Development of a Co-Dominant Cleaved Amplified Polymorphic Sequences Assay for the Rapid Detection and Differentiation of Two Pathogenic Clarireedia spp. Associated with Dollar Spot in Turfgrass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. PCR Primer Design
2.4. PCR Amplification and Optimization
2.5. CAPS Analysis of the PCR Product
2.6. Direct PCR Testing
3. Results
3.1. PCR Primer Design
3.2. Optimization of PCR Primers
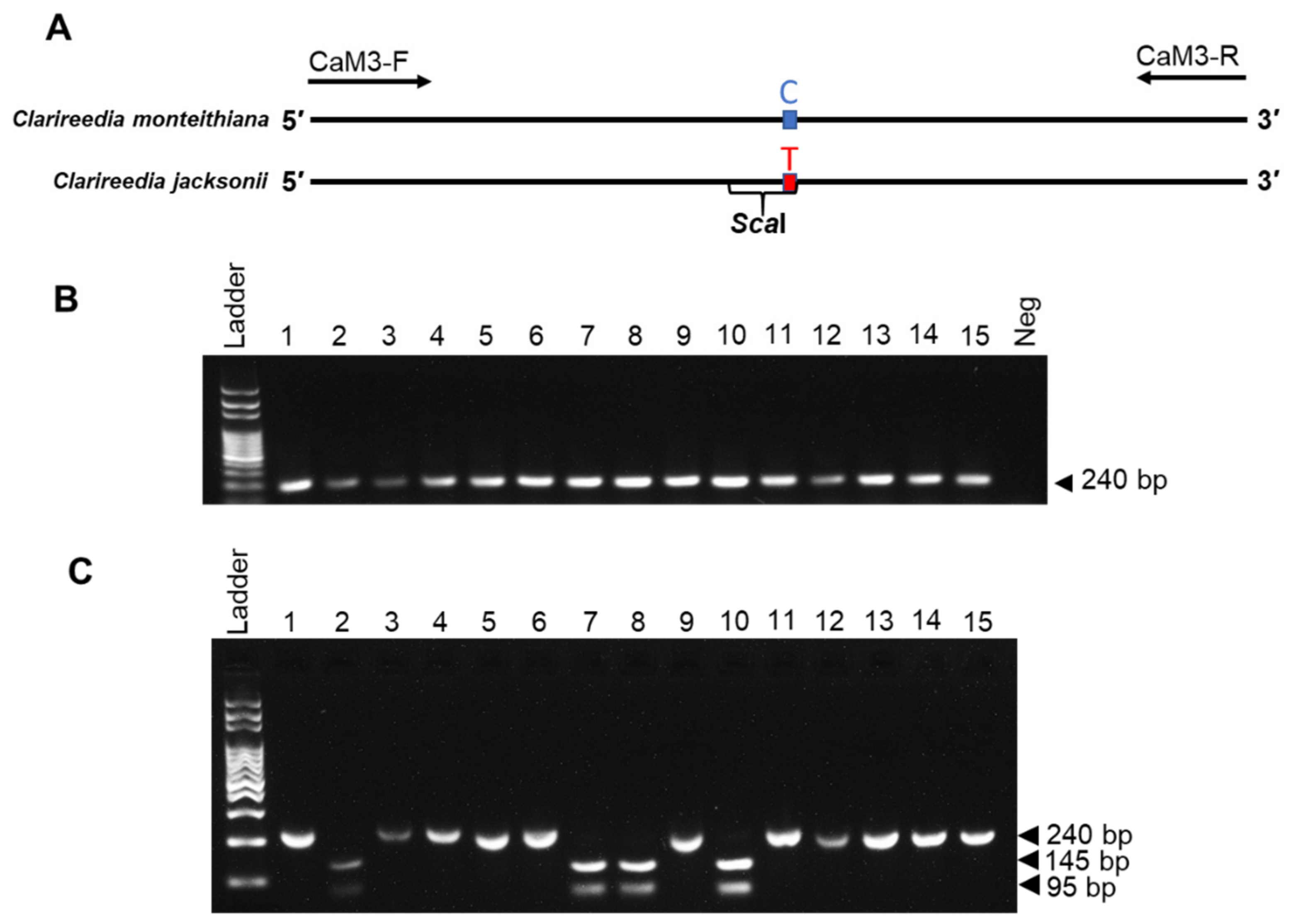
3.3. CAPS Analysis of PCR Fragment
3.4. Direct PCR Testing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chawla, S.; Roshni, A.; Patel, M.; Patil, S.; Shah, H. Turfgrass: A billion dollar industry. In Proceedings of the National Conference on Floriculture for Rural and Urban Prosperity in the Scenerio of Climate Change-2018, Pakyong, India, 16–18 February 2018. [Google Scholar]
- Dousset, B.; Gourmelon, F. Surface temperatures of the Paris Basin during summertime, using satellite remote sensing data. In Proceedings of the 5th International Conference on Urban Climate, Lodz, Poland, 1–5 September 2003. [Google Scholar]
- McPherson, E.G. Cooling urban heat islands with sustainable landscapes. In The Ecological City: Preserving and Restoring Urban Biodiversity; Platt, R.H.R., Rowan, A., Muick, P.C., Eds.; University of Massachusetts Press: Amherst, MA, USA, 1994; pp. 151–171. [Google Scholar]
- Kowalczyk, A.; Twardy, S.; Kuźniar, A. Permanent turf grass as the factor alleviating water erosion in the Carpathian Mountains. J. Water Land Dev. 2011, 15, 41–51. [Google Scholar] [CrossRef]
- Krenitsky, E.; Carroll, M.; Hill, R.; Krouse, J. Runoff and sediment losses from natural and man-made erosion control materials. Crop Sci. 1998, 38, 1042–1046. [Google Scholar] [CrossRef]
- Monteiro, J.A. Ecosystem services from turfgrass landscapes. Urban For. Urban Green. 2017, 26, 151–157. [Google Scholar] [CrossRef]
- Vargas, J.M. Management of Turfgrass Diseases, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 1351434187. [Google Scholar]
- Hu, J.; Zhou, Y.; Geng, J.; Dai, Y.; Ren, H.; Lamour, K. A new dollar spot disease of turfgrass caused by Clarireedia paspali. Mycol. Prog. 2019, 18, 1423–1435. [Google Scholar] [CrossRef]
- Salgado-Salazar, C.; Beirn, L.A.; Ismaiel, A.; Boehm, M.J.; Carbone, I.; Putman, A.I.; Tredway, L.P.; Clarke, B.B.; Crouch, J.A. Clarireedia: A new fungal genus comprising four pathogenic species responsible for dollar spot disease of turfgrass. Fungal Biol. 2018, 122, 761–773. [Google Scholar] [CrossRef]
- Bennett, F. Dollar spot disease of turf and its causal organism Sclerotinia homoeocarpa n. sp. Ann. Appl. Biol. 1937, 24, 236–257. [Google Scholar] [CrossRef]
- Crouch, J.A.; Beirn, L.; Boehm, M.; Carbone, I.; Clarke, B.B.; Kerns, J.P.; Malapi, M.; Mitchell, T.; Venu, R.; Tredway, L. Genome resources for seven fungal isolates that cause dollar spot disease in turfgrass, including Clarireedia jacksonii and C. monteithiana. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Groben, G.; Clarke, B.B.; Murphy, J.A.; Koch, P.L.; Crouch, J.A.; Lee, S.; Zhang, N. Real-time PCR detection of Clarireedia spp., the causal agents of dollar spot in turfgrasses. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Stowell, L.; Gelernter, W. Diagnosis of turfgrass diseases. Annu. Rev. Phytopathol. 2001, 39, 135–155. [Google Scholar] [CrossRef]
- Smiley, R.; Dernoeden, P.; Clarke, B. Compendium of Turfgrass Diseases, 3rd ed.; American Phytopathological Society: St. Paul, MN, USA, 2005; ISBN 9780890543306. [Google Scholar]
- Williams, D.W.; Powell, A.J.; Vincelli, P.; Dougherty, C.T. Dollar Spot on bentgrass influenced by displacement of leaf surface moisture, nitrogen, and clipping removal. Crop Sci. 1996, 36. [Google Scholar] [CrossRef]
- Horvath, B.; Kravchenko, A.; Robertson, G.; Vargas, J., Jr. Geostatistical analysis of dollar spot epidemics occurring on a mixed sward of creeping bentgrass and annual bluegrass. Crop Sci. 2007, 47, 1206–1216. [Google Scholar] [CrossRef]
- Martínez-Espinoza, A.D.; Burpee, L.L.; Waltz, C. Abiotic injuries and disorders of turfgrasses in Georgia. In University of Georgia; Georgia, U.O., Ed.; University of Georgia: Athens, GA, USA, 2009; Volume 1258. [Google Scholar]
- Couch, H.B.; Bloom, J.R. Influence of environment on diseases of turf-grasses. II. Influence of nutrition, pH and soil moisture on Sclerotinia dollar spot. Phytopathology 1960, 50, 761–763. [Google Scholar]
- Baird, P.R. Determining Dollar Spot Fungicide Resistance in Tennessee and Northern Mississippi. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2005. [Google Scholar]
- Little, E.L. 2017 Georgia plant disease loss estimates (102-10). In University of Georgia; Georgia, U.O., Ed.; Annual Publication: Athens, GA, USA, 2019; pp. 1–21. [Google Scholar]
- Walsh, B.; Ikeda, S.S.; Boland, G.J. Biology and management of dollar spot (Sclerotinia homoeocarpa); an important disease of turfgrass. HortScience 1999, 34, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Goodman, D.; Burpee, L. Biological control of dollar spot disease of creeping bentgrass. Phytopathology 1991, 81, 1438–1446. [Google Scholar] [CrossRef]
- Sapkota, S.; Martinez Espinoza, A.; Ali, E.; Vermeer, B.; Bahri, B. Taxonomical identification of Clarireedia species causing dollar spot disease of turfgrass in Georgia. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Aynardi, B.A.; Jiménez-Gasco, M.; Uddin, W. Effects of isolates of Clarireedia jacksonii and Clarireedia monteithiana on severity of dollar spot in turfgrasses by host type. Eur. J. Plant Pathol. 2019, 155, 817–829. [Google Scholar] [CrossRef]
- DeVries, R.E.; Trigiano, R.N.; Windham, M.T.; Windham, A.S.; Sorochan, J.C.; Rinehart, T.A.; Vargas, J.M. Genetic analysis of fungicide-resistant Sclerotinia homoeocarpa isolates from Tennessee and Northern Mississippi. Plant Dis. 2007, 92, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Hsiang, T.; Mahuku, G.S. Genetic variation within and between southern Ontario populations of Sclerotinia homoeocarpa. Plant Pathol. 1999, 48, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.-K.; Niver, A.L.; Rimelspach, J.W.; Boehm, M.J. Fungicide sensitivity of Sclerotinia homoeocarpa from golf courses in Ohio. Plant Dis. 2006, 90, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, J.; Gu, M.; Geng, J.; Guo, C.; Yang, Z.; Lamour, K. Baseline sensitivity and control efficacy of fluazinam against Clarireedia homoeocarpa. J. Crop Prot. 2020, 137, 105290. [Google Scholar] [CrossRef]
- Marvin, J.W.; Kerr, R.A.; McCarty, L.B.; Bridges, W.; Martin, S.B.; Wells, C.E. Curative evaluation of biological control agents and synthetic fungicides for Clarireedia jacksonii. HortScience 2020, 55, 1622–1625. [Google Scholar] [CrossRef]
- Flores, F.; Walker, N. First Report of dollar spot of sandbur caused by Sclerotinia homoeocarpa in Oklahoma. Plant Dis. 2014, 98, 1160. [Google Scholar] [CrossRef] [PubMed]
- Oren, E.; Klingeman, W.; Gazis, R.; Moulton, J.; Lambdin, P.; Coggeshall, M.; Hulcr, J.; Seybold, S.J.; Hadziabdic, D. A novel molecular toolkit for rapid detection of the pathogen and primary vector of thousand cankers disease. PLoS ONE 2018, 13, e0185087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacio-Bielsa, A.; Cambra, M.A.; López, M.M. PCR detection and identification of plant-pathogenic bacteria: Updated review of protocols (1989–2007). J. Plant Pathol. 2009, 249–297. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Bioinformatic tools and guideline for PCR primer design. Afr. J. Biotechnol. 2003, 2, 91–95. [Google Scholar]
- Vincelli, P.; Tisserat, N. Nucleic acid-based pathogen detection in applied plant pathology. Plant Dis. 2008, 92, 660–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaels, S.D.; Amasino, R.M. A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J. 1998, 14, 381–385. [Google Scholar] [CrossRef]
- Kaundun, S.S.; Marchegiani, E.; Hutchings, S.-J.; Baker, K. Derived polymorphic amplified cleaved sequence (dPACS): A novel PCR-RFLP procedure for detecting known single nucleotide and deletion–insertion polymorphisms. Int. J. Mol. Sci. 2019, 20, 3193. [Google Scholar] [CrossRef] [Green Version]
- Szalanski, A.L.; Sui, D.D.; Harris, T.S.; Powers, T.O. Identification of cyst nematodes of agronomic and regulatory concern with PCR-RFLP of ITS1. J. Nematol. 1997, 29, 255–267. [Google Scholar]
- Adachi, M.; Sako, Y.; Ishida, Y. Restriction fragment length polymorphism of ribosomal DNA internal transcribed spacer and 5.8s regions in Japanese Alexandrium species (dinophyceae). J. Phycol. 1994, 30, 857–863. [Google Scholar] [CrossRef]
- Park, E.-J.; Fukuda, S.; Endo, H.; Kitade, Y.; Saga, N. Genetic polymorphism within Porphyra yezoensis (Bangiales, Rhodophyta) and related species from Japan and Korea detected by cleaved amplified polymorphic sequence analysis. Eur. J. Phycol. 2007, 42, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Reale, S.; Doveri, S.; Díaz, A.; Angiolillo, A.; Lucentini, L.; Pilla, F.; Martín, A.; Donini, P.; Lee, D. SNP-based markers for discriminating olive (Olea europaea L.) cultivars. Genome 2006, 49, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Iaffaldano, B.J.; Zhuang, X.; Cardina, J.; Cornish, K. Chloroplast genome resources and molecular markers differentiate rubber dandelion species from weedy relatives. BMC Plant Bio. 2017, 17, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Waliullah, S.; Hudson, O.; Oliver, J.E.; Brannen, P.M.; Ji, P.; Ali, M.E. Comparative analysis of different molecular and serological methods for detection of Xylella fastidiosa in blueberry. PLoS ONE 2019, 14, e0221903. [Google Scholar] [CrossRef] [Green Version]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Stackhouse, T.; Martinez-Espinoza, A.D.; Ali, M.E. Turfgrass disease diagnosis: Past, present, and future. Plants 2020, 9, 1544. [Google Scholar] [CrossRef] [PubMed]
- Susca, A.; Stea, G.; Mulé, G.; Perrone, G. Polymerase chain reaction (PCR) identification of Aspergillus niger and Aspergillus tubingensis based on the calmodulin gene. Food Addit. Contam. 2007, 24, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Mulè, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Roux, K.H. Optimization and troubleshooting in PCR. Cold Spring Harb. Protoc. 2009, 2009, 66. [Google Scholar] [CrossRef] [Green Version]
- Sialer, M.F.; Cillo, F.; Barbarossa, L.; Gallitelli, D. Differentiation of cucumber mosaic virus subgroups by RT-PCR RFLP. J. Plant Path. 1999, 81, 145–148. [Google Scholar]
- Chaffin, A.; Dee, M.; Boggess, S.; Trigiano, R.; Bernard, E.; Gwinn, K. First report of Chaetomium globosum causing a leaf spot of hemp (Cannabis sativa) in Tennessee. Plant Dis. 2020, 104, 1540. [Google Scholar] [CrossRef]
- Ben-Amar, A.; Oueslati, S.; Mliki, A. Universal direct PCR amplification system: A time-and cost-effective tool for high-throughput applications. 3 Biotech 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A.; Newsham, K.K.; Neises, D.J. Filamentous ascomycetes inhabiting the rhizoid environment of the liverwort Cephaloziella varians in Antarctica are assessed by direct PCR and cloning. Mycologia 2003, 95, 457–466. [Google Scholar] [CrossRef]
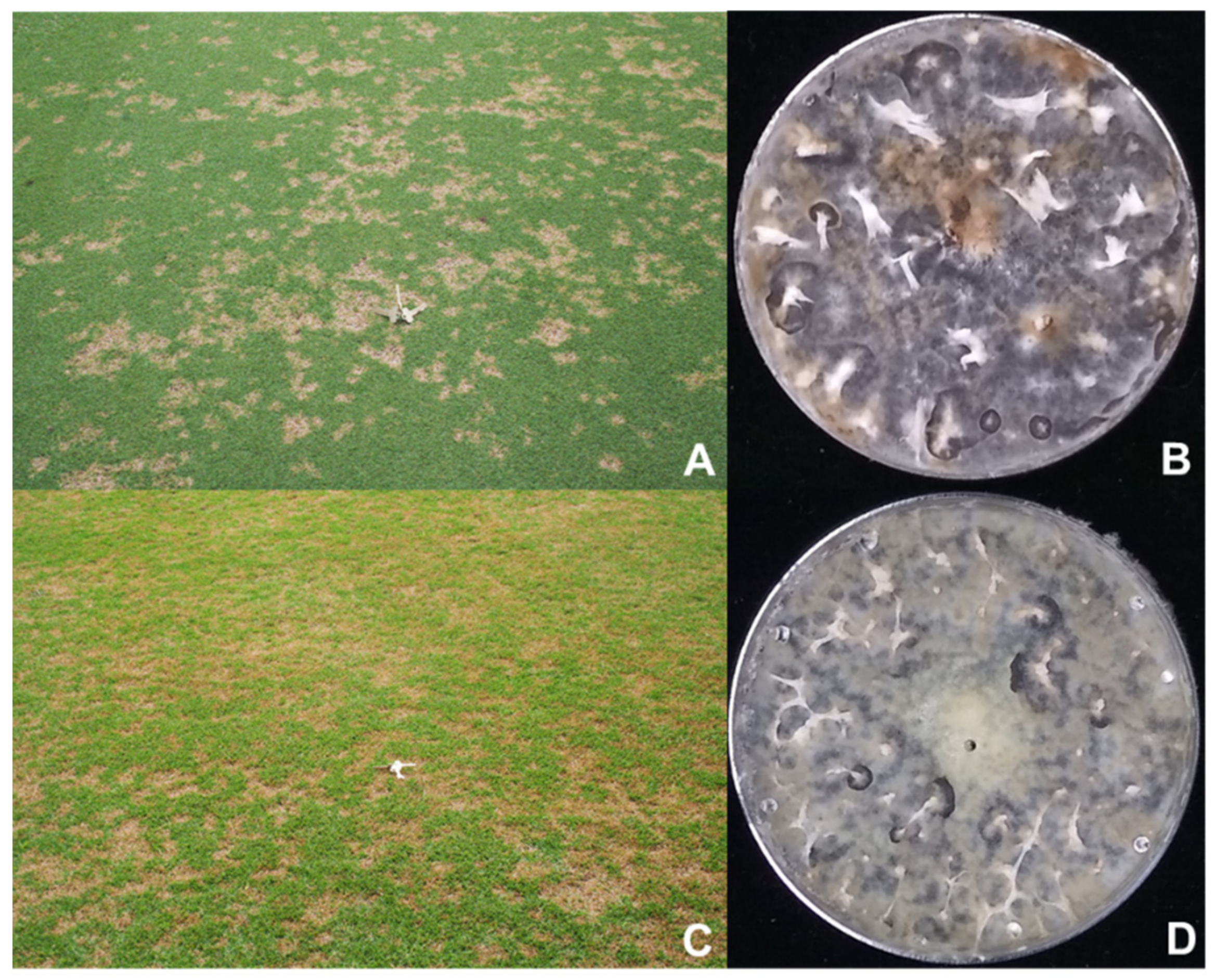


| Pathogen | MDL Name | Grass Species | Grass Type | GA, US County |
|---|---|---|---|---|
| C. monteithiana | 2020-DS2 | Zoysia sp. | Warm | Spalding |
| C. jacksonii | 2020-DS3 | Agrostis stolonifera L. | Cool | Spalding |
| C. monteithiana | 2020-DS4 | Zoysia sp. | Warm | Fulton |
| C. monteithiana | 2020-DS5 | Cynodon dactylon L. | Warm | Cook |
| C. monteithiana | 2020-DS6 | Paspalum vaginatum Swartz | Warm | Cook |
| C. monteithiana | 2020-DS7 | Cynodon dactylon L. | Warm | Spalding |
| C. jacksonii | 2020-DS8 | Digitaria sp. | Warm | Spalding |
| C. jacksonii | 2020-DS10 | Festuca arundinacea Schreber | Cool | Spalding |
| C. monteithiana | 2020-DS11 | Zoysia sp. | Warm | Fulton |
| C. jacksonii | 2020-DS15 | Agrostis stolonifera L. | Cool | Spalding |
| C. monteithiana | 2020-DS16 | Cynodon dactylon L. | Warm | Spalding |
| C. monteithiana | 2020-DS17 | Paspalum vaginatum Swartz | Warm | Spalding |
| C. monteithiana | 2020-DS18 | Zoysia sp. | Warm | Spalding |
| C. monteithiana | 2020-DS19 | Cynodon dactylon L. | Warm | Coweta |
| C. monteithiana | 2020-DS21 | Zoysia sp. | Warm | Upson |
| Primer Set Name | Gene Target | Forward Primer Sequence (F) | Reverse Primer Sequence (R) | Recommended Annealing Temperatures F/R (°C) | Produced Bands at Recommended Temperatures? | Only One, Correctly Sized Band? | Amplified Only Target Samples? (Specificity) | Had a Good CAPS Assay Target? |
|---|---|---|---|---|---|---|---|---|
| MCM1 | Mcm7 | GCTGGAATTTCGATGCCCTAG | AGCCGACCGTTGAAGTTAATG | 66.7/65 | No | - | - | - |
| MCM3 | Mcm7 | CTGTTCAATCCGTCTAAACACG | ACCAGTTGGTCATATTCCGAG | 63.3/62.7 | No | - | - | - |
| ITS1 | ITS | TTTGGCAGGCTGCTGCC | ACCCTGTAACGAGAGGTATGT | 69/59 | No | - | - | - |
| CaM4 | CaM | CTATTTCAGCCCTATGTGAAC | CAGCATGATATTCAAGATCGC | 57.8/62.1 | No | - | - | - |
| MCM2 | Mcm7 | CATCGAGATGGCCGTAGATC | GATGTCGTAGATGTCGCCG | 64.5/64.4 | Yes | No | - | - |
| MCM4 | Mcm7 | GCAAGAATATGCCGGCGAC | CGCCAGACTGCAAGGTCA | 67.7/66.6 | Yes | No | - | - |
| ITS2 | ITS | TTTGGCCGGCTGCTCGA | ACCCTGTAACGAGAGGTATGTGT | 71.8/62.5 | Yes | Yes | No | - |
| ITS3 | ITS | GCTTTGGCAGGCTGCTGGA | GCCCTGTAACGAGGTGTATGT | 71.2/62.3 | Yes | Yes | No | - |
| CaM1 | CaM | AGTCCTCCGCTACCATCC | CAGCATGATATTCAAGATCGC | 61.7/62.1 | Yes | Yes | No | - |
| ITS4 | ITS | CTTTGGCCGGCTGCTCGA | ACCCTGTAACGAGAGGTATGTGT | 72.2/62.5 | Yes | Yes | Yes | No |
| CaM2 | CaM | CTTGGACCACTATCGCGACC | TGCAAACGTCAGTCTACAGC | 66.8/61.7 | Yes | Yes | Yes | No |
| CaM3 | CaM | CTATTTCAGCCCTTTGCGAAG | CAGCATGATATTCAAGGTCGC | 64.7/64.4 | Yes | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stackhouse, T.; Waliullah, S.; Martinez-Espinoza, A.D.; Bahri, B.; Ali, M.E. Development of a Co-Dominant Cleaved Amplified Polymorphic Sequences Assay for the Rapid Detection and Differentiation of Two Pathogenic Clarireedia spp. Associated with Dollar Spot in Turfgrass. Agronomy 2021, 11, 1489. https://doi.org/10.3390/agronomy11081489
Stackhouse T, Waliullah S, Martinez-Espinoza AD, Bahri B, Ali ME. Development of a Co-Dominant Cleaved Amplified Polymorphic Sequences Assay for the Rapid Detection and Differentiation of Two Pathogenic Clarireedia spp. Associated with Dollar Spot in Turfgrass. Agronomy. 2021; 11(8):1489. https://doi.org/10.3390/agronomy11081489
Chicago/Turabian StyleStackhouse, Tammy, Sumyya Waliullah, Alfredo D. Martinez-Espinoza, Bochra Bahri, and Md Emran Ali. 2021. "Development of a Co-Dominant Cleaved Amplified Polymorphic Sequences Assay for the Rapid Detection and Differentiation of Two Pathogenic Clarireedia spp. Associated with Dollar Spot in Turfgrass" Agronomy 11, no. 8: 1489. https://doi.org/10.3390/agronomy11081489







