Spermidine Pretreatments Mitigate the Effects of Saline Stress by Improving Growth and Saline Excretion in Frankenia pulverulenta
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Design and Treatments
- -
- Indole-Acetic acid (1 μM)
- -
- Kinetin (1 μM)
- -
- Gibberellic acid (1 μM)
- -
- Spermidine (0.5 mM)
- -
- Salicylic acid (0.5 mM)
2.3. Growth Parameters
2.4. Determination of Proline
2.5. Ion Content Measurement of Salt Crystals in Surface Leaves of F. pulverulenta
2.6. Analysis of Free Polyamines
2.7. Ethylene Production
2.8. Data Analysis
3. Results
3.1. Effect of Pretreatment PGRs under Saline and Non-Saline Conditions
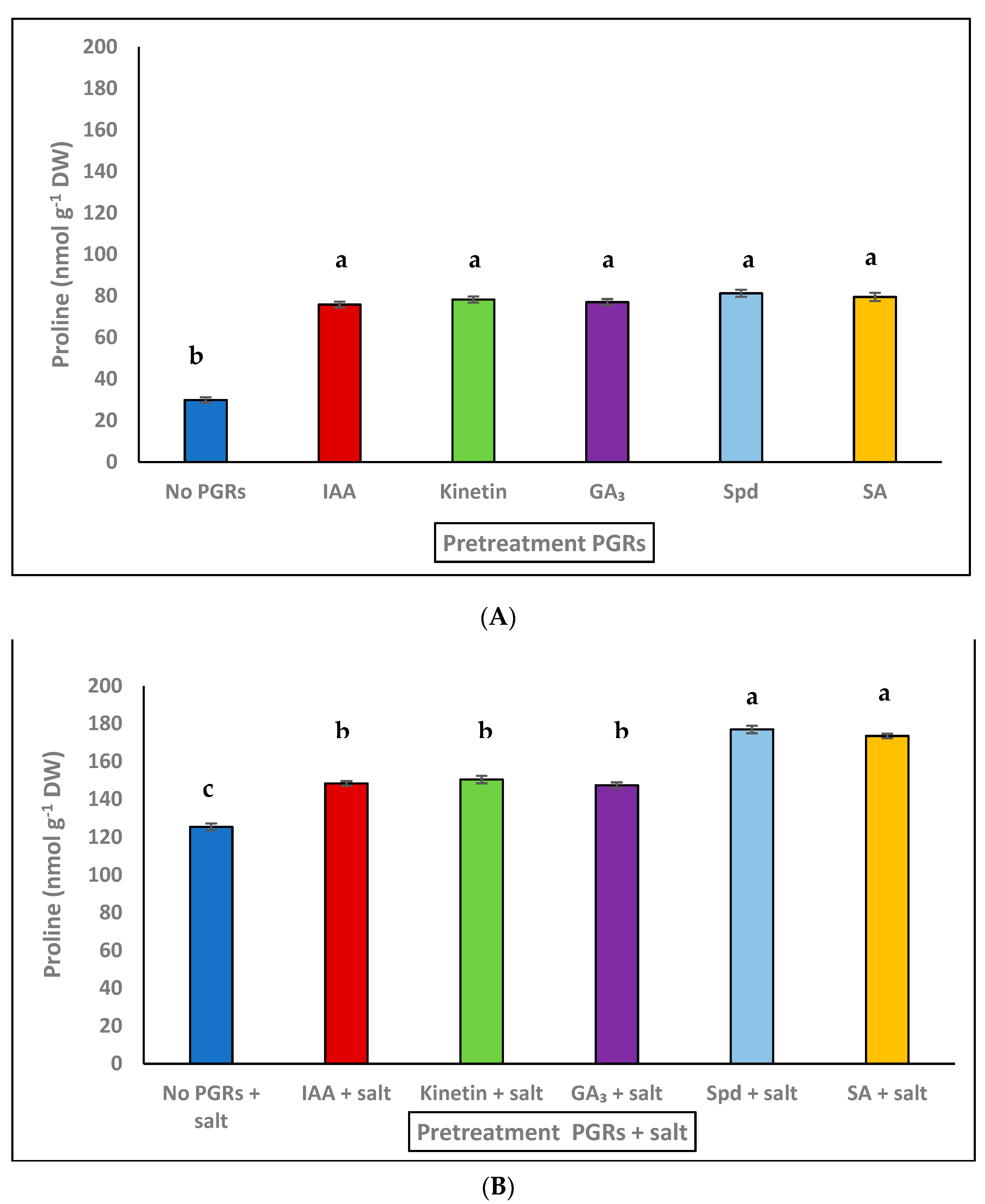
3.2. Effect of Pretreatment PGRs under Saline and Non-Saline Conditions on Proline Accumulation
3.3. Effects of Pretreatment PGRs + Salt on Ionic Content in the Leaf-Washing Solution in F. pulverulenta
3.4. Effect of Pretreatment PGRs on Free Polyamine Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thornton, P.K.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- Leisner, C.P. Review: Climate change impacts on food security-focus on perennial cropping systems and nutritional value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Bueno, M. Adaptation of halophytes to different habitats. In Seed dormancy and Germination, 1st ed.; Jiménez-López, J.C., Ed.; Intech Open: London, UK, 2020; Volume 1, pp. 97–119. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Fernanda Ortuño, M.; Bernal-Vicente, A.; Díaz-Vivancos, P.; Sánchez-Blanco, M.J.; Hernández, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Bueno, M.; Cordovilla, M.P. Ecophysiology and uses of halophytes in diverse habitats. In Handbook of Halophytes: From Molecular to Ecosystems towards Biosaline Agriculture, 1st ed.; Grigore, M.N., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Torre-González, A.; Navarro-León, E.; Albacete, A.; Blasco, B.; Ruíz, J.M. Study of phytohormone profile and oxidative metabolism as key process to identification of salinity response in tomato commercial genotypes. J. Plant Physiol. 2017, 216, 164–173. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, H.; Jiao, Q.; Ma, C.; Wang, P. Phytohormones: Important participators in plant salt tolerance. Int. J. Agric. Biol. 2020, 24, 319–332. [Google Scholar] [CrossRef]
- Szepesi, A. Halotropism: Phytohormonal aspects and potential applications. Front. Plant Sci. 2020, 11, 571025. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Llanes, A.; Reginato, M.; Devinar, G.; Luna, V. What is known about phytohormones in halophytes? A review. Biologia 2018, 73, 727–742. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Consejería de Medio Ambiente. Flora Vascular de Andalucía Oriental, 1st ed.; Junta de Andalucía: Sevilla, Spain, 2009. [Google Scholar]
- Pérez Cuadra, V.; Cambi, V. Morphoanatomical functional traits in xerophytic species of a saline environment. Int. J. Exp. Bot. 2014, 83, 389–396. [Google Scholar]
- Bueno, M.; Lendínez, M.L.; Calero, J.; Cordovilla, M.P. Salinity responses of three halophytes from inland saltmarshes of Jaén (southern Spain). Flora 2020, 266, 151589. [Google Scholar] [CrossRef]
- Easton, I.C.; Kleindorfer, S. Effects of salinity levels and seed mass on germination in Australian species of Frankenia L. (Frankeniaceae). Environ. Exp. Bot. 2009, 65, 345–352. [Google Scholar] [CrossRef]
- Veldkornet, D.A.; Potts, A.J.; Adams, J.B. The distribution of salt marsh macrophyte species in relation to physicochemical variables. S. Afr. J. Bot. 2016, 107, 84–90. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Velasquez Espino, C.; Lao, M.T. Effects of leachate fertigation and the addition of hydrogen peroxide on growth and nutrient balance in Dracaena deremensis potted plants. Agronomy 2021, 11, 127. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Harzallah-Skhiri, F.; Bourgougnon, N.; Aouni, M. Antiviral activity of some Tunisian medicinal plants against Herpes simplex virus type 1. Nat. Prod. Res. 2008, 22, 53–65. [Google Scholar] [CrossRef]
- Lopes, A.; Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Barreira, L.; Varela, J.; Trampetti, F.; Custódio, L. Natural products from extreme marine environments: Searching for potential industrial uses within extremophile plants. Ind. Crop Prod. 2016, 94, 299–307. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Wided, M.K.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. LC-MS identification and preparative HPLC isolation of Frankenia pulverulenta phenolics with antioxidant and neuroprotective capacities in PC12 cell line. Pharm. Biol. 2017, 55, 880–887. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Bull. 1950, 347, 1–32. [Google Scholar]
- Tounekti, T.; Hernández, I.; Müller, M.; Khemira, H.; Munné-Bosch, S. Kinetin applications alleviate salt stress and improve the antioxidant composition of leaf extracts in Salvia officinalis. Plant Physiol. Biochem. 2011, 49, 1165–1176. [Google Scholar] [CrossRef]
- Li, S.; Jin, H.; Zhang, Q. The effect of exogenous spermidine concentration polyamine metabolism and salt tolerance in Zoysiagrass (Zoysia japonica Steud) subjected to short-term salinity stress. Front. Plant Sci. 2016, 7, 1221. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zouhaier, B.; Najla, T.; Abdallah, A.; Wahbi, D.; Wided, C.; Chedly, A.; Abderrazak, S. Salt stress response in the halophyte Limoniastrum guyonianum Boiss. Flora 2015, 217, 1–9. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Aparicio, C.; Cordovilla, M.P. Effect of salinity on polyamines and ethylene in Atriplex prostrata and Plantago coronopus. Biol. Plant 2015, 59, 596–600. [Google Scholar] [CrossRef]
- Amir, R.; Munir, F.; Khan, M.; Iqbal, T. Use of plant hormones for the improvement of plant growth and production under salt stress. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution, 2nd ed.; Akhtar, M.S., Ed.; Springer Nature: Singapore, 2019; Volume 1, pp. 59–90. [Google Scholar]
- Morsi, M.M.; Abdelmigid, H.M.; Aljoudi, N.G.S. Exogenous salicylic acid ameliorates the adverse effects of salt stress on antioxidant system in Rosmarinus officinalis L. Egypt. J. Bot. 2018, 58, 249–263. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; García-Sánchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Rivero, R.M.; Gimeno, J.; Van Deynze, A.; Walia, H.; Blumwald, E. Enhanced cytokinin synthesis in tobacco plants expressing PSARK::IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol. 2010, 51, 1929–1941. [Google Scholar] [CrossRef]
- Pearce, S.; Huttly, A.K.; Prosser, I.M.; Li, Y.D.; Vaughan, S.P.; Gallova, B.; Phillips, A.L. Heterologous expression, and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. Plant Biol. 2015, 15, 130. [Google Scholar] [CrossRef]
- Ashfaq, M.; Khan, S. Role of phytohormones in improving the yield of oilseed crops. In Oils Seed Crops: Yield and Adaptation under Environmental Stress, 1st ed.; Ahmad, P., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; Volume 9, pp. 165–183. [Google Scholar] [CrossRef]
- Ghabriche, R.; Ghnaya, T.; Mnasri, M.; Zaier, H.; Baioui, R.; Vromman, D.; Abdelly, C.; Lutts, S. Polyamine and tyramine involvement in NaCl-induced improvement of Cd resistance in the halophyte Inula chrithmoides L. J. Plant Physiol. 2017, 216, 136–144. [Google Scholar] [CrossRef]
- Thomas, J.C.; Bohnert, H.J. Salt stress perception and plant growth regulators in the halophyte Mesembryanthemum crystallinum. Plant Physiol. 1993, 103, 1299–1304. [Google Scholar] [CrossRef][Green Version]
- Llanes, A.; Bertazza, G.; Palacio, G.; Luna, V. Different sodium salts cause different solute accumulation in the halophyte Prosopis strombulifera. Plant Biol. 2013, 15, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Llanes, A.; Pinamonti, P.; Iparraguirre, J.; Bertazza, G.; Luna, V. Abscisic acid alters carbohydrate accumulation induced by differential response to sodium salts in the halophyte Prosopis strombulifera. Plant Biosyst. 2019, 154, 337–347. [Google Scholar] [CrossRef]
- Tipirdamaz, R.; Gagneul, D.; Duhazé, C.; Aïnouche, A.; Monnier, C.; Özkum, D.; Larher, F. Clustering of halophytes from an inland salt marsh in Turkey according to their ability to accumulate sodium and nitrogenous osmolytes. Environ. Exp. Bot. 2006, 57, 139–153. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Iqbal, M.; Khan, R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Pathak, M.R.; Teixeira, J.A.; Wani, S.H. Polyamines in response to abiotic stress tolerance through transgenic approaches. GM Crop. Food 2014, 5, 87–96. [Google Scholar] [CrossRef]
- Shaygan, M.; Mulligan, D.; Baumgartl, T. The potential of three halophytes (Tecticornia pergranulata, Sclerolaena longicuspis, and Frankenia serpyllifolia) for the rehabilitation of brine-affected soils. Land Degrad. Dev. 2018, 29, 2002–2014. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Vara Prasad, M.N.; Ozturk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. BioMed Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.T. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact of climate change. Environ. Sci. Poll. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef]
- Atzori, G. The potential of edible halophytes as new crops in saline agriculture. The ice plant (Mesembryanthemum crystallinum L.) case study. In Future of Sustainable Agriculture in Saline Environments, 1st ed.; CRC Press: Boca Raton, NY, USA, 2021; pp. 443–460. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Srivastava, A.K.; Pandey, G.K.; Suprasanna, P. Halophytes in biosaline agriculture: Mechanism, utilization, and value addition. Land Degrad. Dev. 2018, 29, 1081–1095. [Google Scholar] [CrossRef]
- Duarte, B.; Caçador, I. Iberian halophytes as agroecological solutions for degraded lands and biosaline agriculture. Sustainability 2021, 13, 1005. [Google Scholar] [CrossRef]
- Christiansen, A.H.C.; Lyra, D.A.; Jørgensen, H. Increasing the value of Salicornia bigelovii green biomass grown in a desert environment through biorefining. Ind. Crop Prod. 2021, 160, 113105. [Google Scholar] [CrossRef]
- Ben Hassine, A.; Ghanem, M.E.; Bouzid, S.; Lutts, S. Abscisic acid has contrasting effects on salt excretion and polyamine concentrations of an inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus. Ann. Bot. 2009, 104, 925–936. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional levels. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Cordovilla, M.P. Polyamines in halophytes. Front. Plant Sci. 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Tomar, P.C.; Lakra, N.; Mishra, S.N. Effect of cadaverine on Brassica juncea (L.) under multiple stress. Indian J. Exp. Biol. 2013, 51, 758–763. [Google Scholar]
- Jancewicz, A.L.; Gibbs, N.M.; Masson, P.H. Cadaverine’s functional role in plant development and environmental response. Front. Plant Sci. 2016, 7, 870. [Google Scholar] [CrossRef]
- Reginato, M.; Abdala, G.I.; Miersch, O.; Ruíz, O.A.; Moschetti, E.; Luna, V. Changes in the levels of jasmonates and free polyamines induced by Na2SO4 and NaCl in roots and leaves of the halophyte Prosopis strombulifera. Biologia 2012, 67, 689–697. [Google Scholar] [CrossRef]


| SLDW (g/Plant) | RDW (g/Plant) | SLWC (%) | RWC (%) |
|---|---|---|---|---|
| Control (-PGRs) | 0.1137 ± 0.0035 b | 0.0075 ± 0.0003 a | 90.11 ± 0.36 b | 65.74 ± 1.29 b |
| IAA | 0.1241 ± 0.0027 b | 0.0080 ± 0.0001 a | 92.22 ± 0.59 ab | 71.90 ± 0.95 a |
| K | 0.1443 ± 0.0031 a | 0.0083 ± 0.0003 a | 92.77 ± 0.37 a | 75.15 ± 0.94 a |
| GA3 | 0.1412 ± 0.0038 a | 0.0080 ± 0.0004 a | 92.13 ± 0.90 ab | 71.29 ± 0.68 a |
| Spd | 0.1483 ± 0.0010 a | 0.0086 ± 0.0003 a | 92.60 ± 0.33 ab | 74.03 ± 0.88 a |
| SA | 0.1440 ± 0.0037 a | 0.0083 ± 0.0003 a | 92.35 ± 0.39 ab | 73.39 ± 0.72 a |
| SLDW (g/plant) | RDW (g/plant) | SLWC (%) | RWC (%) |
| Control (-PGRs) + NaCl | 0.0912 ± 0.0014 b | 0.0057 ± 0.0005 b | 87.81 ± 0.61 b | 58.11 ± 0.53 c |
| IAA + NaCl | 0.0943 ± 0.0021 b | 0.0071 ± 0.0002 ab | 89.21 ± 0.54 ab | 61.10 ± 0.68 bc |
| K + NaCl | 0.1033 ± 0.0036 b | 0.0072 ± 0.0004 ab | 90.19 ± 0.67 ab | 65.04 ± 0.95 ab |
| GA3 + NaCl | 0.0939 ± 0.0041 b | 0.0059 ± 0.0004 b | 89.71 ± 0.19 ab | 60.40 ± 0.75 bc |
| Spd + NaCl | 0.1422 ± 0.0032 a | 0.0082 ± 0.0004 a | 91.48 ± 0.31 a | 68.14 ± 1.23 a |
| SA + NaCl | 0.1344 ± 0.0067 a | 0.0079 ± 0.0005 a | 91.31 ± 0.75 a | 67.49 ± 1.40 a |
| Pretreatment PGRs + Salt | Na+ (mg g−1 FW) | Cl− (mg g−1 FW) |
|---|---|---|
| Control (-PGRs) + NaCl | 5.49 ± 0.15 b | 1.16 ± 0.05 c |
| IAA + NaCl | 5.53 ± 0.20 b | 1.46 ± 0.06 b |
| K + NaCl | 5.69 ± 0.13 b | 1.38 ± 0.08 bc |
| GA3 + NaCl | 5.71 ± 0.10 b | 1.34 ± 0.07 bc |
| Spd + NaCl | 6.45 ± 0.21 a | 1.93 ± 0.04 a |
| SA + NaCl | 5.72 ± 0.09 b | 1.84 ± 0.04 a |
| Put (nmol g−1 DW) | Spd (nmol g−1 DW) | Spm (nmol g−1 DW) | Cad (nmol g−1 DW) |
|---|---|---|---|---|
| Control (-PGRs) | 1.89 ± 0.09 a | 28.47 ± 0.29 a | 7.61 ± 0.22 bc | 0.99 ± 0.07 a |
| IAA | 1.76 ± 0.07 a | 23.82 ± 0.43 bc | 10.51 ± 0.25 ab | 0.73 ± 0.01 b |
| K | 1.45 ± 0.03 bc | 22.91 ± 0.53 c | 11.23 ± 0.89 a | 0.87 ± 0.05 ab |
| GA3 | 1.38 ± 0.05 c | 19.29 ± 0.30 d | 10.45 ± 0.58 ab | 0.75 ± 0.03 b |
| Spd | 1.73 ± 0.08 ab | 25.51 ± 0.25 b | 12.87 ± 0.53 a | 0.91 ± 0.03 ab |
| SA | 1.42 ± 0.05 bc | 24.10 ± 0.36 bc | 12.04 ± 0.78 a | 0.86 ± 0.04 ab |
| Put (nmol g−1 DW) | Spd (nmol g−1 DW) | Spm (nmol g−1 DW) | Cad (nmol g−1 DW) |
| Control (-PGRs + NaCl) | 1.13 ± 0.05 b | 18.08 ± 0.76 d | 19.37 ± 0.51 c | 8.36 ± 0.26 b |
| IAA + NaCl | 1.01 ± 0.04 bc | 21.91 ± 0.37 bc | 20.51 ± 0.71 c | 7.42 ± 0.20 b |
| K + NaCl | 1.48 ± 0.04 a | 21.73 ± 0.44 bc | 21.29 ± 0.69 bc | 10.09 ± 0.21 a |
| GA3 + NaCl | 0.79 ± 0.10 c | 21.08 ± 0.52 c | 21.44 ± 0.66 bc | 8.19 ± 0.27 b |
| Spd + NaCl | 1.02 ± 0.07 bc | 25.91 ± 0.39 a | 25.85 ± 0.99 a | 10.97 ± 0.45 a |
| SA + NaCl | 1.14 ± 0.06 b | 23.97 ± 0.36 ab | 24.75 ± 0.74 ab | 10.64 ± 0.25 a |
| Total PAs (nmol g−1 DW) | Ethylene (nmol g−1 FW h−1) |
|---|---|---|
| Control (-PGRs) | 38.95 ± 0.352 ab | 0.636 ± 0.021 a |
| IAA | 36.83 ± 0.596 b | 0.560 ± 0.026 ab |
| K | 36.64 ± 0.626 b | 0.452 ± 0.031 b |
| GA3 | 31.88 ± 0.301 c | 0.491 ± 0.027 b |
| Spd | 41.01 ± 0.759 a | 0.569 ± 0.025 ab |
| SA | 38.41 ± 0.132 ab | 0.456 ± 0.020 b |
| Total PAs (nmol g−1 DW) | Ethylene (nmol g−1 FW h−1) |
| Control (-PGRs) + NaCl | 46.95 ± 1.077 d | 0.364 ± 0.019 a |
| IAA + NaCl | 51.00 ± 1.154 cd | 0.323 ± 0.025 ab |
| K + NaCl | 54.60 ± 1.193 bc | 0.215 ± 0.019 c |
| GA3 + NaCl | 51.50 ± 0.835 cd | 0.211 ± 0.028 c |
| Spd + NaCl | 63.769 ± 1.908 a | 0.255 ± 0.014 bc |
| SA + NaCl | 60.50 ± 1.121 ab | 0.235 ± 0.016 bc |
| SLDW | RDW | SLWC | RWC | PRO | PUT | SPD | SPM | CAD | Total PAs | C2H2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SLDW | 1 | ||||||||||
| RDW | 0.812 ** | 1 | |||||||||
| SLWC | 0.835 ** | 0.768 ** | 1 | ||||||||
| RWC | 0.872 ** | 0.805 ** | 0.864 ** | 1 | |||||||
| PRO | −0.283 | −0.290 | −0.319 | −0.476 ** | 1 | ||||||
| PUT | 0.3067 | 0.397 * | 0.393 * | 0.516 ** | −0.777 ** | 1 | |||||
| SPD | 0.4055 * | 0.480 ** | 0.373 * | 0.395 * | −0.281 | 0.473 ** | 1 | ||||
| SPM | −0.316 | −0.329 * | −0.373 * | −0.528 ** | 0.972 ** | −0.745 ** | −0.189 | 1 | |||
| CAD | −0.450 ** | −0.427 ** | −0.505 ** | −0.648 ** | 0.937 ** | −0.686 ** | −0.225 | 0.952 ** | 1 | ||
| Total PAs | −0.269 | −0.245 * | −0.334 * | −0.481 ** | 0.904 ** | −0.594 ** | 0.082 | 0.951 ** | 0.937 ** | 1 | |
| C2H2 | 0.365 * | 0.378 * | 0.312 | 0.496 ** | −0.918 ** | 0.697 ** | 0.315 | −0.905 ** | −0.890 ** | −0.832 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, M.; Cordovilla, M.d.P. Spermidine Pretreatments Mitigate the Effects of Saline Stress by Improving Growth and Saline Excretion in Frankenia pulverulenta. Agronomy 2021, 11, 1515. https://doi.org/10.3390/agronomy11081515
Bueno M, Cordovilla MdP. Spermidine Pretreatments Mitigate the Effects of Saline Stress by Improving Growth and Saline Excretion in Frankenia pulverulenta. Agronomy. 2021; 11(8):1515. https://doi.org/10.3390/agronomy11081515
Chicago/Turabian StyleBueno, Milagros, and María del Pilar Cordovilla. 2021. "Spermidine Pretreatments Mitigate the Effects of Saline Stress by Improving Growth and Saline Excretion in Frankenia pulverulenta" Agronomy 11, no. 8: 1515. https://doi.org/10.3390/agronomy11081515
APA StyleBueno, M., & Cordovilla, M. d. P. (2021). Spermidine Pretreatments Mitigate the Effects of Saline Stress by Improving Growth and Saline Excretion in Frankenia pulverulenta. Agronomy, 11(8), 1515. https://doi.org/10.3390/agronomy11081515







