Microbial Resource Limitation in Aggregates in Karst and Non-Karst Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Sampling
2.3. Soil Aggregate Fractionation
2.4. Measurements of Soil Properties and Enzyme Activities
3. Results
3.1. Soil Properties
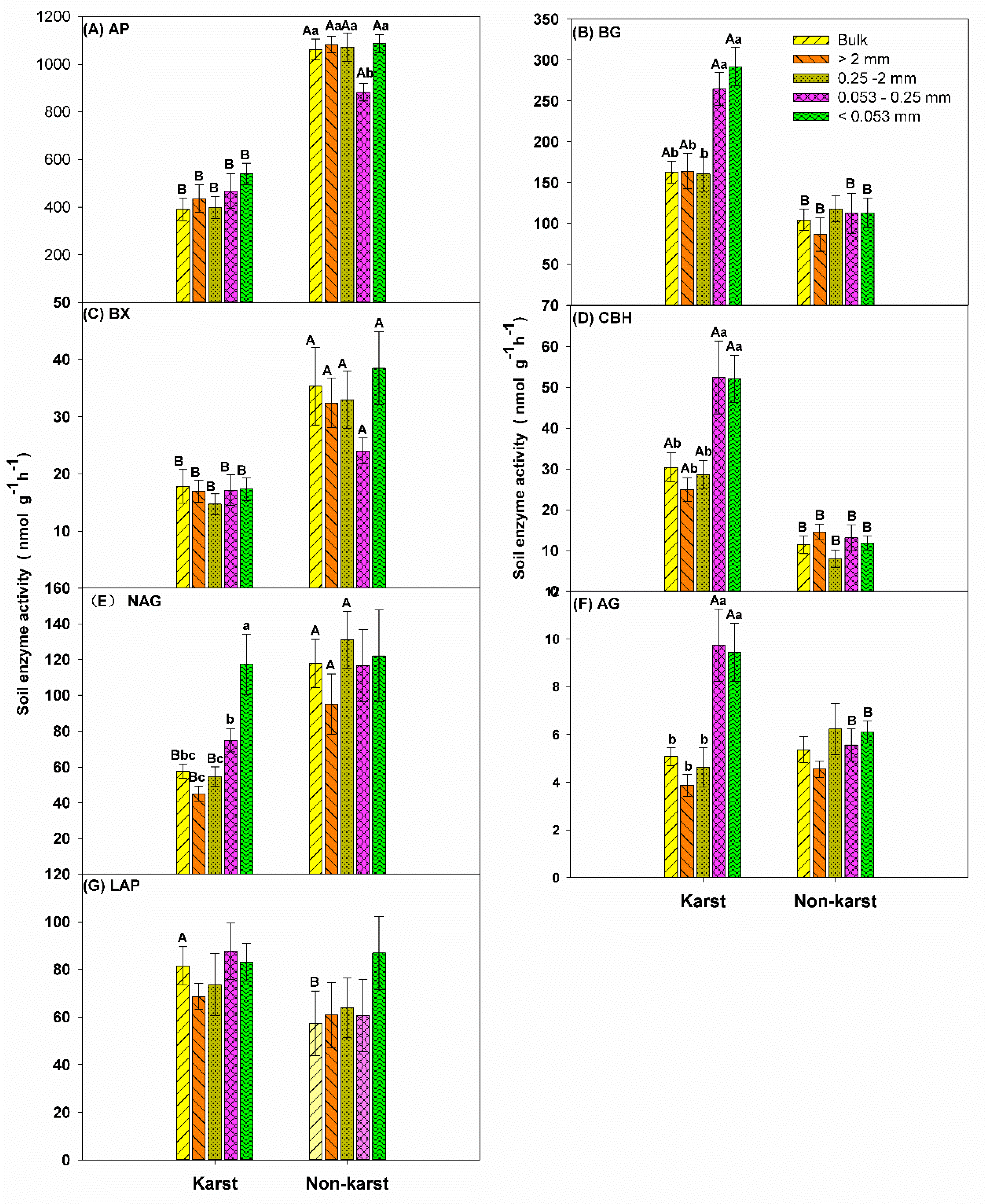
3.2. Soil Enzyme Activity
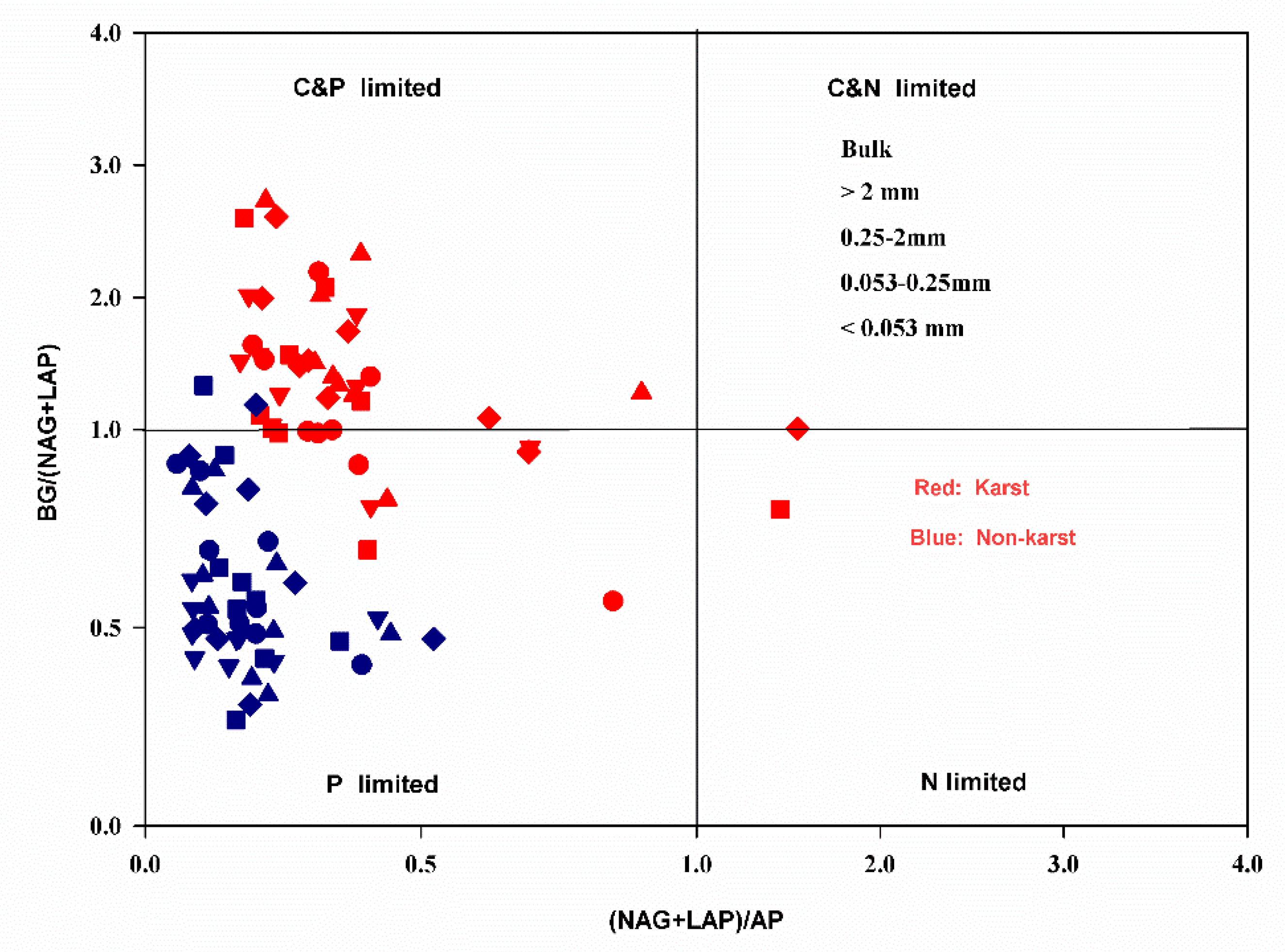
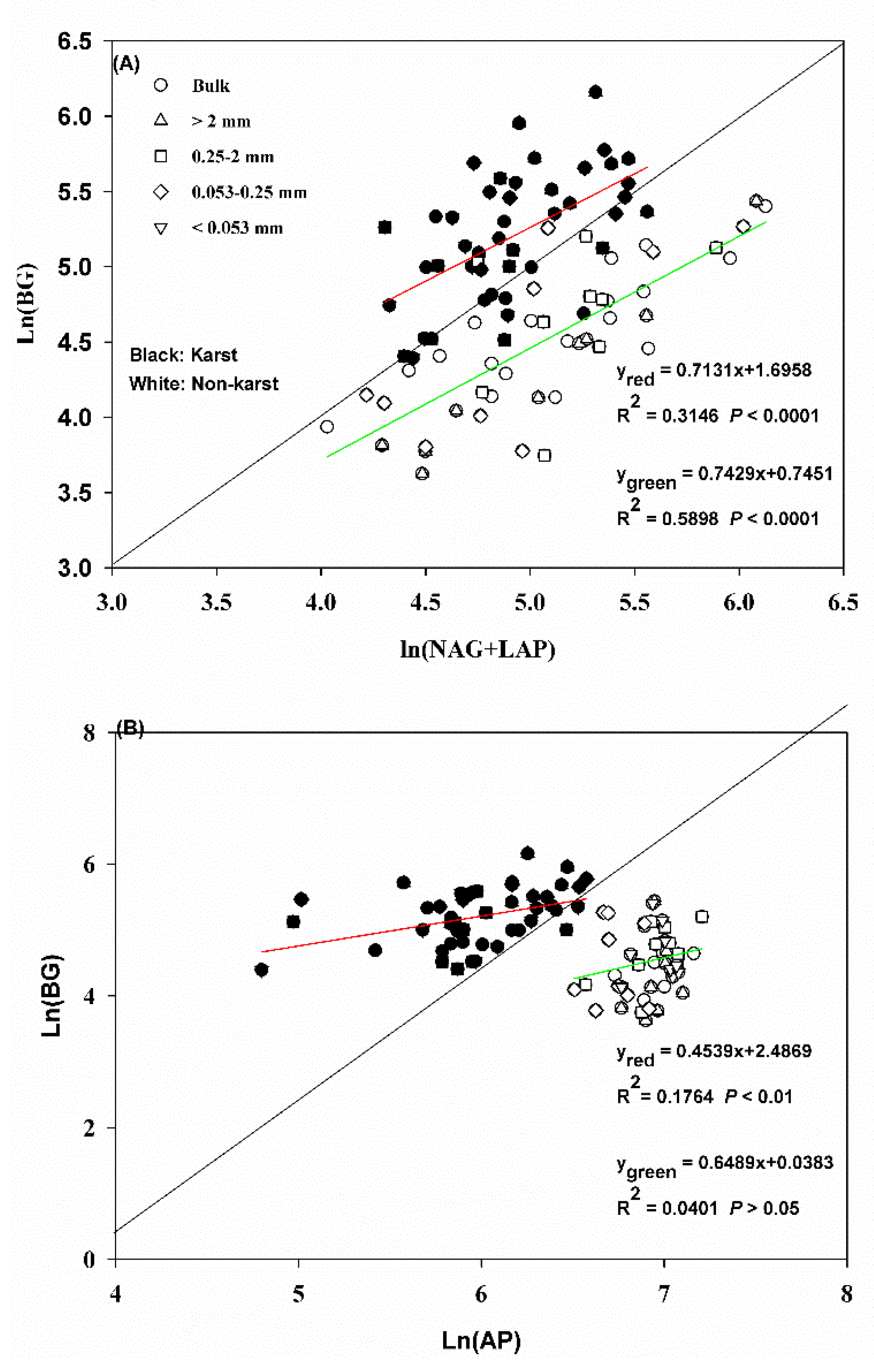
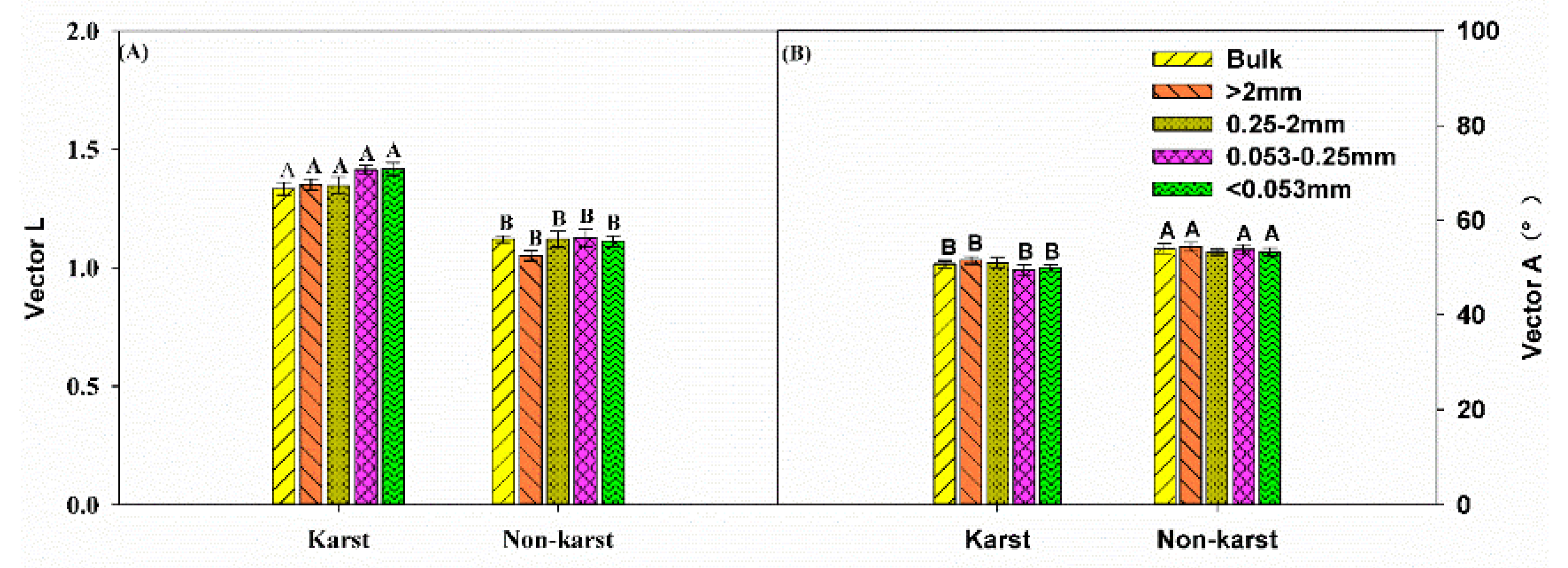
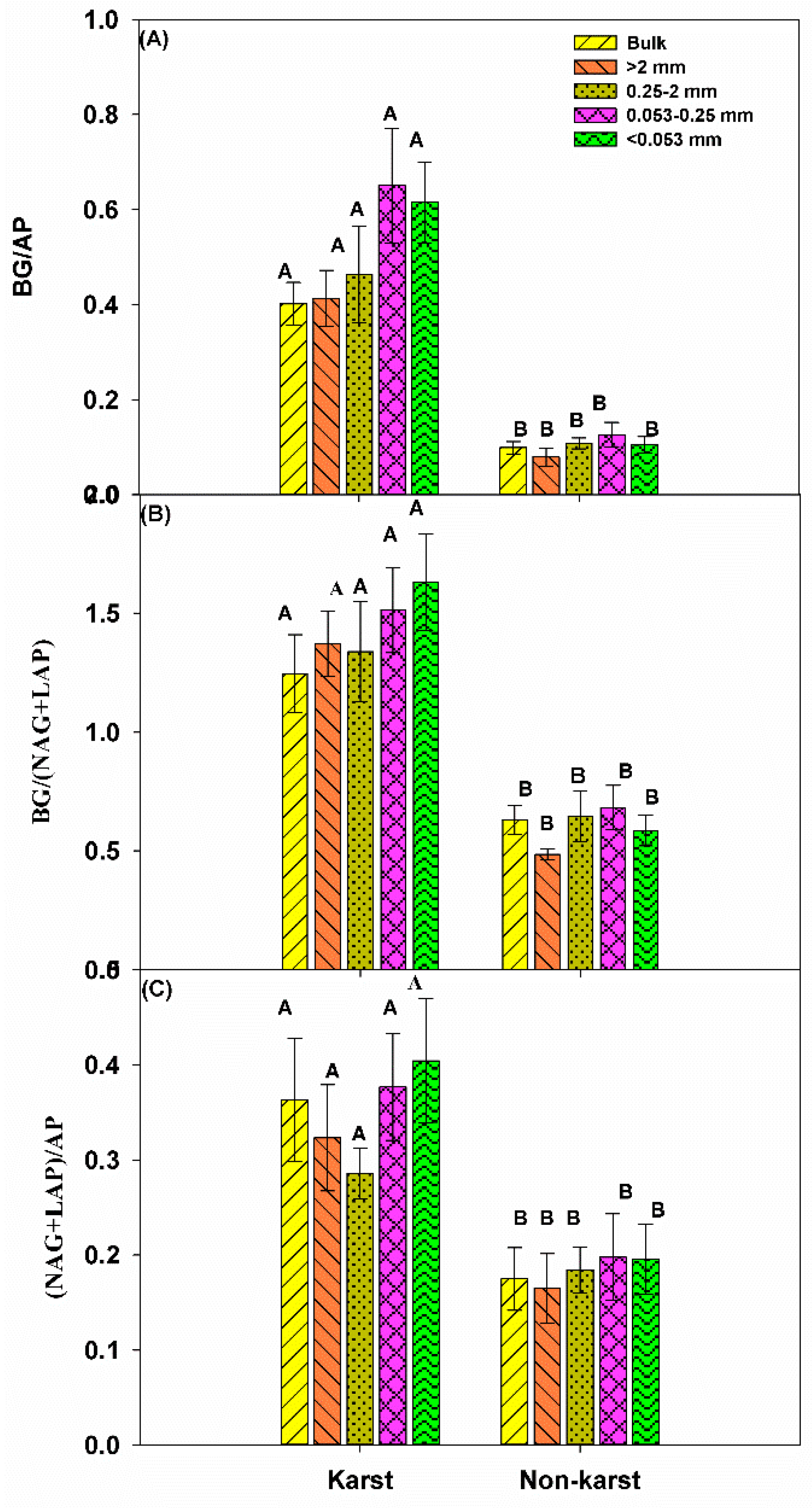
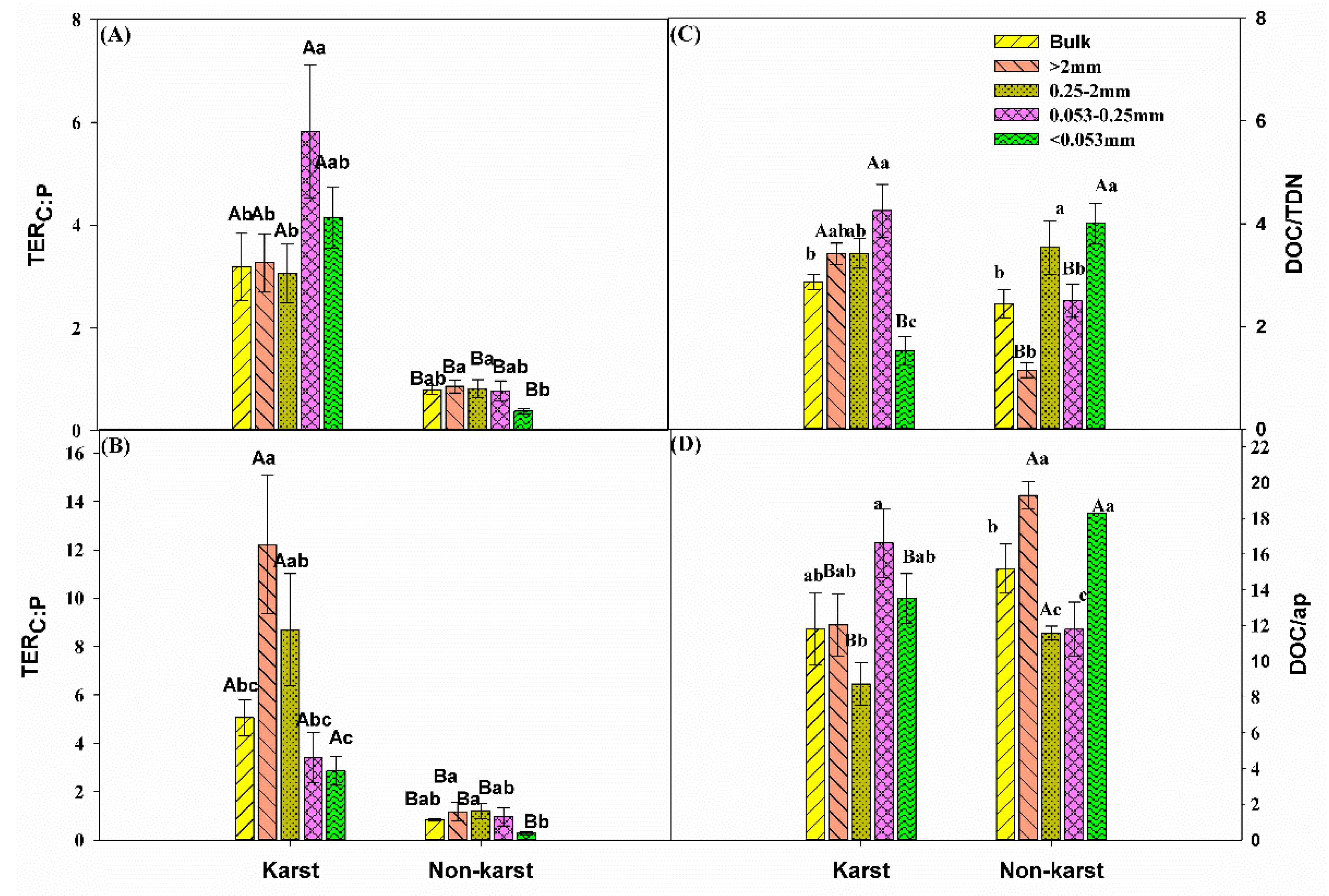
3.3. Exoenzymatic Stoichiometry Ratio
4. Discussion
4.1. Effect of Soil Properties on Individual Enzyme Activity in Karst and Non-Karst Forests
4.2. Microbial Resource Limitation in Karst and Non-Karst Forests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sweeting, M.M. Karst in China: Its Geomorphology and Environment; Reference to a Chapter in an Edited Book; Springer: Berlin, Germany, 1995. [Google Scholar]
- Ahmed, Y.A.R.; Pichler, V.; Homolák, M.; Gömöryová, E.; Nagy, D.; Pichlerová, M.; Gregor, J. High Organic Carbon Stock in a Karstic Soil of the Middle-European Forest Province Persists after Centuries-Long Agroforestry Management. Eur. J. For. Res. 2012, 131, 1669–1680. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Li, D.J.; Yang, L.Q.; Luo, P.; Chen, H.; Xiao, K.C.; Song, T.Q.; He, X.Y.; Chen, H.S.; Wang, K.L. Rapid Recuperation of Soil Nitrogen Following Agricultural Abandonment in a Karst Area, Southwest China. Biogeochemistry 2016, 129, 341–354. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Yang, L.; Luo, P.; Xiao, K.; Chen, H.; Zhang, W.; He, X.; Chen, H.; Wang, K. Dynamics of Soil Organic Carbon and Nitrogen Following Agricultural Abandonment in a Karst Region. J. Geophys. Res. Biogeosci. 2017, 122, 230–242. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, M.H.; Mao, Q.G.; Xiao, K.C.; Wang, K.L.; Li, D.J. Cropland conversion changes the status of microbial resource limitation in degraded karst soil. Geoderma 2019, 352, 197–203. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, L.; Pan, S.; Li, Y.; Kuzyakov, Y.; Xu, J.; Brookes, P.C.; Luo, Y. Feedstock Determines Biochar-Induced Soil Priming Effects by Stimulating the Activity of Specific Microorganisms. Eur. J. Soil Sci. 2018, 69, 521–534. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of Soil Microbial and Enzyme Activities are Linked to Soil C, N and P Stoichiometry in Afforested Ecosystems. For. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Wei, X.M.; Razavi, B.S.; Hu, Y.J.; Xu, X.L.; Zhu, Z.K.; Liu, Y.H.; Kuzyakov, Y.; Li, Y.; Wu, J.S.; Ge, T.D. C/P Stoichiometry of Dying Rice Root Defines the Spatial Distribution and Dynamics of Enzyme Activities in Root-Detritusphere. Biol. Fertil. Soils 2019, 55, 251–263. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Hu, C.; Wu, L.; Duan, Y.H.; Zhang, W.J.; Aziz, T.; Cai, A.D.; Abrar, M.M.; Xu, M.G. Soil and Microbial Biomass Stoichiometry Regulate Soil Organic Carbon and Nitrogen Mineralization in Rice-Wheat Rotation Subjected to Long-Term Fertilization. J. Soils Sediments 2020, 20, 3103–3113. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, Z.K.; Xu, X.L.; Liu, S.L.; Jones, D.L.; Kuzyakov, Y.; Shibistova, O.; Wu, J.S.; Ge, T.D. Carbon and Nitrogen Recycling from Microbial Necromass to Cope with C:N Stoichiometric Imbalance by Priming. Soil Biol. Biochem. 2020, 142, 107720. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. In Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphe; Princeton University Press: Princeton, NJ, USA, 2002; pp. 225–226. [Google Scholar]
- Ge, T.D.; Luo, Y.; Bhupinder, P.S. Resource Stoichiometric and Fertility in Soil. Biol. Fertil. Soils 2020, 56, 1091–1092. [Google Scholar] [CrossRef]
- Wei, X.M.; Zhu, Z.K.; Liu, Y.; Luo, Y.; Deng, Y.W.; Xu, X.L.; Liu, S.L.; Adreas, R.; Olga, S.; Geory, G.; et al. C:N:P Stoichiometry Regulates Soil Organic Carbon Mineralization and Concomitant Shifts in Microbial Community Composition in Paddy Soil. Biol. Fertil. Soils 2020, 56, 1093–1107. [Google Scholar] [CrossRef]
- Ekblad, A.; Nordgren, A. Is Growth of Soil Microorganisms in Boreal Forests Limited by Carbon or Nitro Gen Availability? Plant Soil 2002, 242, 115–122. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Kolka, R.K.; Lehto, L.L.P.; Sebestyen, S.D.; Seifert-Monson, L.R. Ecoenzymatic Stoichiometry and Microbial Processing of Organic Matter in Northern Bogs and Fens Reveals a Common P-limitation between Peatland Types. Biogeochemistry 2014, 120, 203–224. [Google Scholar] [CrossRef]
- Zhu, Z.K.; Ge, T.D.; Luo, Y.; Liu, S.L.; Xu, X.L.; Tong, C.L.; Olga, S.; Georg, G.; Wu, J.S. Microbial Stoichi Ometric Flexibility Regulates Rice Straw Mineralization and its Priming Effect in Paddy Soil. Soil Biol. Biochem. 2018, 121, 67–76. [Google Scholar] [CrossRef]
- Zhu, Z.K.; Zhou, J.; Muhammad, S.; Tang, H.; Liu, S.L.; Zhang, W.J.; Yuan, H.Z.; Zhou, P.; Hattan, A.; Wu, J.S.; et al. Microorganisms Maintain C:N Stoichiometric Balance by Regulating the Priming Effect in Long-Term Fertilized Soils. Appl. Soil Ecol. 2021, 167, 104033. [Google Scholar] [CrossRef]
- Vance, E.D.; Chapin, F.S. Substrate Limitations to Microbial Activity in Taiga Forest Floors. Soil Biol. Biochem. 2001, 33, 173–188. [Google Scholar] [CrossRef]
- Simpson, A.J.; Simpson, M.J.; Emma, S.; Kelleher, B. Microbially Derived Inputs to Soil Organic Matter: Are Current Estimates Too Low? Environ. Sci. Technol. 2007, 41, 8070–8076. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Moorhead, D.; Bertrand, I. Eco-Enzymatic Stoichiometry and Enzymatic Vectors Reveal Differential C, N, P Dynamics in Decaying Litter Along a Land-Use Gradient. Biogeochemistry 2016, 129, 21–36. [Google Scholar] [CrossRef]
- Gregg, J.; Andres, R.J.; Marland, G. China: Emissions Pattern of the World Leader in CO2 Emissions from Fossil Fuel Consumption and Cement Production. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky Desertification in Southwest China: Impacts, Causes, and Restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Xiao, S.S.; Zhang, W.; Ye, Y.Y.; Zhao, J.; Wang, K.L. Soil Aggregate Mediates the Impacts of Land Uses on Organic Carbon, Total Nitrogen, and Microbial Activity in a Karst Ecosystem. Sci. Rep. 2017, 7, 41402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.M.; Chen, H.S.; Zhang, W.; Wang, K.L. Influencing Factors on Soil Nutrients at Different Scales in a Karst Area. Catena 2019, 175, 411–420. [Google Scholar] [CrossRef]
- Castle, S.C.; Sullivan, B.W.; Knelman, J.; Hood, E.; Nemergut, D.R.; Schmidt, S.K.; Cleveland, C.C. Nutrient Limitation of Soil Microbial Activity during the Earliest Stages of Ecosystem Development. Oecologia 2017, 185, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Carvalho, C.J.R.D.; Figueira, A.M.; Ishida, F.Y.; Ometto, J.P.H.B.; Nardoto, G.B.; Sabá, R.T.; Hayashi, S.N.; Leal, E.C.; Vieira, I.C.G.; et al. Recuperation of Nitrogen Cycling in Amazonian Forests Following Agricultural Abandonment. Nature 2007, 447, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.X.; Pan, G.X.; Li, L.Q.; Hu, Z.L.; Wang, X.Z. Leaf N/P Ratio and Nutrient Reuse between Dominant Species and Stands: Predicting Phosphorus Deficiencies in Karst Ecosystems, Southwestern China. Environ. Earth Sci. 2010, 64, 299–309. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.J.; Li, D.; Chen, H.S.; Wang, K.L. Changes in Nitrogen and Phosphorus Limitation during Secondary Succession in a Karst Region in Southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Feng, J.; Wei, K.; Chen, Z.H.; Lü, X.T.; Tian, J.H.; Wang, C.; Chen, L.J. Coupling and Decoupling of Soil Carbon and Nutrient Cycles Across an Aridity Gradient in the Drylands of Northern China: Evidence From Ecoenzymatic Stoichiometry. Glob. Biogeochem. Cycles 2019, 33, 559–569. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Lei, Y.B.; Qin, W.; Korpelainen, H.; Li, C.Y. Revealing Microbial Processes and Nutrient Limitation in Soil through Ecoenzymatic Stoichiometry and Glomalin-Related Soil Proteins in a Retreating Glacier Forefield. Geoderma 2019, 338, 313–324. [Google Scholar] [CrossRef]
- Rosinger, C.; Rousk, J.; Sandén, H. Can Enzymatic Stoichiometry be used to Determine Growth-Limiting Nutrients for Microorganisms?—A Critical Assessment in Two Subtropical Soils. Soil Biol. Biochem. 2019, 128, 115–126. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Bing, H.; Zhou, J.; Wang, J. Vegetation Type rather than Climate Modulates the Variation in Soil Enzyme Activities and Stoichiometry in Subalpine Forests in the Eastern Tibetan Plateau. Geoderma 2020, 374, 114424. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The Implications of Exoenzyme Activity on Microbial Carbon and Nitrogen Limitation in Soil: A Theoretical Model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic Relationships between Microbial Biomass, Respiration, Inorganic Nutrients and Enzyme Activities: Informing Enzyme-Based Decomposition Models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajwa, H.A.; Dell, C.J.; Rice, C.W. Changes in Enzyme Activities and Microbial Biomass of Tallgrass Prairie Soil as Related to Burning and Nitrogen Fertilization. Soil Biol. Biochem. 1999, 31, 769–777. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Creamer, C.; Filley, T.; Dan, C.D.; Diane, E.S. Changes to Soil Organic N Dynamics with Leguminous Woody Plant Encroachment into Grasslands. Biogeochemistry 2013, 113, 307–321. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Waring, B.G. Exploring Relationships between Enzyme Activities and Leaf Litter Decomposition in a Wet Tropical Forest. Soil Biol. Biochem. 2013, 64, 89–95. [Google Scholar] [CrossRef]
- Štursová, M.; Baldrian, P. Effects of Soil Properties and Management on the Activity of Soil Organic Matter Transforming Enzymes and the Quantification of Soil-Bound and Free Activity. Plant Soil 2011, 338, 99–100. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil Moisture is the Major Factor Influencing Microbial Community Structure and Enzyme Activities Across Seven Biogeoclimatic Zones in Western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil Extracellular Enzyme Activities Correspond with Abiotic Factors more than Fungal Community Composition. Biogeochemistry 2014, 117, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Meentemeyer, V. Macroclimate and Lignin Control of Litter Decomposition Rates. Ecology 1978, 59, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Mccaugherty, C.A.; Linkins, A.E. Temperature Responses of Enzymes in two Forest Soils. Soil Biol. Biochem. 1990, 22, 29–33. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Allison, S.D.; Jastrow, J.D. Activities of Extracellular Enzymes in Physically Isolated Fractions of Restored Grassland Soils. Soil Biol. Biochem. 2006, 38, 3245–3256. [Google Scholar] [CrossRef]
- Castle, S.C.; Neff, J.C. Plant Response to Nutrient Availability across Variable Bedrock Geologies. Ecosystems 2008, 12, 101–113. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.J.; Xiao, K.C.; Wang, K.L. Soil Microbial Processes and Resource Limitation in Karst and Non-Karst Forests. Funct. Ecol. 2018, 329, 61–64. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.J.; Chen, H.; Wang, K.L. Dynamics of Soil Organic Carbon in Density Fractions during Postagricultural Succession over Two Lithology Types, Southwest China. J. Environ. Manag. 2017, 201, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, J.; Zhang, Q.; Li, Q.X.; Long, C.Y.; Yang, F.; Chen, Q.; Cheng, X.L. Stimulation of Nitrogen-Hydrolyzing Enzymes in Soil Aggregates Mitigates Nitrogen Constraint for Carbon Sequestration Following Afforestation in Subtropical China. Soil Biol. Biochem. 2018, 123, 136–144. [Google Scholar] [CrossRef]
- Mustafa, A.; Mingang, X.; AliShah, S.A.; Abrar, M.M.; Sun, N.; Wang, B.R.; Cai, Z.J.; Saeed, Q.; Naveed, M.; Mehmood, K.; et al. Soil Aggregation and Soil Aggregate Stability Regulate Organic Carbon and Nitrogen Storage in a Red Soil of Southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Carpentier, A.; Van Kessel, C.; Merckx, R.; Harris, D.; Horwath, W.R.; Lüscher, A. Impact of Elevated CO2 on Soil Organic Matter Dynamics as Related to Changes in Aggregate Turnover and Residue Quality. Plant Soil 2001, 234, 27–36. [Google Scholar] [CrossRef]
- Eliot, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Chen, H.; Xiao, K.; Song, T.; Wang, K. Soil Gross Nitrogen Transformations in Typical Karst and Nonkarst Forests Southwest China. J. Geophys. Res. Biogeosci. 2017, 122, 2831–2840. [Google Scholar] [CrossRef]
- Smith, A.P.; Marín-Spiotta, M.E.; Graaff, M.A.D.; Balser, T.C. Microbial Community Structure Varies across Soil Organic Matter Aggregate Pools during Tropical Land cover Change. Soil Biol. Biochem. 2014, 77, 292–303. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; Reference to a Chapter in an Edited, Book; Taylor & Francis Group: Oxfordshire, UK; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chlororm Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommeren1ng, B.; Chaussod, R.; Brookes, P.C. Measurement of Soil Microbial Biomass C by Fumigation-Extraction—An Automated Procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Fansler, S.J.; Smith, J.L.; Bolton, H.; Bailey, V.L., Jr. Distribution of Two C Cycle Enzymes in Soil Aggregates of a Prairie Chronosequence. Biol. Fertil. Soils 2005, 42, 17–23. [Google Scholar] [CrossRef]
- Marx, M.C.; Kandeler, E.; Wood, M.; Wermbter, N.; Jarvis, S.C. Exploring the Enzymatic Landscape: Distribution and Kinetics of Hydrolytic Enzymes in Soil Particle-Size Fractions. Soil Biol. Biochem. 2005, 37, 35–48. [Google Scholar] [CrossRef]
- Chen, H.; Luo, P.; Wen, L.; Yang, L.; Wang, K.; Li, D. Determinants of Soil Extracellular Enzyme Activity in a Karst Region, Southwest China. Eur. J. Soil Biol. 2017, 80, 69–76. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.J.; Mao, Q.G.; Xiao, K.C.; Wang, K.L. Resource Limitation of Soil Microbes in Karst Ecosystems. Sci. Total Environ. 2018, 650, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic Stoichiometry of Microbial Nutrient Acquisition in Tropical Soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Richard, D.B.; Jacqueline, A.A.P.; Kate, L. The DIRT Experiment: Litter and Root Influences on Forest Soil Organic Matter Stocks and Function. In Forests in Time: The Environmental Consequences of 1000 Years of Change in New England; Yale University Press: Newhaven, UK; London, UK, 2004; Chapter 15. [Google Scholar]
- Emma, J.S. Using Experimental Manipulation to Assess the Roles of Leaf Litter in the Functioning of Forest Ecosystems. Biol. Rev. Camb Philos. Soc. 2006, 81, 1–31. [Google Scholar]
- Hobbies, S.; Vitousek, P. Nutrient Limitation of Decomposition in Hawaiian Forests. Ecology 2000, 81, 1867–1877. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Reference to Chapters in an Edited Book; Springer: New York, NY, USA, 2002. [Google Scholar]
- Dorodnikov, M.; Blagodatskaya, E.; Blagodatsky, S.; Marhan, S.; Fangmeier, A.; Kuzyakov, Y. Stimulation of Microbial Extracellular Enzyme Activities by Elevated CO2 Depends on Soil Aggregate Size. Glob. Chang. Biol. 2009, 15, 1603–1614. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; Chen, A.; Xiao, M.; Deng, Y.; Ye, R.; Zhu, Z.; Inubushi, K.; Wu, J.; Ge, T. Legacy effect of elevated CO2 and N fertilization on mineralization and retention of rice (Oryza sativa L.) rhizodeposit-C in paddy soil aggregates. Soil Ecol. Lett. 2020. [Google Scholar] [CrossRef]
- Amato, M.; Ladd, J.N. Decomposition of C-14-Labeled Glucose and Legume Material in Soils—Propeties Influencing the Accumulation of Organic Residue-C and Microbial Biomass-C. Soil Biol. Biochem. 1992, 24, 455–464. [Google Scholar] [CrossRef]
- Dick, R.P.; Myrold, D.D.; Kerle, E.A. Microbial Biomass and Soil Enzyme Activities in Compacted and Rehabilitated Skid Trail Soils. Soil Sci. Soc. 1998, 52, 512–516. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wedin, D.A. Soil Carbon, Nutrients, and Mycorrhizae during Conversion of Dry Tropical Forest to Grassland. Ecol. Appl. 1997, 7, 171. [Google Scholar] [CrossRef]
- Finzi, A.C.; Chales, C.; Nico, V.B. Canopy Tree Soil Interactions within Temperate Forests: Species Effects on Soil Carbon and Nitrogen. Ecol. Appl. 1998, 8, 440–446. [Google Scholar]
- Li, D.; Wen, L.; Jiang, S.; Song, T.; Wang, K. Responses of Soil Nutrients and Microbial Communities to Three Restoration Strategies in a Karst Area, Southwest China. J. Environ. Manag. 2018, 207, 456–464. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, H.; Wang, Y.Q.; Mao, Q.G.; Zheng, M.H.; Su, Y.R.; Xiao, K.C.; Wang, K.L.; Li, D.J. Responses of Soil Microbial Resource Limitation to Multiple Fertilization Strategies. Soil Tillage Res. 2020, 196, 104474. [Google Scholar] [CrossRef]
- McDowell, W.H.; Nadelhoffer, K.J.; Magill, A.; Berntson, G.; Kamakea, M.; Mcnulty, S.G.; Currie, W.; Rustad, L.E.; Fernadez, I.J. Nitrogen Saturation in Temperate Forest Ecosystems. BioScience 1998, 48, 921–934. [Google Scholar]
- Kamble, P.N.; Bååth, E. Induced N-Limitation of Bacterial Growth in Soil: Effect of Carbon Loading and N Status in Soil. Soil Biol. Biochem. 2014, 74, 11–20. [Google Scholar] [CrossRef]
- Lutzow, M.V.; KogelKnabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of Organic Matter in Temperate Soils: Mechanisms and Their Relevance under Different Soil Conditions—A Review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Kaiser, M.; Walter, K.; Ellerbrock, R.H.; Sommer, M. Effects of Land Use and Mineral Characteristics on the Organic Carbon Content, and the Amount and Composition of Na-Pyrophosphate-Soluble Organic Matter, in Subsurface Soils. Eur. J. Soil Sci. 2011, 62, 226–236. [Google Scholar] [CrossRef]
- Di, X.Y.; An, X.J.; Dong, H.; Tang, H.M.; Xiao, B.H. The Distribution and Evolution of Soil Organic Matter in the Karst Region, Guizhou Province Southwestern China. Environ. Earth Sci. 2015, 43, 697–708. [Google Scholar]


| Location | Aggregates | SOC (g/kg) | TN (g/kg) | C:N Ratio | DOC (mg/kg) | TDN (mg/kg) | Ap (mg/kg) | MBC (mg/kg) | MBN (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Karst | Bulk | 44.3 ± 2.1Aab | 7.3 ± 0.5Aab | 6.2 ± 0.2Aa | 79.4 ± 7.2Bcd | 27.3 ± 1.0Bb | 8.2 ± 1.2Ab | 675 ± 68.1Aa | 170.8 ± 21.3Aab |
| >2 mm | 39.7 ± 1.3Ab | 6.5 ± 0.4Aab | 6.2 ± 0.3Aa | 88.9 ± 4.7Abc | 26.2 ± 0.4Bb | 8.8 ± 1.4Ab | 827.7 ± 79.9Aa | 222.8 ± 20.7Aa | |
| 0.25–2 mm | 45 ± 2.2Aab | 6.9 ± 0.7Aab | 7.7 ± 1.5Aa | 96.4 ± 7.8Ab | 28.7 ± 1.9Ab | 12.3 ± 1.4Aa | 711.2 ± 60.3Aa | 203.6 ± 21.2Aa | |
| 0.053–0.25 mm | 50 ± 1.9Aa | 8.4 ± 0.4Aa | 6.0 ± 0.2Ba | 122.6 ± 3.0Aa | 31.5 ± 3Aab | 8.1 ± 0.8Ab | 627.5 ± 78.5Aab | 124.9 ± 33.6Bb | |
| <0.053 mm | 44.9 ± 2.3Aab | 5.6 ± 1.5Ab | 7.9 ± 1.9Aa | 65.0 ± 0.0Ad | 52.6 ± 7.5Aa | 5.3 ± 0.7Ab | 443.5 ± 42.8Ab | 114.5 ± 14.4Bb | |
| Non-karst | Bulk | 17.4 ± 1.6Ba | 2.6 ± 0.2Bab | 6.8 ± 0.6Aab | 101.4 ± 5.0Aa | 47.0 ± 6.6Aab | 7.2 ± 0.8Ab | 446.4 ± 41.2Ba | 176.5 ± 20.1Aa |
| >2 mm | 15.9 ± 0.9Ba | 2.6 ± 0.4Bab | 6.9 ± 0.9Aab | 53.2 ± 0.0Bb | 52.7 ± 6.9Aa | 2.8 ± 0.1Bc | 511.7 ± 91.6Ba | 174.8 ± 17.8Aa | |
| 0.25–2 mm | 16.2 ± 1.0Ba | 3.1 ± 0.4Ba | 5.8 ± 0.6Ab | 108.6 ± 3.7Aa | 34.8 ± 3.9Ab | 9.4 ± 0.0Ba | 403.5 ± 41.9Ba | 210.3 ± 22.8Aa | |
| 0.053–0.25 mm | 14.8 ± 1.8Ba | 1.8 ± 0.3Bb | 8.8 ± 0.5Aa | 92.2 ± 11.9Ba | 37.2 ± 1.3Ab | 7.8 ± 0.2Ab | 425.8 ± 80.1Aa | 225.8 ± 22.1Aa | |
| <0.053 mm | 18.4 ± 1.6Ba | 2.3 ± 0.4Aab | 7.6 ± 1.1Aab | 69.6 ± 0.0Ab | 19.3 ± 2.6Bc | 3.8 ± 0.0Bc | 223.7 ± 16.5Bb | 210.4 ± 17.0Aa |
| Variable | TERC;N | TERC;P | Vector | Vector | BG/ | (NAG + LAP)/ | BG/AP |
|---|---|---|---|---|---|---|---|
| Length | Angle | (NAG + LAP) | AP | ||||
| SOC | 0.057 | 0.533 ** | 0.533 ** | −0.496 ** | 0.398 ** | 0.536 ** | 0.630 ** |
| TN | −0.018 | 0.417 ** | 0.424 ** | −0.271 ** | 0.398 ** | 0.324 ** | 0.452 ** |
| CN | 0.096 | −0.302 ** | −0.310 ** | 0.099 | −0.369 ** | −0.157 * | −0.275 ** |
| DOC | −0.173 * | 0.318 ** | 0.284 ** | −0.23 ** | 0.211 ** | 0.240 ** | 0.315 ** |
| TDN | −0.084 | 0.241 ** | 0.251 ** | −0.212 ** | 0.181 * | 0.225 ** | 0.287 ** |
| AP | 0.12 | 0.163 * | 0.396 ** | −0.376 ** | 0.287 ** | 0.386 ** | 0.448 ** |
| MBC | 0.204 ** | 0.680 ** | 0.514 ** | −0.435 ** | 0.385 ** | 0.476 ** | 0.586 ** |
| MBN | −0.243 ** | 0.449 ** | 0.332 ** | −0.243 ** | 0.272 ** | 0.267 ** | 0.362 ** |
| MBP | 0.12 | 0.163 * | 0.396 ** | −0.376 ** | 0.287 ** | 0.386 ** | 0.448 ** |
| DOC/TDN | −0.134 | −0.097 | −0.158 * | 0.122 | −0.133 | −0.14 | −0.176 * |
| DOC/ap | −0.214 ** | 0.135 | −0.11 | 0.177 * | −0.06 | −0.178 * | −0.157 * |
| BC:P | 0.129 | 0.500 ** | 0.158 * | –0.066 | 0.152 * | 0.096 | 0.152 * |
| BC:N | 0.640 ** | 0.199 ** | 0.192 ** | −0.215 ** | 0.125 | 0.239 ** | 0.232 ** |
| BN:P | −0.315 ** | 0.210 ** | −0.023 | 0.112 | 0.017 | −0.098 | −0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shahbaz, M.; Zhran, M.; Chen, A.; Zhu, Z.; Galal, Y.G.M.; Ge, T.; Li, Y. Microbial Resource Limitation in Aggregates in Karst and Non-Karst Soils. Agronomy 2021, 11, 1591. https://doi.org/10.3390/agronomy11081591
Wang Y, Shahbaz M, Zhran M, Chen A, Zhu Z, Galal YGM, Ge T, Li Y. Microbial Resource Limitation in Aggregates in Karst and Non-Karst Soils. Agronomy. 2021; 11(8):1591. https://doi.org/10.3390/agronomy11081591
Chicago/Turabian StyleWang, Yunqiu, Muhammad Shahbaz, Mostafa Zhran, Anlei Chen, Zhenke Zhu, Yehia Galal Mohamed Galal, Tida Ge, and Yuhong Li. 2021. "Microbial Resource Limitation in Aggregates in Karst and Non-Karst Soils" Agronomy 11, no. 8: 1591. https://doi.org/10.3390/agronomy11081591







