---|---|---|---|---|---|---|---|---|---|
| Location (L) | 1 | 30,426 ** | 66,110 ** | 333.3 ** | 156.8 ** | 1031 ** | 20,858 ** | 7641 ** | 15,967 ** |
| Replication/L | 4 | 8.56 | 12.17 | 10.44 | 0.74 | 3.60 | 49.00 | 28.44 | 10.02 |
| Sowing date (D) | 1 | 6131 ** | 11,544 ** | 846.8 ** | 446.9 ** | 505.4 ** | 8157 ** | 8480 ** | 6005 ** |
| L × D | 1 | 6.04 | 2.20 | 3.81 | 7.22 * | 2.35 | 4.95 | 0.08 | 4.37 |
| Error | 4 | 3.58 | 10.46 | 8.15 | 0.37 | 2.76 | 29.26 | 7.31 | 8.00 |
| Genotype (G) | 20 | 56.59 ** | 126.9 ** | 61.63 ** | 3.17 ** | 24.13 ** | 302.9 ** | 119.5 ** | 142.7 ** |
| GCA | 5 | 15.79 ** | 156.7 ** | 30.85 ** | 3.89 ** | 11.34 ** | 425.9 ** | 94.66 ** | 46.53 ** |
| SCA | 15 | 70.18 ** | 117.0 ** | 71.89 ** | 2.93 ** | 28.40 ** | 261.9 ** | 127.8 ** | 174.8 ** |
| G × L | 20 | 73.42 ** | 52.37 ** | 5.78 * | 7.52 ** | 20.15 ** | 188.7 ** | 92.98 ** | 170.9 ** |
| GCA × L | 5 | 53.88 ** | 30.12 ** | 7.19 * | 6.63 ** | 24.95 ** | 161.7 ** | 121. 0 ** | 155.6 ** |
| SCA × L | 15 | 79.93 ** | 59.79 ** | 5.31 * | 7.82 ** | 18.55 ** | 197.7 ** | 83.64 ** | 175.9 ** |
| G × D | 20 | 5.56 ** | 20.46 ** | 6.70 ** | 0.60 * | 2.12 * | 49.15 ** | 14.03 ** | 11.29 ** |
| GCA × D | 5 | 8.23 ** | 12.63 | 5.98 | 0.59 | 1.74 | 67.72 ** | 11.82 * | 8.52 * |
| SCA × D | 15 | 4.68 ** | 23.07 ** | 6.95 ** | 0.61 * | 2.24 * | 42.95 ** | 14.77 ** | 12.21 ** |
| G × D × L | 20 | 3.02 ** | 15.39 ** | 10.23 ** | 0.61 * | 2.24 * | 84.78 ** | 15.90 ** | 9.95 ** |
| GCA × L × D | 5 | 3.92 * | 7.92 | 13.60 ** | 0.57 | 4.41 ** | 78.15 ** | 8.25 | 3.71 |
| SCA × L × D | 15 | 2.72 * | 17.88 ** | 9.11 ** | 0.62 * | 1.51 | 86.99 ** | 18.45 ** | 12.03 ** |
| Pooled Error | 160 | 1.43 | 7.39 | 2.95 | 0.33 | 1.22 | 7.25 | 4.25 | 3.73 |
| GCA/SCA | 0.23 | 1.34 | 0.43 | 1.33 | 0.40 | 1.63 | 0.74 | 0.27 | |
| GCA × L/SCA × L | 0.67 | 0.50 | 1.35 | 0.85 | 1.35 | 0.82 | 1.45 | 0.88 | |
| GCA × D/SCA × D | 1.76 | 0.55 | 0.86 | 0.96 | 0.78 | 1.58 | 0.80 | 0.70 |
| Trait | Environment | Parents | LSD (gi) 0.05 | LSD (gi) 0.01 | |||||
|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | ||||
| DTH | E1 | 0.10 | −1.53 ** | −0.28 | 0.39 | 0.97 ** | 0.35 | 0.49 | 0.66 |
| E2 | −0.54 * | −1.17 ** | 1.33 ** | −0.08 | −0.17 | 0.63 * | 0.48 | 0.64 | |
| E3 | 1.06 ** | 0.89 ** | 0.14 | −0.99 ** | 0.43 | −1.53 ** | 0.44 | 0.58 | |
| E4 | 0.67 ** | 0.92 ** | 0.42 * | −0.79 ** | 0.21 | −1.42 ** | 0.40 | 0.53 | |
| Combined | 0.32 ** | −0.22 * | 0.40 ** | −0.37 ** | 0.36 ** | −0.49 ** | 0.22 | 0.29 | |
| PH | E1 | −0.38 | 0.51 | 2.56 ** | −3.60 ** | 0.64 | 0.27 | 1.18 | 1.58 |
| E2 | 0.57 | 0.73 | 1.23 * | −1.85 ** | −0.81 | 0.13 | 1.07 | 1.43 | |
| E3 | 0.18 | 1.09 * | 0.66 | −1.90 ** | 1.27 ** | −1.31 ** | 0.94 | 1.25 | |
| E4 | −0.05 | 1.42 ** | 0.91 * | −1.54 ** | 0.71 | −1.44 ** | 0.88 | 1.18 | |
| Combined | 0.08 | 0.94 ** | 1.34 ** | −2.22 ** | 0.45 | −0.58 * | 0.50 | 0.66 | |
| CHLC | E1 | 0.36 | 1.08 ** | 0.41 | −0.61 * | −0.42 | −0.82 ** | 0.61 | 0.81 |
| E2 | 0.52 | −0.71 * | 0.85 * | −0.39 | 0.30 | −0.56 | 0.68 | 0.92 | |
| E3 | 0.58 | −0.20 | 0.47 | 0.02 | −0.36 | −0.51 | 0.69 | 0.92 | |
| E4 | −0.08 | 0.56 | 1.13 ** | 0.95 ** | −0.76 * | −1.80 ** | 0.61 | 0.81 | |
| Combined | 0.34* | 0.18 | 0.71 ** | −0.01 | −0.31 | −0.92 ** | 0.32 | 0.42 | |
| SL | E1 | −0.13 | 0.71 ** | −0.13 | −0.03 | −0.34 ** | −0.08 | 0.22 | 0.30 |
| E2 | −0.09 | 0.74 ** | −0.56 ** | −0.24 * | −0.24 * | 0.39 ** | 0.22 | 0.30 | |
| E3 | −0.36 ** | −0.17 | −0.18 | 0.21 * | 0.30 ** | 0.20 * | 0.18 | 0.25 | |
| E4 | −0.25 * | −0.25 * | −0.13 | 0.14 | 0.27 * | 0.21 | 0.24 | 0.32 | |
| Combined | −0.21 ** | 0.26 ** | −0.25 ** | 0.02 | 0.00 | 0.18 ** | 0.11 | 0.14 | |
| NSPP | E1 | 1.26 ** | −0.26 | 0.43 | −1.23 ** | −0.41 | 0.22 | 0.44 | 0.59 |
| E2 | 1.44 ** | 0.21 | −0.21 | −0.56 * | −0.88 ** | −0.01 | 0.52 | 0.70 | |
| E3 | −0.40 * | −0.04 | 0.37 | 0.50 * | −0.17 | −0.26 | 0.39 | 0.52 | |
| E4 | −0.77 ** | 0.29 * | 0.62 ** | −0.14 | −0.39 ** | 0.39 ** | 0.27 | 0.37 | |
| Combined | 0.38 ** | 0.05 | 0.30 ** | −0.36 ** | −0.46 ** | 0.09 | 0.20 | 0.27 | |
| NGPS | E1 | 3.74 ** | −0.10 | 0.27 | −2.22 ** | 2.62 ** | −4.32 ** | 1.04 | 1.39 |
| E2 | 5.57 ** | 0.61 | −2.48 ** | −2.56 ** | 1.07 * | −2.22 ** | 0.96 | 1.28 | |
| E3 | 2.60 ** | 0.63 | −5.19 ** | 0.11 | 3.70 ** | −1.85 ** | 1.04 | 1.39 | |
| E4 | −0.04 | 1.01 | −1.98 ** | 1.11 * | −0.60 | 0.50 | 1.02 | 1.36 | |
| Combined | 2.97 ** | 0.54 * | −2.35 ** | −0.89 ** | 1.70 ** | −1.97 ** | 0.49 | 0.65 | |
| TGW | E1 | −2.02 ** | 1.60 ** | −0.89 | 0.54 | 1.10 * | −0.33 | 0.91 | 1.22 |
| E2 | −3.19 ** | 0.80 | 0.27 | 0.14 | 1.63 ** | 0.35 | 0.86 | 1.15 | |
| E3 | −0.24 | 0.04 | 1.42 ** | 1.92 ** | −3.01 ** | −0.12 | 0.71 | 0.95 | |
| E4 | −1.10 ** | 0.04 | 0.21 | 2.06 ** | −2.24 ** | 1.03 ** | 0.59 | 0.78 | |
| Combined | −1.64 ** | 0.62 ** | 0.25 | 1.16 ** | −0.63 ** | 0.23 | 0.38 | 0.50 | |
| GYPP | E1 | 1.57 ** | −1.76 ** | 0.28 | −2.21 ** | 2.23 ** | −0.10 | 0.86 | 1.15 |
| E2 | 1.52 ** | −0.69 | −0.05 | −1.50 ** | 1.64 ** | −0.92 * | 0.79 | 1.06 | |
| E3 | −0.14 | 1.42 ** | −0.10 | 1.35 ** | −0.53 | −2.01 ** | 0.67 | 0.90 | |
| E4 | −0.85 ** | 2.63 ** | −0.10 | 0.91 ** | −0.81 ** | −1.79 ** | 0.55 | 0.74 | |
| Combined | 0.53 ** | 0.40 * | 0.01 | −0.36 * | 0.63 ** | −1.21 ** | 0.35 | 0.47 | |
| Crosses | Days to Heading | Plant Height | Chlorophyll Content | Spike Length | ||||||||||||||||
| E1 | E2 | E3 | E4 | Comb. | E1 | E2 | E3 | E4 | Comb. | E1 | E2 | E3 | E4 | Comb. | E1 | E2 | E3 | E4 | Comb. | |
| P1 × P2 | −0.51 | 0.99 | −3.21 ** | −2.01 ** | −1.18 ** | −0.72 | −0.41 | −4.97 ** | −4.80 ** | −2.72 ** | −1.2 | −0.41 | −4.19 ** | −2.15 * | −1.99 ** | −0.68 * | −0.42 | −0.29 | −0.30 | −0.42 ** |
| P1 × P3 | 0.91 | 0.16 | −6.46 ** | −8.18 ** | −3.39 ** | −8.47 ** | −9.29 ** | −2.04 | −3.00 * | −5.70 ** | 0.06 | −2.73 ** | 0.44 | −2.41* * | −1.16 ** | −0.34 | 0.26 | 1.21 ** | 0.94 ** | 0.52 ** |
| P1 × P4 | −3.09 ** | −0.76 | 1.99 ** | 2.70 ** | 0.21 | 4.68 ** | 2.46 | 5.86 ** | 4.52 ** | 4.38 ** | 2.70 ** | 1.90 * | 2.07 * | 3.56 ** | 2.56 ** | 0.44 | −0.45 | 0.07 | −0.01 | 0.01 |
| P1 × P5 | 1.66 * | 0.33 | −1.09 | −0.64 | 0.07 | 4.78 ** | 4.09 ** | −0.31 | 0.25 | 2.20 ** | −0.74 | 1.52 | 1.75 | 2.00 * | 1.13 * | 1.53 ** | 1.47 ** | −0.52 * | −0.35 | 0.53 ** |
| P1 × P6 | 2.62 ** | 1.87 ** | −0.46 | −0.35 | 0.92 ** | −0.52 | 4.14 ** | −4.07 ** | −1.12 | −0.39 | 0.65 | 4.60 ** | 3.13 ** | 1.21 | 2.40 ** | 1.61 ** | 1.22 ** | −1.86 ** | −1.30 ** | −0.09 |
| P2 × P3 | 5.54 ** | 5.12 ** | 1.37 * | 2.24 ** | 3.57 ** | 2.86 | 3.05 * | −2.62 * | 0.58 | 0.97 | 1.96 * | 4.47 ** | 2.86 ** | 3.74 ** | 3.26 ** | 0.88 ** | −0.40 | −1.22 ** | −1.09 ** | −0.46 ** |
| P2 × P4 | 1.87 ** | 0.2 | −5.51 ** | −4.55 ** | −2.00 ** | −0.32 | 2.46 | −2.72 * | −1.97 | −0.64 | 0.48 | 1.13 | 1.35 | −1.52 | 0.36 | 0.43 | 0.94 ** | 0.64 * | 0.66 * | 0.67 ** |
| P2 × P5 | 5.95 ** | 4.62 ** | 0.41 | 0.78 | 2.94 ** | 1.89 | −6.34 ** | 1.28 | 2.55 * | −0.16 | −0.41 | −2.72 ** | −0.81 | 1.22 | −0.68 | 1.12 ** | −0.29 | −0.80 ** | −0.69 * | −0.16 |
| P2 × P6 | 0.24 | −0.84 | 3.04 ** | 0.07 | 0.63 * | −3.07 | −1.12 | 3.77 ** | 2.17 | 0.44 | 0.19 | −2.26 * | −0.19 | 0.96 | −0.33 | −0.01 | 0.32 | 0.15 | 0.14 | 0.15 |
| P3 × P4 | 0.29 | 0.7 | 1.58 * | 0.95 | 0.88 ** | −9.59 ** | −8.41 ** | −3.96 ** | −0.78 | −5.68 ** | −5.77 ** | −3.72 ** | −4.99 ** | −6.08 ** | −5.14 ** | −0.07 | −1.10 ** | −0.11 | −0.13 | −0.35 * |
| P3 × P5 | −7.96 ** | −4.21 ** | 0.16 | 0.28 | −2.93 ** | −0.08 | 2.42 | 3.71 ** | 2.74 * | 2.20 ** | −1.15 | −0.44 | −3.28 ** | 0.46 | −1.10 * | 0.23 | 0.10 | −0.65 * | −0.67 * | −0.25 |
| P3 × P6 | 0.66 | −0.34 | −2.88 ** | −2.76 ** | −1.33 ** | 5.50 ** | −5.19 ** | 2.28 | 1.09 | 0.92 | 3.30 ** | 4.91 ** | 2.87 ** | 2.67 ** | 3.44 ** | −0.38 | −1.09 ** | 2.56 ** | 2.18 ** | 0.82 ** |
| P4 × P5 | 2.04 ** | −1.80 ** | −3.71 ** | −4.85 ** | −2.08 ** | 0.86 | −3.50 * | −2.90 * | −3.51 ** | −2.26 ** | −0.27 | 0.46 | 0.18 | 1.74 * | 0.53 | −0.36 | −0.22 | 1.56 ** | 1.40 ** | 0.59 ** |
| P4 × P6 | −1.67 * | −0.59 | −3.76 ** | −2.55 ** | −2.14 ** | −6.31 ** | −0.44 | −2.49 | −1.11 | −2.59 ** | −2.88 ** | −5.19 ** | −3.67 ** | −1.16 | −3.22 ** | −0.04 | 0.53 | 0.02 | −0.03 | 0.12 |
| P5 × P6 | −1.26 | −0.17 | −3.51 ** | −3.22 ** | −2.04 ** | 4.42 ** | 1.52 | −0.65 | −0.92 | 1.09 | 0.68 | 0.63 | 1.38 | −3.78 ** | −0.27 | −0.49 | 0.73 * | 0.26 | 0.29 | 0.2 |
| LSD Sij 0.05 | 1.35 | 1.31 | 1.2 | 1.09 | 0.60 | 3.24 | 2.94 | 2.57 | 2.43 | 1.37 | 1.66 | 1.88 | 1.89 | 1.67 | 0.87 | 0.61 | 0.61 | 0.50 | 0.65 | 0.29 |
| LSD Sij 0.01 | 1.8 | 1.75 | 1.6 | 1.45 | 0.80 | 4.33 | 3.94 | 3.44 | 3.25 | 1.82 | 2.22 | 2.51 | 2.53 | 2.23 | 1.15 | 0.82 | 0.82 | 0.68 | 0.87 | 0.39 |
| Crosses | Number of Spikes per Plant | Number of Grains per Spike | Thousand-Grain Weight | Grain Yield per Plant | ||||||||||||||||
| E1 | E2 | E3 | E4 | Comb. | E1 | E2 | E3 | E4 | Comb. | E1 | E2 | E3 | E4 | Comb. | E1 | E2 | E3 | E4 | Comb. | |
| P1 × P2 | −2.09 ** | −1.22 | −0.86 | −0.48 | −1.16 ** | −0.38 | −1.97 | 0.76 | 3.30 * | 0.43 | −0.95 | −3.15 * | −8.43 ** | −8.18 ** | −5.17 ** | −4.24 ** | −4.31 ** | −4.50 ** | −2.10 ** | −3.79 ** |
| P1 × P3 | −1.17 | −2.19 ** | −2.47 ** | −3.16 ** | −2.25 ** | 1.02 | 2.79 * | −7.34 ** | −3.38 * | −1.73 * | −0.3 | −0.45 | 3.71 ** | 0.96 | 0.98 | −0.39 | −0.58 | −6.03 ** | −2.09 ** | −2.27 ** |
| P1 × P4 | −1.87 ** | −1.90 * | 0.05 | 1.40 ** | −0.58 * | 2.75 | 3.45 * | −8.25 ** | −0.8 | −0.71 | −0.45 | −6.78 ** | −2.13 * | −0.56 | −2.48 ** | −0.47 | −0.46 | −0.37 | −2.52 ** | −0.95 |
| P1 × P5 | 3.72 ** | 3.53 ** | −0.62 | 0.29 | 1.73 ** | 2.46 | 3.62 ** | 0.79 | 0.82 | 1.92 ** | 0.45 | −1.77 | 0.09 | 1.56 | 0.08 | 8.06 ** | 6.73 ** | −0.15 | 3.58 ** | 4.56 ** |
| P1 × P6 | 4.68 ** | 5.22 ** | 0.56 | 0.88 * | 2.83 ** | 8.40 ** | 9.71 ** | −7.32 ** | −1.19 | 2.40 ** | 2.38 | 0.45 | 3.47 ** | −0.52 | 1.44 ** | 10.29 ** | 7.96 ** | −1.77 | 2.45 ** | 4.73 ** |
| P2 × P3 | 4.47 ** | 2.15 ** | 1.93 ** | 1.93 ** | 2.62 ** | 4.99 ** | 6.65 ** | −0.67 | −3.10 * | 1.97 ** | −0.98 | 2.23 | 0.73 | 2.44 ** | 1.10 * | 10.49 ** | 9.01 ** | 1.97 * | −0.67 | 5.20 ** |
| P2 × P4 | 3.45 ** | 3.10 ** | −0.47 | 0.20 | 1.57 ** | 6.47 ** | 5.67 ** | −6.96 ** | −2.74 | 0.61 | 3.86 ** | 6.32 ** | 8.67 ** | 4.81 ** | 5.91 ** | 7.01 ** | 7.25 ** | 2.20 * | 4.48 ** | 5.24 ** |
| P2 × P5 | 0.90 | 1.42 | −0.94 | −0.71 | 0.17 | 0.57 | −9.83 ** | −9.01 ** | −0.56 | −4.71 ** | 0.001 | −0.80 | 4.79 ** | 1.61 * | 1.40 ** | 7.20 ** | 6.93 ** | −2.42 * | −2.11 ** | 2.40 ** |
| P2 × P6 | −0.60 | 0.69 | 0.79 | 0.16 | 0.26 | −8.09 ** | −6.77 ** | −5.29 ** | 0.87 | −4.82 ** | 3.29 * | −0.65 | 0.02 | 3.54** | 1.55 ** | −8.26 ** | −9.17 ** | 1.93 * | 0.96 | −3.64 ** |
| P3 × P4 | −2.20 ** | −0.55 | −0.67 | 0.06 | −0.84 ** | −11.66 ** | −15.64 ** | −2.01 | 0.14 | −7.29 ** | −6.45 ** | −12.58 ** | 1.90 | 0.7 5 | −4.10 ** | −8.45 ** | −10.78 ** | 1.48 | −2.06 ** | −4.95 ** |
| P3 × P5 | −0.75 | −1.35 | 1.09 * | 1.94 ** | 0.23 | 3.33 * | −3.84 ** | 7.53 ** | 2.85 * | 2.47 ** | 0.28 | 2.13 | 3.96 ** | 4.96 ** | 2.84 ** | −4.15 ** | −6.86 ** | 10.26 ** | 8.41 ** | 1.91 ** |
| P3 × P6 | −1.96 ** | −3.26** | −0.11 | −0.67 | −1.50 ** | 8.01 ** | 2.42 | 2.58 | 5.08 ** | 4.52 ** | 0.25 | −0.38 | −6.48 ** | −4.16 ** | −2.69 ** | 2.64 * | 0.86 | −0.6 | −1.96 * | 0.23 |
| P4 × P5 | −0.10 | −1.04 | 0.04 | 0.57 | −0.13 | 4.75 ** | 4.21 ** | −0.68 | −1.58 | 1.68 * | 1.52 | 3.23 ** | −3.26 ** | −2.29 ** | −0.20 | −0.48 | −0.26 | 0.23 | −3.11 ** | −0.9 |
| P4 × P6 | −1.03 | 0.26 | −0.28 | 0.92* | −0.03 | −8.97 ** | −14.43 ** | −11.09 ** | −4.17 ** | −9.67 ** | −7.51 ** | −7.49 ** | −3.25 ** | 2.71 ** | −3.89 ** | −2.22 | −2.44 * | −7.87 ** | −4.25 ** | −4.19 ** |
| P5 × P6 | −0.33 | −0.10 | 0.08 | −0.02 | −0.09 | −2.28 | 1.57 | −4.27 ** | 2.41 | −0.64 | 2.50 * | 3.59 ** | 2.09 * | 1.61 * | 2.44 ** | −1.75 | −0.41 | 3.06 ** | −1.02 | −0.03 |
| LSD Sij 0.05 | 1.21 | 1.44 | 1.06 | 0.75 | 0.56 | 2.85 | 2.64 | 2.86 | 2.79 | 1.36 | 2.50 | 2.36 | 1.94 | 1.61 | 1.04 | 2.36 | 2.17 | 1.84 | 1.51 | 0.97 |
| LSD Sij 0.01 | 1.62 | 1.93 | 1.42 | 1.01 | 0.74 | 3.81 | 3.53 | 3.83 | 3.73 | 1.8 | 3.34 | 3.16 | 2.60 | 2.15 | 1.38 | 3.16 | 2.9 | 2.46 | 2.02 | 1.29 |
| Cross | Days to Heading | Plant Height | Chlorophyll Content | Spike Length | ||||||||||||
| M.P | B.P | M.P | B.P | M.P | B.P | M.P | B.P | |||||||||
| Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | |
| P1 × P2 | −1.93 ** | −0.55 | 1.60 | 1.79 | −4.65 ** | −4.59 ** | −3.84 * | −3.44 | −4.62 * | −0.21 | −5.76 ** | −0.90 | −2.38 | −3.50 | −6.86 ** | −9.35 ** |
| P1 × P3 | −5.16 ** | −6.96 ** | −4.36 ** | −5.97 ** | −8.30 ** | −11.18 ** | −5.66 ** | −8.19 ** | 0.82 | −3.78 | −1.36 | −7.21 ** | 6.82 ** | 6.01 | 6.58 * | 5.17 |
| P1 × P4 | −3.09 ** | −1.38 | −2.45 ** | −1.28 | 2.80 | 2.46 | 4.31 * | 3.96 | 3.50 | 5.93 ** | 0.95 | 1.01 | 5.80 ** | 0.98 | 4.49 | 0.47 |
| P1 × P5 | −1.77 * | −2.55 ** | −1.68 * | −2.13 * | 3.61 * | 2.28 | 5.18 ** | 3.13 | 1.66 | 6.86 ** | 1.53 | 6.08 * | 7.07 ** | 9.11 ** | 5.94 * | 8.10 * |
| P1 × P6 | −0.85 | −1.39 | 0.57 | −1.28 | −3.63 * | 1.35 | −2.51 | 3.01 | 7.21 ** | 10.44 ** | 4.27 | 8.15 ** | 1.97 | 4.54 | 0.23 | 2.28 |
| P2 × P3 | 4.37 ** | 4.85 ** | 7.19 ** | 8.48 ** | −2.19 | −0.98 | −0.25 | 1.11 | 4.18 * | 10.74 ** | 3.14 | 7.51 ** | 0.92 | −9.47 ** | −3.93 | −14.32 ** |
| P2 × P4 | −2.23 ** | −3.49 ** | 0.60 | −1.12 | −4.80 ** | −2.03 | −2.56 | 0.61 | −0.93 | −2.33 | −2.22 | −6.23 ** | 6.72 ** | 8.04 ** | 3.05 | 1.98 |
| P2 × P5 | 3.95 ** | 3.15 ** | 7.78 ** | 6.03 ** | 3.00 | −3.29 | 5.47 ** | −1.29 | −2.06 | −0.73 | −3.11 | −0.77 | 3.23 | −3.78 | −0.51 | −8.82 ** |
| P2 × P6 | 2.25 ** | −0.87 | 4.39 ** | 1.34 | −0.42 | −0.11 | 1.61 | 2.77 | 1.54 | −0.04 | −2.38 | −2.77 | 2.50 | 4.28 | −0.57 | 0.03 |
| P3 × P4 | −1.32 | −1.58 * | −1.14 | −0.64 | −11.66 ** | −10.47 ** | −7.74 ** | −6.06 ** | −15.23 ** | −13.46 ** | −15.49 ** | −14.45 ** | 4.01 | −5.39 | 2.49 | −5.66 |
| P3 × P5 | −6.38 ** | −4.83 ** | −5.49 ** | −4.23 ** | 2.11 | 0.51 | 6.68 ** | 4.79 * | −6.53 ** | 0.60 | −8.43 ** | −2.29 | 2.32 | −1.85 | 1.01 | −1.98 |
| P3 × P6 | −3.24 ** | −4.33 ** | −2.68 ** | −3.21 ** | 2.45 | −5.62 ** | 6.65 ** | −0.79 | 7.38 ** | 10.30 ** | 2.24 | 4.25 | 12.56 ** | 7.76 * | 10.40 ** | 6.26 |
| P4 × P5 | −3.37 ** | −7.32 ** | −2.64 ** | −7.02 ** | −1.75 | −6.68 ** | −1.71 | −6.10 ** | −3.75 * | 0.29 | −6.00 ** | −3.68 | 9.15 ** | 10.02 ** | 8.94 ** | 9.55 ** |
| P4 × P6 | −5.51 ** | −5.12 ** | −4.79 ** | −4.91 ** | −7.73 ** | −3.01 | −7.45 ** | −2.85 | −8.76 ** | −9.54 ** | −13.38 ** | −15.43 ** | 4.25 * | 8.10 ** | 3.76 | 6.29 |
| P5 × P6 | −4.72 ** | −4.99 ** | −3.26 ** | −4.49 ** | 3.92 * | 0.12 | 4.28 * | 0.90 | 3.13 | −2.81 | 0.18 | −5.50 * | 2.75 | 10.82 ** | 2.08 | 9.43 ** |
| Number of Spikes per Plant | Number of Grains per Spike | Thousand-Grain Weight | Grain Yield per Plant | |||||||||||||
| M.P | B.P | M.P | B.P | M.P | B.P | M.P | B.P | |||||||||
| Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | Rec | Late | |
| P1 × P2 | −3.90 | 2.52 | −11.32 ** | −2.36 | −4.32 * | 3.18 | −6.54 ** | 0.94 | −7.49 ** | −18.65 ** | −8.07 ** | −20.28 ** | −10.37 ** | −4.63 | −17.06 ** | −6.89 |
| P1 × P3 | −11.11 ** | −19.50 ** | −12.02 ** | −23.47 ** | −5.41 * | 2.06 | −15.38 ** | −2.37 | 2.06 | −6.64 * | −1.05 | −8.05 ** | −7.84 * | −5.70 | −12.34 ** | −12.63 * |
| P1 × P4 | −7.28 * | 3.05 | −9.39 ** | −2.35 | −11.90 ** | 0.25 | −12.74 ** | −3.06 | −4.84 * | −18.46 ** | −10.13 ** | −23.07 ** | −4.90 | −10.99 * | −4.98 | −19.97 ** |
| P1 × P5 | 11.20 ** | 20.65 ** | 4.41 | 10.43 * | 1.69 | 8.40 ** | −0.24 | 7.46 * | 3.37 | −1.91 | −0.21 | −7.58 * | 23.30 ** | 44.72 ** | 13.93 ** | 42.09 ** |
| P1 × P6 | 16.11 ** | 26.91 ** | 11.79 ** | 23.84 ** | −5.97 ** | 11.56 ** | −10.90 ** | 9.32 ** | 4.62 * | −6.48 * | 2.43 | −8.11 ** | 12.90 ** | 30.29 ** | 8.61 * | 28.35 ** |
| P2 × P3 | 22.22 ** | 15.64 ** | 11.74 ** | 4.97 | 1.14 | −0.07 | −7.58 ** | −6.39 * | 1.67 | 7.51 ** | −0.83 | 3.79 | 32.36 ** | 23.63 ** | 28.57 ** | 17.16 ** |
| P2 × P4 | 11.75 ** | 22.20 ** | 5.38 | 21.56 ** | −10.38 ** | −5.29 * | −11.62 ** | −6.41 * | 13.28 ** | 12.87 ** | 7.62 ** | 4.48 | 17.58 ** | 26.58 ** | 8.71 * | 16.31 ** |
| P2 × P5 | 7.55 * | 14.35 ** | 5.57 | 9.67 * | −9.14 ** | −12.26 ** | −9.55 ** | −14.90 ** | 11.51 ** | 9.02 ** | 6.99 ** | 4.73 | 24.56 ** | 29.28 ** | 24.37 ** | 23.99 ** |
| P2 × P6 | 7.09 * | 13.48 ** | 2.46 | 10.69 * | −19.57 ** | −9.01 ** | −22.03 ** | −12.73 ** | 5.36 * | 6.08 * | 3.80 | 2.17 | −8.64 * | −20.21 ** | −12.27 ** | −20.93 ** |
| P3 × P4 | −11.35 ** | −3.43 | −14.23 ** | −12.75 ** | −17.38 ** | −23.36 ** | −25.45 ** | −29.00 ** | −6.89 ** | −19.29 ** | −9.37 ** | −22.73 ** | −12.88 ** | −42.35 ** | −17.20 ** | −44.20 ** |
| P3 × P5 | 1.88 | 1.59 | −5.26 | −11.17 ** | 10.89 ** | −2.68 | 0.92 | −6.12 * | 6.85 ** | 13.10 ** | 0.12 | 5.04 | 24.39 ** | 7.57 | 20.65 ** | −2.00 |
| P3 × P6 | −5.85 | −13.87 ** | −10.24 ** | −20.00 ** | 4.28 | 5.13 | −1.90 | 2.58 | −7.87 ** | −7.50 ** | −8.79 ** | −7.72 ** | 4.91 | −11.71 * | 3.68 | −17.04 ** |
| P4 × P5 | −0.16 | 5.62 | −4.16 | 1.80 | −3.02 | −3.78 | −3.94 | −7.74 ** | −0.41 | 1.88 | −9.02 ** | −9.11 ** | 4.45 | −10.09 * | −3.56 | −20.47 ** |
| P4 × P6 | −4.73 | 10.88 ** | −6.17 | 7.61 | −27.97 ** | −25.48 ** | −31.12 ** | −29.34 ** | −12.95 ** | −9.82 ** | −16.09 ** | −13.47 ** | −23.17 ** | −29.82 ** | −26.15 ** | −36.04 ** |
| P5 × P6 | 2.96 | 7.75 | 0.31 | 0.91 | −10.05 ** | 2.81 | −13.19 ** | 1.62 | 7.31 ** | 11.42 ** | 1.50 | 3.25 | 9.59* | −0.59 | 5.09 | −3.83 |
| Tait | Env. | Genetic Components | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | H1 | H2 | h2 | F | E | (H1/D) 0.5 | (H2/4H1) | KD/KR | h2/H2 | r | r2 | h2 (b.s) | h2 (n.s) | ||
| DTH | E1 | 17.07 | 58.36 * | 46.29 * | 7.73 | 28.21 | 0.54 | 1.85 | 0.20 | 2.62 | 0.17 | −0.95 | 0.89 | 95.67 | 3.71 |
| E2 | 11.79 * | 29.99 * | 21.62 * | 3.89 | 18.17 | 0.60 | 1.60 | 0.18 | 2.87 | 0.18 | −0.9 | 0.82 | 91.38 | 14.12 | |
| E3 | 3.54 * | 50.10 ** | 48.54 ** | 73.25 ** | 0.35 | 0.48 | 3.76 | 0.24 | 1.03 | 1.51 | 0.6 | 0.37 | 96.82 | 15.84 | |
| E4 | 1.49 | 50.73 ** | 49.39 ** | 73.51 ** | −1.13 | 0.58 | 5.83 | 0.24 | 0.88 | 1.49 | 0.8 | 0.64 | 96.1 | 13.27 | |
| PH | E1 | 21.54 | 115.17 ** | 87.07 ** | 0.75 | 29.66 | 3.19 | 2.31 | 0.19 | 1.85 | 0.01 | 0.79 | 0.63 | 90.87 | 28.58 |
| E2 | 16.6 | 101.61 ** | 83.33 ** | 30.35 | 30.47 | 3.03 | 2.47 | 0.21 | 2.18 | 0.36 | 0.92 | 0.85 | 88.38 | 8.48 | |
| E3 | 4.47 | 43.67 ** | 41.28 ** | 13.49 ** | −0.41 | 2.00 | 3.13 | 0.24 | 0.97 | 0.33 | −0.68 | 0.47 | 87.48 | 22.78 | |
| E4 | 3.98 | 22.43 ** | 21.74 ** | 0.64 | −1.57 | 1.86 * | 2.37 | 0.24 | 0.85 | 0.03 | 0.06 | 0.00 | 82.16 | 29.95 | |
| CHLC | E1 | 1.93 | 20.76 * | 17.57 * | 0.41 | 2.54 | 0.85 | 3.28 | 0.21 | 1.50 | 0.02 | 0.19 | 0.04 | 87.03 | 19.75 |
| E2 | 1.75 | 40.00 ** | 35.06 ** | 0.08 | 4.41 | 1.12 | 4.78 | 0.22 | 1.72 | 0 | −0.4 | 0.16 | 89.87 | 10.32 | |
| E3 | 2.2 | 29.95 ** | 26.19 ** | −0.64 | 5.43 | 1.48 | 3.69 | 0.22 | 2.01 | −0.02 | 0.3 | 0.09 | 82.15 | 3.19 | |
| E4 | 7.59 * | 30.22 ** | 28.36 ** | −0.43 | 5.22 | 0.83 | 2 | 0.23 | 1.42 | −0.02 | −0.58 | 0.33 | 91.72 | 21.08 | |
| SL | E1 | 0.6 | 2.73 ** | 2.41 ** | 2.21 ** | 0.39 | 0.11 | 2.14 | 0.22 | 1.36 | 0.92 | −0.91 | 0.83 | 88.55 | 27.00 |
| E2 | 0.87 ** | 3.53 ** | 2.39 ** | 0.31 | 0.89 | 0.13 | 2.01 | 0.17 | 1.68 | 0.13 | −0.25 | 0.06 | 89.71 | 43.62 | |
| E3 | 0.42 | 5.84 ** | 4.76 ** | 0.12 | 1.02 | 0.09 | 3.75 | 0.20 | 1.97 | 0.02 | −0.74 | 0.55 | 94.33 | 15.80 | |
| E4 | 0.15 | 3.89 ** | 3.21 ** | 0.09 | 0.48 | 0.13 | 5.03 | 0.21 | 1.91 | 0.03 | −0.72 | 0.52 | 88.43 | 16.14 | |
| NSPP | E1 | 3.74 * | 28.20 ** | 24.17 ** | 3.71 | 4.44 | 0.49 | 2.75 | 0.21 | 1.55 | 0.15 | −0.02 | 0.00 | 94.03 | 20.28 |
| E2 | 3.63 | 28.43 ** | 22.16 ** | 3.06 | 6.4 | 0.66 | 2.8 | 0.19 | 1.92 | 0.14 | −0.1 | 0.01 | 91.74 | 21.98 | |
| E3 | 0.53 | 4.16 | 3.38 | 0.29 | 0.82 | 0.44 | 2.81 | 0.2 | 1.76 | 0.09 | 0.28 | 0.08 | 71.17 | 15.93 | |
| E4 | 1.30 | 7.03 * | 6.17 * | 1.56 | 0.99 | 0.17 | 2.32 | 0.22 | 1.39 | 0.25 | 0.37 | 0.13 | 92.55 | 25.36 | |
| NGPS | E1 | 5.51 | 170.98 ** | 146.78 ** | 18.05 | −20.64 | 2.72 | 5.57 | 0.21 | 0.5 | 0.12 | −0.59 | 0.34 | 95.79 | 38.97 |
| E2 | 3.48 | 294.60 ** | 237.60 ** | 21.97 | −4.98 | 2.42 | 9.2 | 0.20 | 0.86 | 0.09 | −0.22 | 0.05 | 97.44 | 34.62 | |
| E3 | 90.68 ** | 261.58 ** | 203.55 ** | 395.44 ** | 110.99 ** | 2.49 | 1.7 | 0.19 | 2.13 | 1.94 | 0.52 | 0.27 | 96.55 | 26.11 | |
| E4 | 14.60 ** | 31.95 ** | 23.83 ** | −1.11 | 19.88 * | 3.12* | 1.48 | 0.19 | 2.71 | −0.05 | 0.57 | 0.32 | 70.25 | 13.5 | |
| TGW | E1 | 8.92 | 51.38 * | 36.78 | −0.65 | 14.41 | 2.41 | 2.4 | 0.18 | 2.01 | −0.02 | 0.8 | 0.65 | 85.06 | 28.18 |
| E2 | 12.17 | 138.82 * | 102.73 | 38.04 | 29.73 | 2.09 | 3.38 | 0.18 | 2.13 | 0.37 | 0.8 | 0.64 | 94.36 | 25.02 | |
| E3 | 21.37 * | 86.65 ** | 77.21 ** | 4.55 | 20.39 | 1.25 | 2.01 | 0.22 | 1.62 | 0.06 | −0.32 | 0.1 | 95.15 | 20.22 | |
| E4 | 12.24 | 59.53 ** | 48.85 ** | 12.51 | 12.39 | 0.78 | 2.21 | 0.21 | 1.6 | 0.26 | −0.21 | 0.04 | 95.73 | 28.83 | |
| GYPP | E1 | 12.45 | 182.21 ** | 159.44 ** | 34.38 ** | 17.98 | 1.74 | 3.83 | 0.22 | 1.47 | 0.22 | −0.71 | 0.50 | 96.54 | 17.16 |
| E2 | 9.72 | 183.77 ** | 154.93 ** | 0.98 | 26.21 | 1.54 | 4.35 | 0.21 | 1.90 | 0.01 | −0.63 | 0.39 | 96.69 | 13.29 | |
| E3 | 24.85 * | 107.82 ** | 74.99 * | 0.42 | 49.53 | 1.07 | 2.08 | 0.17 | 2.83 | 0.01 | 0.23 | 0.05 | 95.54 | 17.07 | |
| E4 | 15.44 * | 54.35 ** | 45.58 ** | 0.21 | 14.34 | 0.71 | 1.88 | 0.21 | 1.66 | 0.00 | 0.67 | 0.45 | 95.84 | 28.96 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamara, M.M.; Ibrahim, K.M.; Mansour, E.; Kheir, A.M.S.; Germoush, M.O.; Abd El-Moneim, D.; Motawei, M.I.; Alhusays, A.Y.; Farid, M.A.; Rehan, M. Combining Ability and Gene Action Controlling Grain Yield and Its Related Traits in Bread Wheat under Heat Stress and Normal Conditions. Agronomy 2021, 11, 1450. https://doi.org/10.3390/agronomy11081450
Kamara MM, Ibrahim KM, Mansour E, Kheir AMS, Germoush MO, Abd El-Moneim D, Motawei MI, Alhusays AY, Farid MA, Rehan M. Combining Ability and Gene Action Controlling Grain Yield and Its Related Traits in Bread Wheat under Heat Stress and Normal Conditions. Agronomy. 2021; 11(8):1450. https://doi.org/10.3390/agronomy11081450
Chicago/Turabian StyleKamara, Mohamed M., Khaled M. Ibrahim, Elsayed Mansour, Ahmed M. S. Kheir, Mousa O. Germoush, Diaa Abd El-Moneim, Mohamed I. Motawei, Ahmed Y. Alhusays, Mona Ali Farid, and Medhat Rehan. 2021. "Combining Ability and Gene Action Controlling Grain Yield and Its Related Traits in Bread Wheat under Heat Stress and Normal Conditions" Agronomy 11, no. 8: 1450. https://doi.org/10.3390/agronomy11081450









