Does Complex Soil Enhance Grain Yield under Cropping System?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Field Management
2.3. Sample Collection
2.4. Licorice Quality
2.5. Soil Properties
2.6. Data Analyses
3. Results
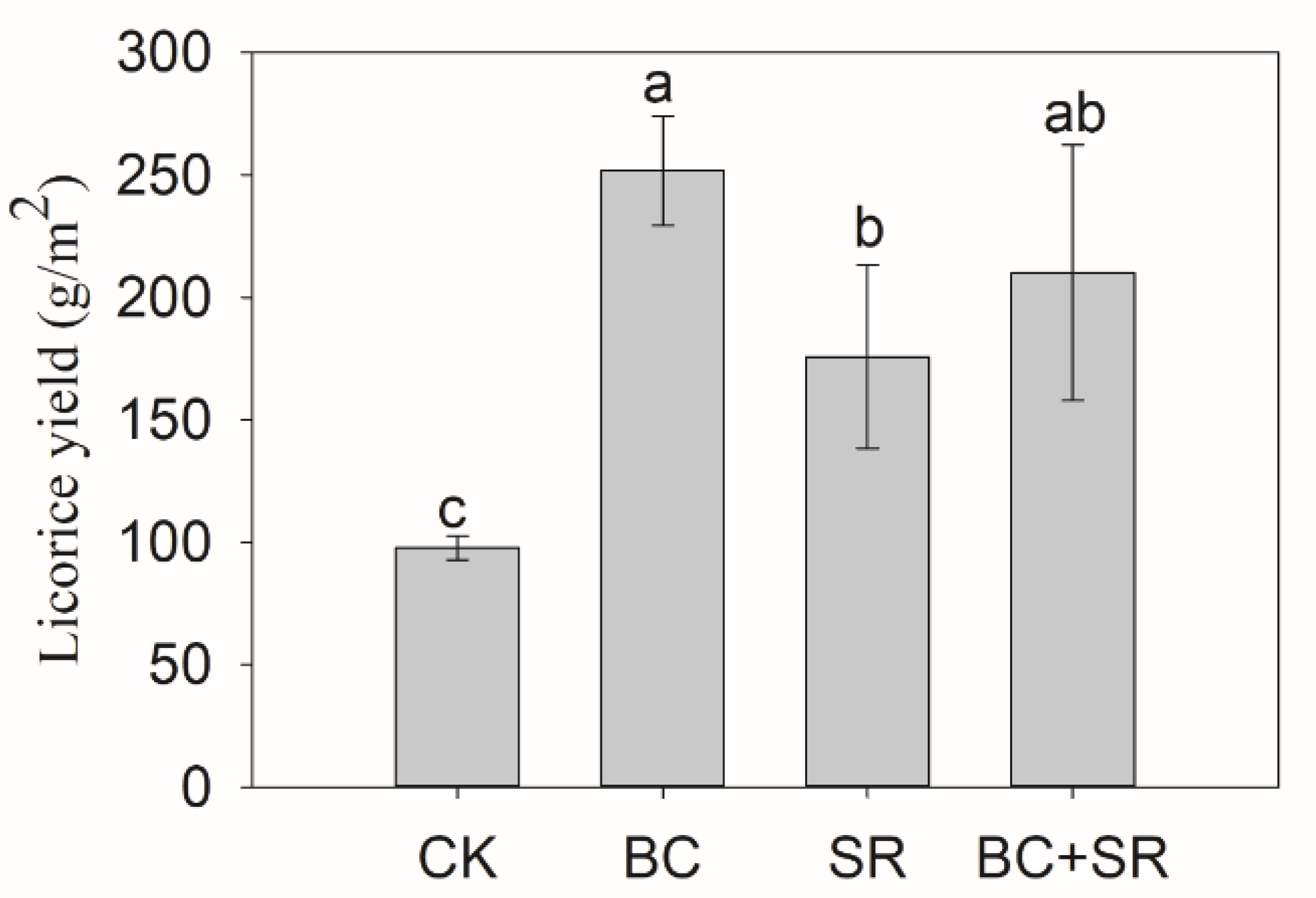
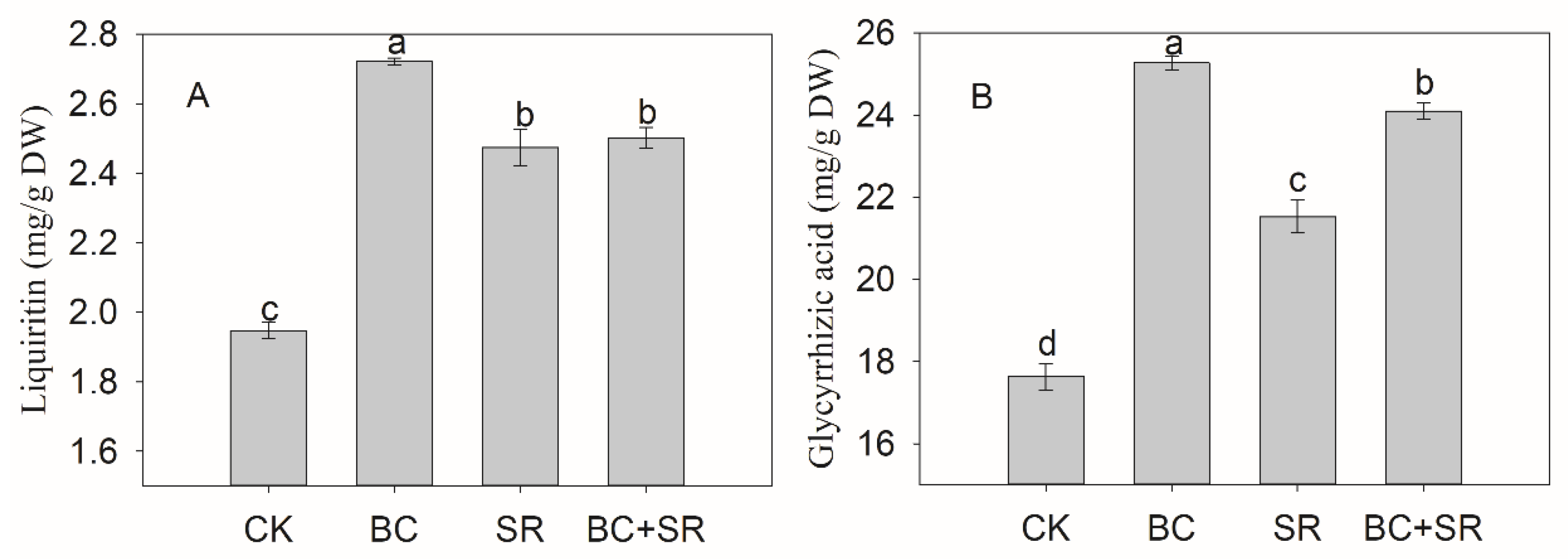
3.1. Licorice Biomass and Quality
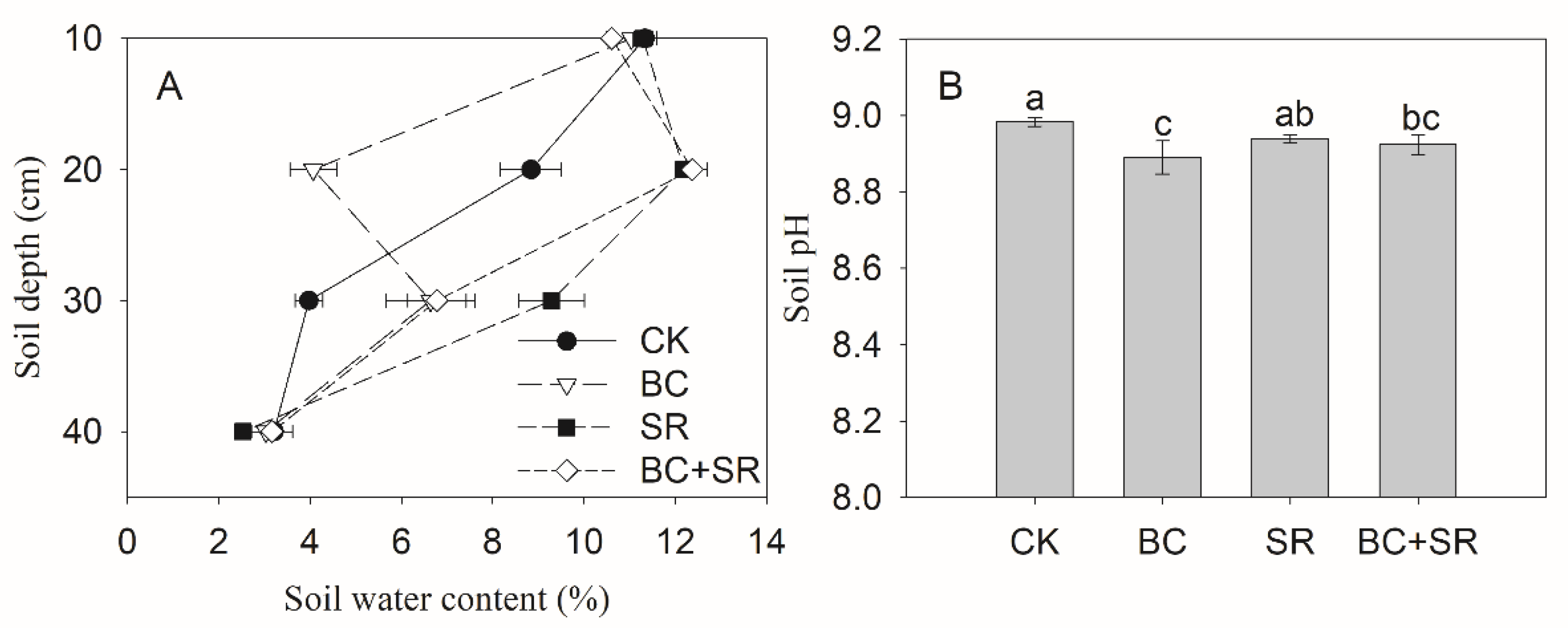
3.2. Soil Water and pH
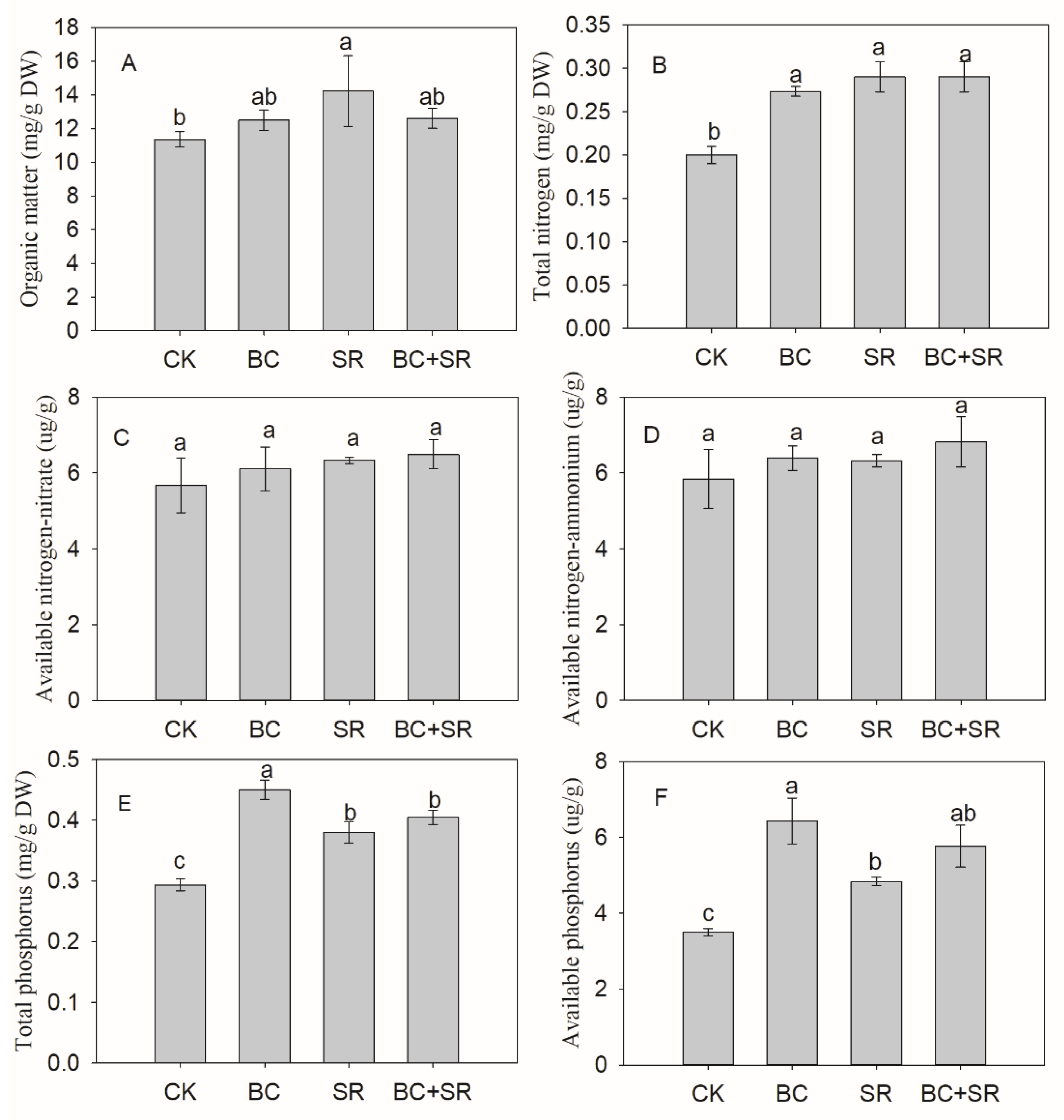
3.3. Soil Nutrients
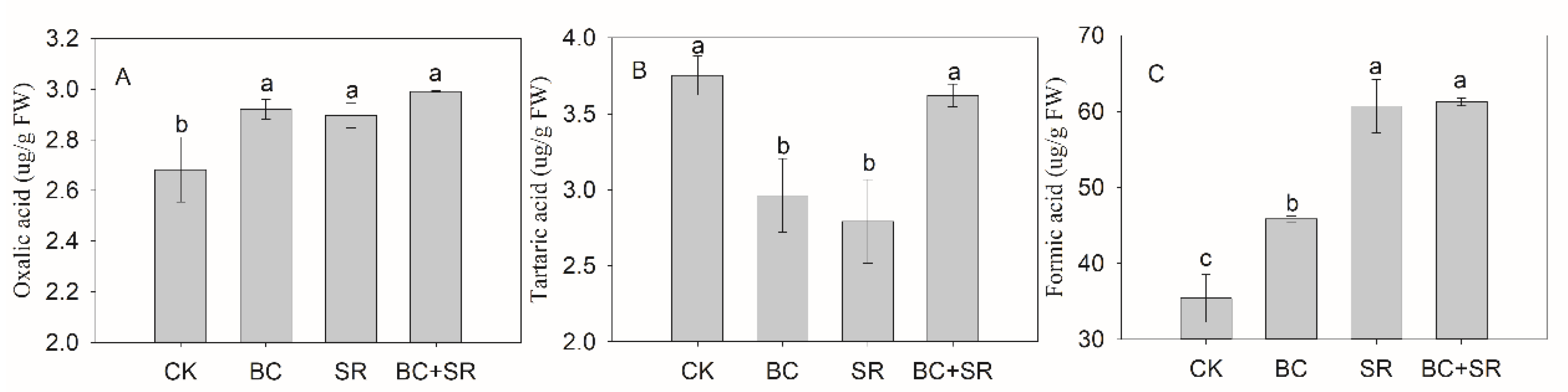
3.4. Soil Organic Acid
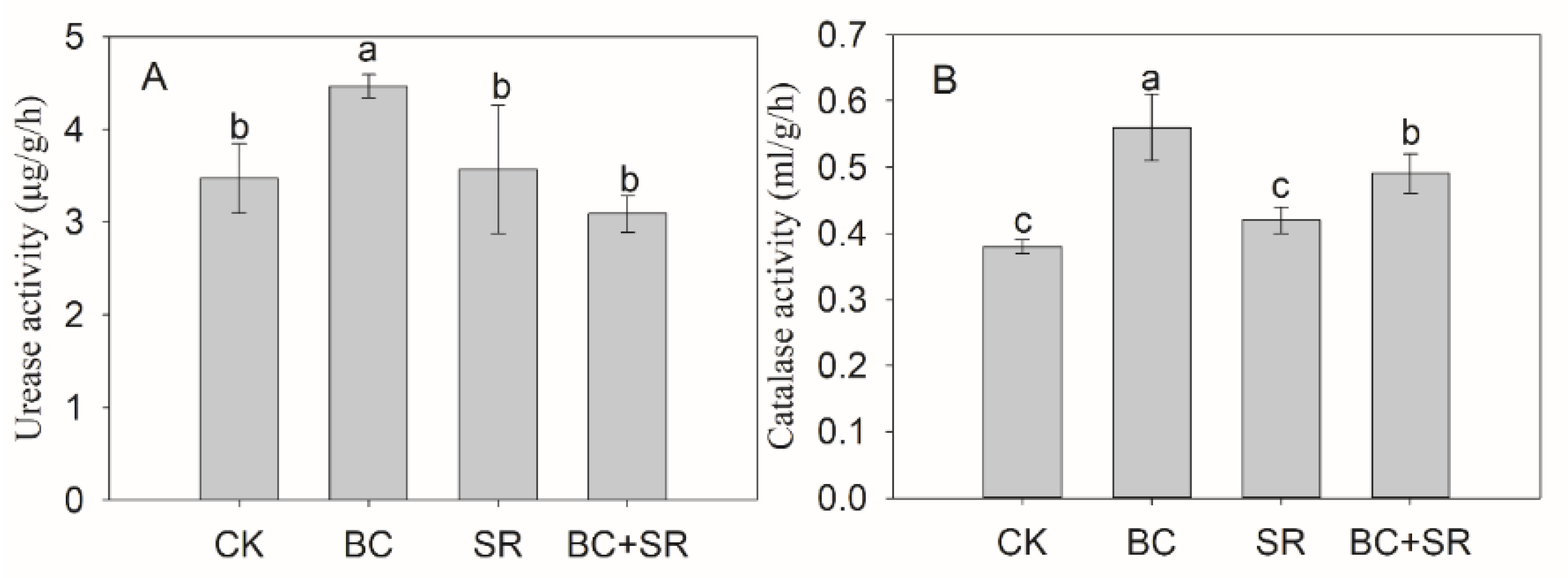
3.5. Soil Enzyme Activity
3.6. Correlation Analysis
4. Discussion
4.1. Effects of Soft Rocks and Biochar on SWC in Topsoil
4.2. Effect of Biochar Application on Soil Enzyme Activity
4.3. Soil Amendment Effect on Soil AP Content
4.4. Effects of Biochar and Soft rocks on Licorice Yield and Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Li, Y. Revitalize the world’s countryside. Nature 2017, 548, 275–277. [Google Scholar] [CrossRef]
- Sultana, N.; Ikeda, T.; Kashem, M.A. Effect of foliar spray of nutrient solutions on photosynthesis, dry matter accumulation and yield in seawater-stressed rice. Environ. Exp. Bot. 2001, 46, 129–140. [Google Scholar] [CrossRef]
- Yang, Y.; Hasi, E.; Sun, B.; Huishi, D.U.; Zhao, Y. Effects of Vegetation Restoration in Different Types on Soil Nutrients in Southern Edge of Mu Us Sandy Land. Agric. Sci. Technol. 2012, 13, 1708–1712. [Google Scholar]
- Sun, Y.; Zhang, N.; Yan, J.; Zhang, S. Effects of Soft Rock and Biochar Applications on Millet (Setaria italica L.) Crop Performance in Sandy Soil. Agronomy 2020, 10, 669. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.; Li, J.; Lu, Y.; Xu, Y. Improvement of aeolian sandy soil in Mu Us, China with soft montmorillonite clay stone. Agron. J. 2021. [Google Scholar] [CrossRef]
- Han, J.C.; Liu, Y.S.; Luo, L.T. Research on the Core Technology of Remixing Soil by Soft Rock and Sand in the Maowusu Sand Land Region. China Land Sci. 2012, 26, 87–94. [Google Scholar]
- Sun, Z.; Han, J. Effect of soft rock amendment on soil hydraulic parameters and crop performance in Mu Us Sandy Land, China. Field Crops Res. 2018, 222, 85–93. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, X.; Lei, Y.; Tang, L.; Liu, M.; Nie, X.; Hu, X. Research Advances in Soil-borne Potato Diseases. Chin. Potato J. 2014, 28, 111–116. [Google Scholar]
- Chen, Q.; Liu, Z.; Zhou, J.; Xu, X.; Zhu, Y. Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize—Wheat rotation on China’s Loess Plateau. Sci. Total Environ. 2021, 775, 145930. [Google Scholar] [CrossRef]
- Jiang, R.; Yang, J.Y.; Drury, C.F.; He, W.; Smith, W.N.; Grant, B.B.; He, P.; Zhou, W. Assessing the impacts of diversified crop rotation systems on yields and nitrous oxide emissions in Canada using the DNDC model. Sci. Total Environ. 2021, 759, 143433. [Google Scholar] [CrossRef]
- Agomoh, I.V.; Drury, C.F.; Yang, X.; Phillips, L.A.; Reynolds, W.D. Crop rotation enhances soybean yields and soil health indicators. Soil Sci. Soc. Am. J. 2021. [Google Scholar] [CrossRef]
- Hou, N.; Huang, J.; Geng, D.; Wang, N.; Yang, P.; Zhao, S. Compositions and metabolic footprints of soil nematode communities under different alfalfa-crop planting patterns in semi-arid region of the Loess Plateau, Northwest China. Chin. J. Appl. Ecol. 2021, 32, 1825–1834. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Ebrahimi, M.; Abadía, J.; Najafabadi, A.S.; Igartua, E.; Khanahmadi, M. Evaluation of glycyrrhizin contents in licorice (Glycyrrhiza glabra L.) under drought and soil salinity conditions using nutrient concentrations and biochemical traits as biomarkers. Acta Physiol. Plant. 2020, 42, 103. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, Y.; Chi, X.; Li, X.; Yang, G. Analysis on international trade competitiveness of licorice extract. Chin. Tradit. Herb. Drugs 2020, 51, 1970–1976. [Google Scholar]
- Li, A.; Zhang, M.; Chen, Y.; Sun, H.; Wu, Y.; Yan, L. Effects of Glycyrrhiza Uralensis Plantation on Soil Texture and Contents of soil Carbon and Nitrogen in Wind Erosion Region of Northwest China. J. Soil Water Conserv. 2016, 30, 286–290. [Google Scholar]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Hack, M.E.A.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Marappan, G.; et al. Use of Licorice (Glycyrrhiza glabra) Herb as a Feed Additive in Poultry: Current Knowledge and Prospects. Animals 2019, 9, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Nie, C.; Zhang, W.; Liu, Y. Effect of feeding different proportions of licorice stem and leaves to substitute roughage made by the fully mixed pellet feed on growth performance, blood index and immune index of Bashbah sheep. J. Shihezi Univ. Nat. Sci. 2018, 36, 705–711. [Google Scholar]
- Chen, D.; Wang, S.; Xiong, B.; Cao, B.; Deng, X. Carbon/Nitrogen Imbalance Associated with Drought-Induced Leaf Senescence in Sorghum bicolor. PLoS ONE 2015, 10, e0137026. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2010. [Google Scholar]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2013, 4, 17–22. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Song, X.; Razavi, B.S.; Ludwig, B.; Zamanian, K.; Zang, H.; Kuzyakov, Y.; Dippold, M.A.; Gunina, A. Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci. Total Environ. 2020, 735, 139393. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Chen, L.; Zhang, S.; Zheng, Q.; Ullah, S.; Zhou, W.; Wang, X. Combined biochar and nitrogen fertilizer change soil enzyme and microbial activities in a 2-year field trial. Eur. J. Soil Biol. 2020, 99, 103212. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, J.; Liu, P. Effect of Bacterial Manure on the Properties of Complex Soil and Growth of Ryegrass. Agronomy 2021, 11, 568. [Google Scholar] [CrossRef]
- Sun, Z.; Han, J.; Wang, H. Soft rock for improving crop yield in sandy soil in the Mu Us desert, China. Arid Land Res. Manag. 2019, 33, 136–154. [Google Scholar]
- Jorge, P.F. Biochar modifies the thermodynamic parameters of soil enzyme activity in a tropical soil. J. Soils Sediments 2015, 15, 578–583. [Google Scholar]
- Kaurin, A.; Cernilogar, Z.; Lestan, D. Revitalisation of metal-contaminated, EDTA-washed soil by addition of unpolluted soil, compost and biochar: Effects on soil enzyme activity, microbial community composition and abundance. Chemosphere 2018, 193, 726–736. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, W.; Gulaqa, A.; Cui, Y.; Zhou, Y.; Weng, W.; Wang, X.; Liu, Q.; Jin, F. Effects of Peanut Shell Biochar on Soil Nutrients, Soil Enzyme Activity, and Rice Yield in Heavily Saline-Sodic Paddy Field. J. Soil Sci. Plant Nut. 2021, 21, 655–664. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Zhang, H.; Wang, M.; Liang, Z. Assessment of Ammonia Volatilization Losses and Nitrogen Utilization during the Rice Growing Season in Alkaline Salt-Affected Soils. Sustainability 2017, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Gömöryová, E.; Střelcová, K.; Škvarenina, J.; Gömöry, D. Responses of soil microorganisms and water content in forest floor horizons to environmental factors. Eur. J. Soil Biol. 2013, 55, 71–76. [Google Scholar] [CrossRef]
- Ogunkunle, C.O.; Falade, F.O.; Oyedeji, B.J.; Akande, F.O.; Vishwakarma, V.; Alagarsamy, K.; Ramachandran, D.; Fatoba, P.O. Short-Term Aging of Pod-Derived Biochar Reduces Soil Cadmium Mobility and Ameliorates Cadmium Toxicity to Soil Enzymes and Tomato. Environ. Toxicol. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiao, W.; Gao, D.; Dai, Y.; Deng, J.; Yang, G.; Han, X.; Ren, G. Relationship between soil nutrient properties and biological activities along a restoration chronosequence of Pinus tabulaeformis plantation forests in the Ziwuling Mountains, China. Catene 2018, 161, 85–95. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Hedo, J.; Cerdá, A.; Candel-Pérez, D.; Viñegla, B. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. ssp. salzmannii) Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Stephanie, M.C. Plant–soil feedbacks: A meta-analytical review. Ecol. Lett. 2010, 11, 980–992. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, Y.; Meng, F.; Zhu, Y. Effect of organic acids on the activation of phosphorus in vegetable garden soils. Acta Ecol. Sin. 2005, 25, 1171–1177. [Google Scholar]
- Schneider, K.; van Straaten, P.; Heidinger, R.M.D.; Glasauer, S.; Trevors, J.; Fallow, D.; Smith, P.S. Comparing phosphorus mobilization strategies using Aspergillus niger for the mineral dissolution of three phosphate rocks. J. Appl. Microbiol. 2010, 108, 366–374. [Google Scholar] [CrossRef]
- Zhang, D.S.; Wang, Y.Y.; Tang, L.; Zheng, Y.; Zuo, J. Effects of wheat and fababean intercropping on available phosphorus of red soils and its relationship with rhizosphere soil pH. Plant Nutr. Fertil. Sci. 2013, 19, 131–137. [Google Scholar]
- Wuest, S.B. Seasonal Variation in Soil Bulk Density, Organic Nitrogen, Available Phosphorus, and pH. Soil Sci. Soc. Am. J. 2015, 79, 1188–1197. [Google Scholar] [CrossRef]
- Sun, H.; Dan, A.; Feng, Y.; Vithanage, M.; Mandal, S.; Shaheen, S.M.; Rinklebe, J.; Shi, W.; Wang, H. Floating duckweed mitigated ammonia volatilization and increased grain yield and nitrogen use efficiency of rice in biochar amended paddy soils. Chemosphere 2019, 237, 124532. [Google Scholar] [CrossRef] [PubMed]

| Glycyrrhizic Acid | Liquiritin | Soluble Sugar | Starch | SWC10 | SWC20 | SWC30 | SWC40 | Catalase | Urease | Oxalic Acid | Formic Acid | pH | SOM | TN | Nitrate | Ammonium | TP | AP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | 0.87 ** | 0.83 ** | 0.21 | 0.54 | −0.44 | −0.29 | 0.52 | −0.10 | 0.67 * | 0.60 * | 0.80 ** | 0.47 | −0.61 * | 0.39 | 0.59 * | 0.27 | 0.23 | 0.91 ** | 0.74 ** |
| glycyrrhizic acid | 0.96 ** | 0.11 | 0.53 | −0.44 | −0.46 | 0.32 | 0.01 | 0.720 ** | 0.60 * | 0.76 ** | 0.36 | −0.76 ** | 0.38 | 0.65 * | 0.27 | 0.24 | 0.94 ** | 0.77 ** | |
| liquiritin | 0.10 | 0.37 | −0.56 | −0.41 | 0.18 | 0.17 | 0.56 | 0.63 * | 0.75 ** | 0.31 | −0.645 * | 0.45 | 0.58 * | 0.19 | 0.13 | 0.88 ** | 0.61 * | ||
| soluble sugar | 0.45 | −0.27 | 0.76 ** | 0.65 * | −0.33 | −0.13 | −0.42 | 0.64 * | 0.89 ** | −0.11 | 0.66 * | 0.76 ** | 0.45 | 0.29 | 0.23 | 0.17 | |||
| starch | −0.02 | −0.01 | 0.82 ** | −0.50 | 0.730 ** | 0.19 | 0.59* | 0.599 * | −0.74 ** | 0.39 | 0.776 ** | 0.56 | 0.595 * | 0.72 ** | 0.82 ** | ||||
| SWC10 | −0.13 | −0.10 | 0.02 | −0.05 | −0.21 | −0.53 | −0.40 | 0.10 | −0.48 | −0.33 | −0.28 | −0.38 | −0.44 | −0.11 | |||||
| SWC20 | 0.36 | −0.19 | −0.54 | −0.73 ** | 0.17 | 0.625 * | 0.34 | 0.30 | 0.24 | 0.30 | 0.18 | −0.32 | −0.34 | ||||||
| SWC30 | −0.54 | 0.54 | −0.16 | 0.67 * | 0.80 ** | −0.52 | 0.30 | 0.74 ** | 0.56 | 0.56 | 0.55 | 0.64 * | |||||||
| SWC40 | −0.18 | 0.23 | −0.06 | −0.38 | −0.02 | −0.05 | −0.36 | −0.13 | −0.634 * | −0.10 | −0.52 | ||||||||
| Catalase | 0.48 | 0.47 | 0.18 | −0.85 ** | −0.01 | 0.44 | 0.36 | 0.40 | 0.80 ** | 0.83 ** | |||||||||
| Urease | 0.17 | −0.24 | −0.40 | 0.19 | 0.00 | −0.05 | −0.03 | 0.57 | 0.37 | ||||||||||
| oxalic acid | 0.804 ** | −0.58 * | 0.67 * | 0.86 ** | 0.42 | 0.25 | 0.83 ** | 0.61 * | |||||||||||
| formic acid | −0.35 | 0.59 * | 0.84 ** | 0.608 * | 0.51 | 0.50 | 0.44 | ||||||||||||
| pH | −0.12 | −0.62 * | −0.616 * | −0.45 | −0.80 ** | −0.67 * | |||||||||||||
| SOM | 0.66 * | 0.10 | 0.02 | 0.47 | 0.26 | ||||||||||||||
| TN | 0.48 | 0.46 | 0.72 ** | 0.68 * | |||||||||||||||
| nitrate | 0.725 ** | 0.41 | 0.25 | ||||||||||||||||
| ammonium | 0.37 | 0.46 | |||||||||||||||||
| TP | 0.84 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Liu, P. Does Complex Soil Enhance Grain Yield under Cropping System? Agronomy 2021, 11, 1502. https://doi.org/10.3390/agronomy11081502
Yan J, Liu P. Does Complex Soil Enhance Grain Yield under Cropping System? Agronomy. 2021; 11(8):1502. https://doi.org/10.3390/agronomy11081502
Chicago/Turabian StyleYan, Jiakun, and Puling Liu. 2021. "Does Complex Soil Enhance Grain Yield under Cropping System?" Agronomy 11, no. 8: 1502. https://doi.org/10.3390/agronomy11081502







