New Insight into the Identity of Italian Grapevine Varieties: The Case Study of Calabrian Germplasm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction and Microsatellite Analysis
2.3. Genetic Diversity Assessment
2.4. Morphological Evaluation and Chemical Characterization of Berries
2.5. Chemical and Sensorial Analysis of Wines
2.6. Statistical Analysis
3. Results
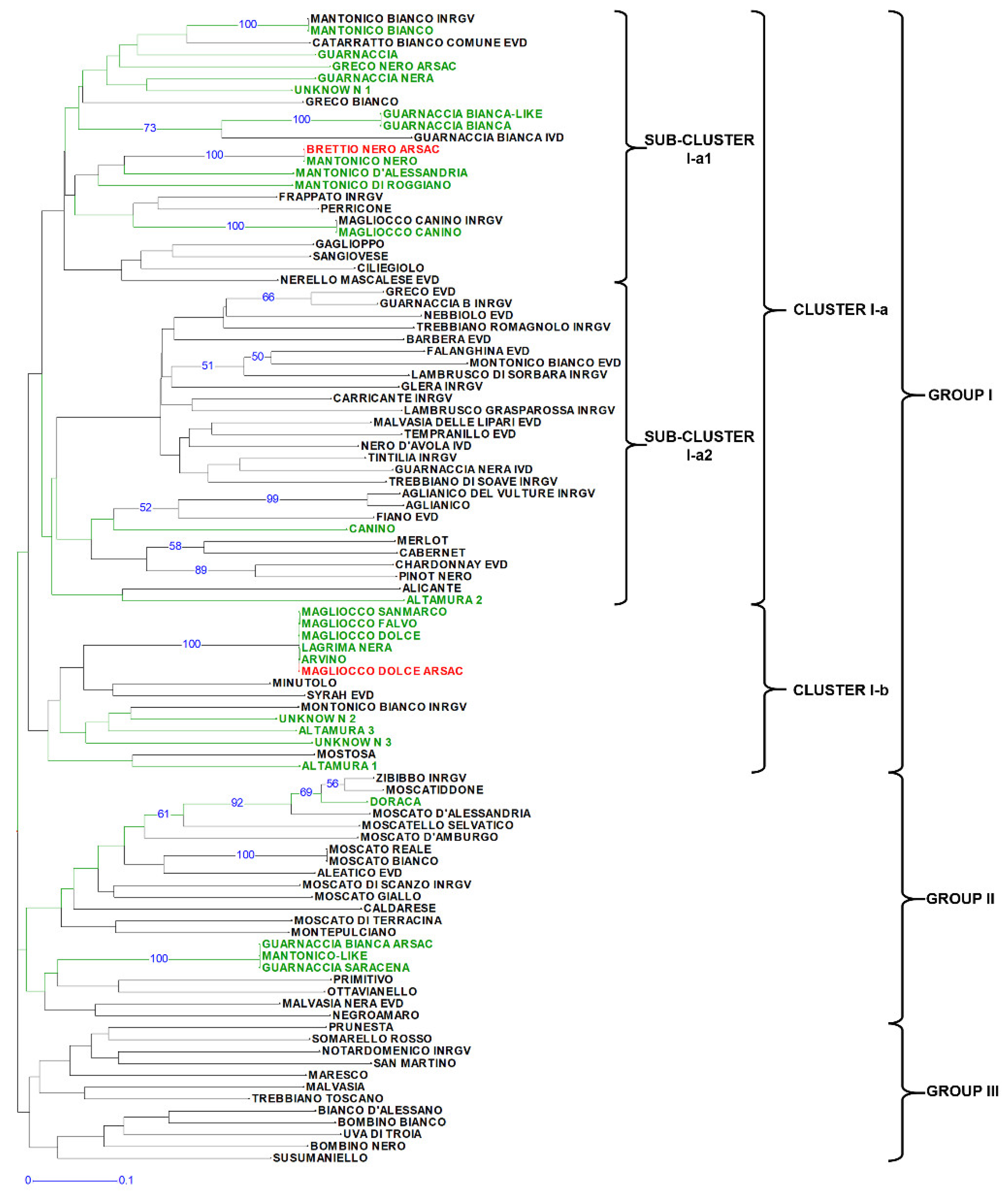
3.1. Genetic Diversity Analysis
3.2. Ampelographic Characterization and Chemical Analysis of Berries
3.3. Oenological Parameters and Wine Sensory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bonis, M. Terra D’Uve Vini e Vitigni in Calabria Dall’antichità All’Ottocento: Notizie, Curiosità, Immagini; Le Nuvole: Cosenza, Italy, 2003. [Google Scholar]
- Sunseri, F.; Lupini, A.; Mauceri, A.; de Lorenzis, G.; Araniti, F.; Brancadoro, L.F.; Dattola, A.; Gullo, G.; Zappia, R.; Mercatiet, F. Single nucleotide polymorphism profiles reveal an admixture genetic structure of grapevine germplasm from Calabria, Italy, uncovering its key role for the diversification of cultivars in the Mediterranean Basin. Aust. J. Grape Wine Res. 2018, 24, 345–359. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Mercati, F.; Bergamini, C.; Cardone, M.F.; Lupini, A.; Mauceri, A.; Caputo, A.R.; Abbate, L.; Barbagallo, M.G.; Antonacci, D.; et al. SNP genotyping elucidates the genetic diversity of Magna Graecia grapevine germplasm and its historical origin and dissemination. BMC Plant Biol. 2019, 20, 7. [Google Scholar] [CrossRef]
- Mateus, N.; Proença, S.; Ribeiro, P.; Machado, J.M.; De Freitas, V. Grape and wine polyphenolic composition of red Vitis vinifera varieties concerning vineyard altitude. Cienc. Y Tecnol. Aliment. 2001, 3, 102–110. [Google Scholar] [CrossRef]
- ISTAT. Available online: http://www.istat.it (accessed on 30 March 2021).
- Ministerial, D. October, 18: Disciplinare di Produzione dei vini a Denominazione di Origine Controllata “Terre di Cosenza”, Approvato con D.M. 18.10.2011, Gazzetta Ufficiale n. 256–03.11.2011. Modificato con DM 23.11.2015, Pubblicato sul sito Ufficiale del Mipaaf Sezione Prodotti DOP e IGP—Vini DOP e IGP. Available online: http://www.politicheagricole.it (accessed on 12 March 2021).
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, including Their Origins and Flavours; HarperCollins: New York, NY, USA, 2012. [Google Scholar]
- Vezzulli, S.; Leonardelli, L.; Malossini, U.; Stefanini, M.; Velasco, R.; Moser, C. Pinot blanc and Pinot gris arose as independent somatic mutations of Pinot noir. J. Exp. Bot. 2012, 63, 6359–6369. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.; Matus, J.T.; Pinto-Carnide, O.; Carrasco, D.; Arroyo-García, R.; Castro, I. Genetic analysis of a white-to-red berry skin color reversion and its transcriptomic and metabolic consequences in grapevine (Vitis vinifera cv. ‘Moscatel Galego’). BMC Genom. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespan, M.; Storchi, P.; Migliaro, D. Grapevine cultivar Mantonico bianco is the second parent of the Sicilian Catarratto. Am. J. Enol. Vitic. 2017, 68, 258–262. [Google Scholar] [CrossRef]
- Schneider, A.; Raimondi, S.; Pirolo, C.S.; Torello Marinoni, D.T.; Ruffa, P.; Venerito, P.; La Notte, P. Genetic characterization of grape cultivars from Apulia (Southern Italy) and synonymies in other Mediterranean regions. Am. J. Enol. Vitic. 2014, 65, 244–249. [Google Scholar] [CrossRef]
- Raimondi, S.; Ruffa, P.; De Lorenzis, G.; Imazio, S.; Fiori, S.; Failla, O.; Schneider, A. Detection of grapevine synonyms in Lombardy and Piedmont regions (northern Italy). Vitis 2015, 54, 31–36. [Google Scholar]
- Miazzi, M.M.; D’Agostino, N.; Gadaleta, S.; Di Rienzo, V.; Fanelli, V.; Sabetta, W.; Montemurro, C.; Taranto, F. Genotyping-by-sequencing-derived single-nucleotide polymorphism catalog from a grapevine (Vitis vinifera L.) germplasm collection that includes the most representative Apulian autochthonous cultivars. Acta Hortic. 2019, 1248, 69–76. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrano, A.; Micheletti, D.; Lorenzi, S.; Neale, D.; Grando, M.S. Genomic signatures of different adaptations to environmental stimuli between wild and cultivated Vitis vinifera L. Hortic. Res. 2018, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.L.; Zhao, H.L.; Li, Q.; Zhang, G.H.; Jiang, J.F.; Liu, C.H.; Yu, Y.H. Genome-wide association study of berry-related traits in grape [Vitis vinifera L.] based on genotyping-by-sequencing markers. Hortic. Res. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfino, P.; Zenoni, S.; Imanifard, Z.; Tornielli, G.B.; Bellin, D. Selection of candidate genes controlling veraison time in grapevine through integration of meta-QTL and transcriptomic data. BMC Genom. 2019, 20, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamini, C.; Cardone, M.F.; Anaclerio, A.; Perniola, R.; Pichierri, A.; Genghi, R.; Alba, V.; Forleo, L.R.; Caputo, A.R.; Montemurro, C.; et al. Validation assay of p3_VvAGL11 marker in a wide range of genetic background for early selection of stenospermocarpy in Vitis vinifera L. Mol. Biotechnol. 2013, 54, 1021–1030. [Google Scholar] [CrossRef]
- Zini, E.; Dolzani, C.; Stefanini, M.; Gratl, V.; Bettinelli, P.; Nicolini, D.; Betta, G.; Dorigatti, C.; Velasco, R.; Letschka, T.; et al. R-loci arrangement versus downy and powdery mildew resistance level: A Vitis hybrid survey. Int. J. Mol. Sci. 2019, 20, 3526. [Google Scholar] [CrossRef] [Green Version]
- Massonnet, M.; Fasoli, M.; Tornielli, G.B.; Altieri, M.; Sandri, M.; Zuccolotto, P.; Paci, P.; Gardiman, M.; Zenoni, S.; Pezzotti, M. Ripening transcriptomic program in red and white grapevine varieties correlates with berry skin anthocyanin accumulation. Plant Physiol. 2017, 174, 2376–2396. [Google Scholar] [CrossRef] [Green Version]
- Volpicella, M.; Leoni, C.; Costanza, A.; Fanizza, I.; Placido, A.; Ceci, L.R. Genome walking by Next Generation Sequencing approaches. Biology 2012, 1, 495–507. [Google Scholar] [CrossRef]
- Galet, P.; Morton, L.T.; Adams, L.D. A Practical Ampelography: Grapevine Identification; Comstock: New York, NY, USA, 1979. [Google Scholar]
- OIV. Second Edition of the OIV Descriptor List for Grape Varieties and Vitis Species. Paris, France—International Plant Genetic Resources Institute, Rome. 2009. Available online: http://www.oiv.org (accessed on 20 February 2021).
- This, P.; Lacombe, T.; Thomas, M. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- Tautz, D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989, 17, 6463–6471. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular Approaches to Agri-Food Traceability and Authentication: An Updated Review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef]
- Pellerone, F.I.; Edwards, K.J.; Thomas, M.R. Grapevine microsatellite repeats: Isolation, characterisation and use for genotyping of grape germplasm from Southern Italy. Vitis 2001, 40, 179–186. [Google Scholar]
- Gristina, A.S.; De Michele, R.; Garfì, G.; La Mantia, T.; Fontana, I.; Spinelli, P.; Motisi, A.; Carimi, F. Urgent need for preservation of grapevine (Vitis vinifera L. subsp. vinifera) germplasm from small circum-Sicilian islands as revealed by SSR markers and traditional use investigations. Genet. Resour. Crop Evol. 2016, 64, 1395–1415. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, V.; Savoia, M.A.; Gadaleta, S.; Piarulli, L.; Montemurro, C.; La Notte, P.; Miazzi, M.M.; Bruno, M.; Falbo, M.; Petrillo, F.; et al. Molecular characterization of wine grape cultivars from Calabria. Acta Hortic. 2019, 1248, 281–286. [Google Scholar] [CrossRef]
- Italian National Registry of Grape Varieties. Available online: http://catalogoviti.politicheagricole.it/catalogo.php (accessed on 26 February 2021).
- Torchio, F.; Cagnasso, E.; Gerbi, V.; Rolle, L. Mechanical properties, phenolic composition and extractability indices of Barbera grapes of different soluble solids contents from several growing areas. Anal. Chim. Acta 2010, 660, 183–189. [Google Scholar] [CrossRef]
- Fanelli, V.; Volpicella, M.; Giampetruzzi, A.; Saldarelli, P.; Leoni, C.; Ceci, L.R.; Di Rienzo, V.; Venerito, P.; Taranto, F.; Giannini, P.; et al. Valorization of autochthonous Apulian grapevine cultivars for spumante production. Acta Hortic. 2019, 1248, 457–462. [Google Scholar] [CrossRef]
- Pastore, C.; Fontana, M.; Raimondi, S.; Ruffa, P.; Filippetti, I.; Schneider, A. Genetic characterization of grapevine varieties from Emilia-Romagna (Northern Italy) discloses unexplored genetic resources. Am. J. Enol. Vitic. 2020, 71, 334–343. [Google Scholar] [CrossRef]
- Italian Vitis Database. Available online: https://vitisdb.it/ (accessed on 26 February 2021).
- European Vitis Database. Available online: http://www.eu-vitis.de/index.php (accessed on 26 February 2021).
- Spadoni, A.; Sion, S.; Gadaleta, S.; Savoia, M.; Piarulli, L.; Fanelli, V.; Di Rienzo, V.; Taranto, F.; Miazzi, M.M.; Montemurro, C.; et al. A simple and rapid method for genomic DNA extraction and microsatellite analysis in tree plants. J. Agric. Sci. Technol. 2019, 21, 1215–1226. [Google Scholar]
- Thomas, M.R.; Cain, P.; Scott, N.S. DNA typing of grapevines: A universal methodology and database for describing cultivars and evaluating genetic relatedness. Plant Mol. Biol. 1994, 25, 939–949. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.E.; Dangl, G.S.; Meredith, C.P. Development and characterization of additional microsatellite DNA markers for grape. Am. J. Enol. Vitic. 1999, 50, 243–246. [Google Scholar]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glössl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program Cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- DARwin6. Available online: https://darwin.cirad.fr/ (accessed on 12 March 2021).
- Di Stefano, R.; Cravero, M.C. Metodi per lo studio dei polifenoli dell’uva. Riv. Di Vitic. E Di Enol. 1991, 44, 37–45. [Google Scholar]
- Official Journal of the European Union. Council Regulation (EC) No 1234/2007 of 22 October 2007 Establishing a Common Organisation of Agricultural Markets and on Specific Provisions for Certain Agricultural Products (Single CMO Regulation). Available online: https://eur-lex.europa.eu/ (accessed on 26 February 2021).
- Di Stefano, R.; Cravero, M.C.; Gentilini, N. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico 1989, 25, 83–89. [Google Scholar]
- Vitis International Variety Catalogue. Available online: https://www.vivc.de/ (accessed on 23 July 2021).
- Alleweldt, G.; Dettweıler, E. Ampelographic studies to characterize grapevine varieties. Vignevini 1986, 13, 56–59. [Google Scholar]
- Santıago, J.L.; Boso, S.; del Carmen Martínez, M.; Pınto-Carnıde, O.; Ortız, J.M. Ampelographic comparison of grape cultivars (Vitis vinifera L.) grown in Northwestern Spain and Northern Portugal. Am. J. Enol. Vitic. 2005, 56, 287–290. [Google Scholar]
- Coombe, B.G.; McCarthy, M.G. Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 2000, 6, 131–135. [Google Scholar] [CrossRef]
- González-Neves, G.; Charamelo, D.; Balado, J.; Barreiro, L.; Bochicchio, R.; Gatto, G.; Gil, G.; Tessore, A.; Carbonneau, A.; Moutounet, M. Phenolic potential of Tannat, Cabernet-Sauvignon and Merlot grapes and their correspondence with wine composition. Anal. Chim. Acta 2004, 513, 191–196. [Google Scholar] [CrossRef]
- Noble, A.C. Bitterness and astringency in wine. In Bitterness in Foods and Beverages, Development in Food Science; Rouseff, R.L., Ed.; Elsevier: New York, NY, USA, 1990; pp. 145–158. [Google Scholar]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waterset, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Z.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar]
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Ivanova, V.; Dörnyei, Á.; Márk, L.; Vojnoski, B.; Stafilov, T.; Stefova, M.; Kilár, F. Polyphenolic content of Vranec wines produced by different vinification conditions. Food Chem. 2011, 124, 316–325. [Google Scholar] [CrossRef]



| Accession | Sampling Region | Accession | Database Used to Retrieve the Molecular Profile |
|---|---|---|---|
| Altamura 1 | Calabria | Aglianico del Vulture | INRGV |
| Altamura 2 | Calabria | Carricante | INRGV |
| Altamura 3 | Calabria | Frappato | INRGV |
| Arvino | Calabria | Glera | INRGV |
| Brettio Nero ARSAC | Calabria | Guarnaccia b. | INRGV |
| Canino | Calabria | Lambrusco di Sorbara | INRGV |
| Doraca | Calabria | Lambrusco Grasparossa | INRGV |
| Greco Nero ARSAC | Calabria | Magliocco Canino | INRGV |
| Guarnaccia | Calabria | Mantonico Bianco | INRGV |
| Guarnaccia Bianca | Calabria | Montonico Bianco | INRGV |
| Guarnaccia Bianca ARSAC | Calabria | Moscato di Scanzo | INRGV |
| Guarnaccia Saracena | Calabria | Notardomenico | INRGV |
| Guarnaccia Bianca-like | Calabria | Tintilia | INRGV |
| Guarnaccia Nera | Calabria | Trebbiano di Soave | INRGV |
| Lagrima Nera | Calabria | Trebbiano Romagnolo | INRGV |
| Magliocco Dolce ARSAC | Calabria | Zibibbo | INRGV |
| Magliocco Canino | Calabria | Guarnaccia Nera | IVD |
| Magliocco Dolce | Calabria | Guarnaccia Bianca | IVD |
| Magliocco Falvo | Calabria | Nero d’Avola | IVD |
| Magliocco Sanmarco | Calabria | Aleatico | EVD |
| Mantonico Nero | Calabria | Barbera | EVD |
| Mantonico Bianco | Calabria | Catarratto Bianco comune | EVD |
| Mantonico d’Alessandria | Calabria | Chardonnay | EVD |
| Mantonico di Roggiano | Calabria | Greco | EVD |
| Mantonico-like | Calabria | Falanghina | EVD |
| Unknown 1 | Calabria | Fiano | EVD |
| Unknown 2 | Calabria | Malvasia delle Lipari | EVD |
| Unknown 3 | Calabria | Malvasia Nera | EVD |
| Caldarese | Puglia | Montonico Bianco | EVD |
| Bianco d’Alessano | Puglia | Nebbiolo | EVD |
| Bombino Bianco | Puglia | Nerello Mascalese | EVD |
| Bombino Nero | Puglia | Syrah | EVD |
| Maresco | Puglia | Tempranillo | EVD |
| Minutolo | Puglia | ||
| Moscatello selvatico | Puglia | ||
| Moscatiddone | Puglia | ||
| Moscato Reale | Puglia | ||
| Negroamaro | Puglia | ||
| Ottavianello | Puglia | ||
| Primitivo | Puglia | ||
| Prunesta | Puglia | ||
| San Martino | Puglia | ||
| Somarello Rosso | Puglia | ||
| Susumaniello | Puglia | ||
| Trebbiano Toscano | Puglia | ||
| Uva di Troia | Puglia | ||
| Ciliegiolo | Abruzzo | ||
| Gaglioppo | Abruzzo | ||
| Montepulciano | Abruzzo | ||
| Moscato Bianco | Abruzzo | ||
| Moscato d’Alessandria | Abruzzo | ||
| Moscato di Terracina | Abruzzo | ||
| Mostosa | Abruzzo | ||
| Malvasia | Toscana | ||
| Merlot | Toscana | ||
| Moscato d’Amburgo | Toscana | ||
| Pinot Nero | Toscana | ||
| Sangiovese | Toscana | ||
| Cabernet | Veneto | ||
| Moscato Giallo | Veneto | ||
| Alicante | Sicilia | ||
| Perricone | Sicilia | ||
| Greco Bianco | Lazio | ||
| Aglianico | Basilicata |
| SSR Marker | Size Range | Na | Ne | I | Ho | He | F | PIC | F (Null) |
|---|---|---|---|---|---|---|---|---|---|
| VVS2 | 131–155 | 10 | 4.82 | 1.83 | 0.87 | 0.79 | −0.09 | 0.77 | −0.050 |
| VVMD5 | 225–251 | 11 | 5.12 | 1.88 | 0.81 | 0.80 | −0.01 | 0.78 | −0.007 |
| VVMD7 | 233–263 | 8 | 4.24 | 1.68 | 0.76 | 0.76 | 0.00 | 0.73 | 0.005 |
| VVMD21 | 228–266 | 9 | 3.88 | 1.64 | 0.77 | 0.74 | −0.03 | 0.71 | −0.011 |
| VVMD25 | 239–269 | 11 | 4.73 | 1.82 | 0.79 | 0.79 | −0.01 | 0.76 | −0.010 |
| VVMD27 | 174–193 | 8 | 4.79 | 1.75 | 0.82 | 0.79 | −0.04 | 0.77 | −0.023 |
| VVMD28 | 218–270 | 14 | 7.58 | 2.20 | 0.93 | 0.87 | −0.07 | 0.85 | −0.035 |
| VVMD32 | 241–273 | 9 | 6.07 | 1.97 | 0.77 | 0.84 | 0.07 | 0.82 | 0.038 |
| VRZAG21 | 179–215 | 7 | 4.07 | 1.54 | 0.82 | 0.75 | −0.09 | 0.72 | −0.049 |
| VRZAG62 | 178–204 | 10 | 7.03 | 2.04 | 0.91 | 0.86 | −0.06 | 0.84 | −0.030 |
| VRZAG64 | 135–189 | 11 | 5.98 | 1.94 | 0.89 | 0.83 | −0.07 | 0.81 | −0.034 |
| VRZAG79 | 236–260 | 12 | 6.44 | 2.05 | 0.82 | 0.84 | 0.02 | 0.83 | 0.010 |
| Total | 120 | ||||||||
| Mean | 10 | 5.40 | 0.83 | 0.81 | −0.03 | 0.78 | |||
| Min. | 7 | 3.88 | 1.54 | 0.76 | 0.74 | −0.09 | 0.71 | −0.050 | |
| Max. | 14 | 7.58 | 2.20 | 0.93 | 0.87 | 0.07 | 0.85 | 0.038 |
| OIV Code | Characteristic | Levels | Magliocco Dolce | Brettio Nero |
|---|---|---|---|---|
| 001 | young shoot: aperture of tip | 1 closed, 3 half open, 5 fully open | 5 | 5 |
| 003 | young shoot: intensity of anthocyanin coloration on prostrate hairs of tip | 1 none or very low, 3 low, 5 medium, 7 high, 9 very high | 1 | 1 |
| 004 | young shoot: density of prostrate hairs on tip | 1 none or very low, 3 low, 5 medium, 7 high, 9 very high | 5 | 5 |
| 007 | shoot: color of dorsal side of internodes | 1 green, 2 green and red, 3 red | 1 | 1 |
| 008 | shoot: color of ventral side of internodes | 1 green, 2 green and red, 3 red | 1 | 1–2 |
| 016 | Shoot: number of consecutive tendrils | 1 green, 2 green and red, 3 red | 1 | 1 |
| 051 | Young leaf: color of the upper side of blade (4th leaf) | 1 green, 2 yellow, 3 bronze, 4 copper-reddish | 1 | 1 |
| 053 | Young leaf: density of prostrate hairs between main veins on lower side of blade (4th leaf) | 1 none or very low, 3 low, 5, medium, 7 high, 9 very high | 5–7 | 5–7 |
| 065 | Mature leaf: size of blade | 1 very small, 3, small, 5 medium, 7 large, 9 very large | 5–7 | 5–7 |
| 067 | Mature leaf: shape of blade | 1 cordate, 2 wedge-shaped, 3 pentagonal, 4 circular, 5 kidney-shaped | 3 | 3 |
| 068 | Mature leaf: number of lobes | 1 one, 2 three, 3 five, 4 seven, 5 more than seven | 2–3 | 2–3 |
| 070 | Mature leaf: area of anthocyanin coloration of main veins on upper side of blade | 1 absent, 2 only at the petiolar point, 3 upto the 1st bifurcation, 4 up to the 2nd bifurcation, 5 beyond the 2nd bifurcation | 1 | 2 |
| 072 | Mature leaf: goffering of blade | 1 absent or very weak, 3 weak, 5 medium, 7 strong, 9 very strong | 3 | 1 |
| 074 | Mature leaf: profile of blade in cross section | 1 flat, 2 V-shaped, 3 involute, 4 revolute, 5 twisted | 2–5 | 5 |
| 075 | Mature leaf: blistering of upper side of blade | 1 absent or very weak, 2 weak, 3 medium, 4 strong, 9 very strong | 5–7 | 5 |
| 076 | Mature leaf: shape of teeth | 1 both sides concave, 2 both sides straight, 3 both sides convex, 4 one side concave on side convex, 5 mixture between both sides straight and both sides convex | 5 | 5 |
| 078 | Mature leaf: length of teeth compared with their width | 1 very short, 3 short, 5 medium, 7 long, 9 very long | 5 | 3–5 |
| 079 | Mature leaf: degree of opening/overlapping of petiole sinus | 1 very wide open, 3 open, 5 closed, 7 overlapped, 9 strongly overlapped | 5 | 7 |
| 080 | Mature leaf: shape of base of petiole sinus | 1 U-shaped, 2 brace-shaped, 3 V-shaped | 3 | 3 |
| 081-1 | Mature leaf: teeth in the petiole sinus | 1 none, 9 present | 1 | 1 |
| 081-2 | Mature leaf: petiole sinus base limited by veins | 1 not limited, 3 on one side, 3 on both sides | 1 | 1 |
| 083-2 | Mature leaf: teeth in the upper lateral sinuses | 1 none, 9 present | 1 | 1 |
| 084 | Mature leaf: density of prostrate hairs between the main veins on lower side of blade | 1 none or very low, 3 low, 5 medium, 7 high, 9 very high | 3–5 | 5 |
| 087 | Mature leaf: density of erect hairs on main veins on lower side of blade | 1 none or very low, 3 low, 5 medium, 7 high, 9 very high | 1 | 1 |
| 151 | Flower: sexual organs | 1 fully developed stamens and no gynoecium, 2 fully developed stamens and reduced gynoecium, 3 fully developed stamens and fully developed gynoecium, 4 reflexed stamens and fully developed gynoecium | 3 | 3 |
| 152 | Inflorescence: insertion of 1st inflorescence | 1 up to the 2nd node, 2 3rd and 4th node, 3 from the 5th node on | 1–2 | 1–2 |
| 153 | Inflorescence: number of inflorescences per shoot | 1 up to 1 inflorescence, 2 1,1 to 2 inflorescences, 3 2,1 to 3 inflorescences, 4 more than 3 inflorescences | 2 | 2 |
| 202 | Bunch: length (peduncle excluded) | 1 very short, 3 short, 5 medium, 7 long, 9 very long. | 3–5 | 5–7 |
| 204 | Bunch: density | 1 very loose, 3 loose, 5 medium, 7 dense, 9 very dense | 5–7 | 5–7 |
| 206 | Bunch: length of peduncle of primary bunch | 1 very short, 3 short, 5 medium, 7 long, 9 very long | 3–5 | 3–5 |
| 208 | Bunch: shape | 1 cylindrical, 2 conical, 3 funnel shaped | 2 | 2 |
| 209 | Bunch: number of wings of the primary bunch | 1 absent, 2 1–2 wings, 3 3–4 wings, 4 5–6 wings, 5 more than 6 wings | 1–2 | 1–2 |
| 220 | Berry: length | 1 very short, 3 short, 5 medium, 7 long, 9 very long | 3 | 5–7 |
| 223 | Berry: shape | 1 obloid, 2 globose, 3 broad ellipsoid, 4 narrow ellipsoid, 5 cylindrical, 6 obtuse ovoid, 7 ovoid, 8 obovoid, 9 horn shaped, 10 finger shaped | 2 | 3 |
| 225 | Berry: color of skin | 1 green yellow, 2 rose, 3 red, 4, grey, 5dark red violet, 6 blue black | 6 | 6 |
| 228 | Berry: thickness of skin | 1 very thin, 3 thin, 5 medium, 7 thick, 9 very thick | 7 | 7 |
| 235 | Berry: firmness of flesh | 1 soft, 2 slightly firm, 3 very firm | 2 | 2 |
| 236 | Berry: particularity of flavor | 1 none, 2 muscat, 3 foxy, 4 herbaceous, 5 other flavor | 1 | 1 |
| 241 | Berry: formation of seeds | 1 none, 2 rudimentary, 3 complete | 3 | 3 |
| 351 | Vigor of shoot growth | 1 very weak, 3 weak, 5 medium, 7 strong, 9 very strong | 5 | 5 |
| 352 | Growth of axillary shoots | 1 absent or very weak, 3 weak, 5 medium, 7 strong, 9 very strong | 5 | 5 |
| 353 | Length of internodes | 1 very short, 3 short, 5 medium, 7 long, 9 very long | 3–5 | 3 |
| 502 | Bunch: weight of a single bunch | 1 very low, 3 low, 5 medium, 7 high, 9 very high | 3 | 3 |
| 503 | Berry: single berry weight | 1 very low, 3 low, 5 medium, 7 high, 9 very high | 1–3 | 1–3 |
| 504 | Yield per m² | 1 very low, 3 low, 5 medium, 7 high, 9 very high | 5 | 5 |
| Parameter | Magliocco Dolce | Brettio Nero |
|---|---|---|
| Alcoholic degree (%, ± v/v) | 14.02 ± 0.59 | 13.37 ± 0.64 |
| Total acidity (g/L) | 6.55 ± 0.32 | 6.51 ± 0.52 |
| pH | 3.48 ± 0.17 | 3.52 ± 0.16 |
| Density (20°/20°) | 1.04 ± 0.07 | 1.05 ± 0.07 |
| Total dry extract (g/L) | 32.24 ± 1.63 | 28.67 ± 0.27 |
| Volatile acidity (g/L) | 0.5 ± 0.03 | 0.39 ± 0.13 |
| Ashes (g/L) | 3.15 ± 0.01 | 2.95 ± 0.06 * |
| Malic acid (g/L) | 3.11 ± 0.06 | 3.73 ± 0.16 * |
| Tartaric acid (g/L) | 3.28 ± 1.17 | 2.93 ± 1.16 |
| Citric acid (g/L) | 0.1 ± 0.04 | 0.16 ± 0.01 |
| Glycerol (g/L) | 9.68 ± 0.48 | 8.95 ± 0.82 |
| Potassium (g/L) | 1.39 ± 0.35 | 1.44 ± 0.27 |
| Sulfates (g/L) | 0.42 ± 0.13 | 0.51 ± 0.07 |
| Color intensity | 8.82 ± 0.69 | 7.23 ± 1.74 |
| Color tonality | 0.85 ± 0.32 | 0.92 ± 0.42 |
| Total anthocyanins (mg/L) | 171.81 ± 21.15 | 82.87 ± 31.44 |
| Total flavonoids (mg/ L) | 973.12 ± 314.93 | 715.44 ± 75.37 |
| Total polyphenols (mg/ L) | 2207.23 ± 309.91 | 1759.63 ± 505.08 |
| Proanthocyanidins (mg/ L) | 2370.49 ± 1280.70 | 1709.40 ± 915.09 |
| Flavans (mg/ L) | 471.13 ± 227.00 | 756.52 ± 402.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanelli, V.; Roseti, V.; Savoia, M.A.; Miazzi, M.M.; Venerito, P.; Savino, V.N.; Pirolo, C.; La Notte, P.; Falbo, M.; Petrillo, F.; et al. New Insight into the Identity of Italian Grapevine Varieties: The Case Study of Calabrian Germplasm. Agronomy 2021, 11, 1538. https://doi.org/10.3390/agronomy11081538
Fanelli V, Roseti V, Savoia MA, Miazzi MM, Venerito P, Savino VN, Pirolo C, La Notte P, Falbo M, Petrillo F, et al. New Insight into the Identity of Italian Grapevine Varieties: The Case Study of Calabrian Germplasm. Agronomy. 2021; 11(8):1538. https://doi.org/10.3390/agronomy11081538
Chicago/Turabian StyleFanelli, Valentina, Vincenzo Roseti, Michele Antonio Savoia, Monica Marilena Miazzi, Pasquale Venerito, Vito Nicola Savino, Costantino Pirolo, Pierfederico La Notte, Maurizio Falbo, Fabio Petrillo, and et al. 2021. "New Insight into the Identity of Italian Grapevine Varieties: The Case Study of Calabrian Germplasm" Agronomy 11, no. 8: 1538. https://doi.org/10.3390/agronomy11081538
APA StyleFanelli, V., Roseti, V., Savoia, M. A., Miazzi, M. M., Venerito, P., Savino, V. N., Pirolo, C., La Notte, P., Falbo, M., Petrillo, F., & Montemurro, C. (2021). New Insight into the Identity of Italian Grapevine Varieties: The Case Study of Calabrian Germplasm. Agronomy, 11(8), 1538. https://doi.org/10.3390/agronomy11081538









