The Impact of Parthenium Weed-Amended Substrates on the Germination and Early Growth of a Range of Pasture and Crop Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Impact of Soil from a Parthenium Weed-Infested Area on Germination and Growth of Test Plant Seedlings (Experiment 1)
2.2. Impact of Parthenium Weed Leaf Litter on Test Plant Germination and Growth (Experiment 2)
3. Results
3.1. Impact of Soil from a Parthenium Weed-Infested Area on Germination and Growth of Test Plant Seedlings (Experiment 1)
3.2. The Impact of Parthenium Weed Leaf Litter on Germination and Growth of Test Plants (Experiment 2)
4. Discussion
4.1. Impact of Soil from a Parthenium Weed-Infested Area on Germination and Growth of Test Plant Seedlings (Experiment 1)
4.2. Impact of Parthenium Weed Leaf Litter on Test Plant Germination and Growth (Experiment 2)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Navie, S.; McFadyen, R.; Panetta, F.; Adkins, S.; Shepherd, R. A comparison of the growth and phenology of two introduced biotypes of Parthenium hysterophorus. In Proceedings of the 11th Australian Weeds Conference, Victoria, Australia, 30 September–3 October 1996; pp. 313–316. [Google Scholar]
- Shi, B.; Aslam, Z.; Adkins, S. The Invasive Potential of Parthenium weed: A Role for Allelopathy. In New Developments in Allelopathy Research; Price, J.E., Ed.; Nova Science Publishers: New York, NY, USA, 2015. [Google Scholar]
- Adkins, S.; Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar] [CrossRef]
- Chippendale, J.; Panetta, F. The cost of parthenium weed to the Queensland cattle industry. Plant Prot. Q. 1994, 9, 73–76. [Google Scholar]
- Navie, S.C.; McFadyen, R.E.; Panetta, F.D.; Adkins, S.W. The effect of CO2 enrichment on the growth of a C3 weed (Parthenium hysterophorus L.) and its competitive interaction with a C4 grass (Cenchrus ciliaris L.). Plant Prot. Q. 2005, 20, 61–66. [Google Scholar] [CrossRef]
- Belgeri, A.; Navie, S.C.; Vivian-Smith, G.; Adkins, S.W. Early recovery signs of an Australian grassland following the management of Parthenium hysterophorus L. Flora-Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed; Academic Press: Orlando, FL, USA; London, UK, 1984. [Google Scholar]
- Kanchan, S.D.; Jayachandra. Allelopathic effects of Parthenium hysterophorus L. I. exudation of inhibitors through roots. Plant Soil 1979, 53, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Adkins, S.; Sowerby, M. Allelopathic potential of the weed, Parthenium hysterophorus L., in Australia. Plant Prot. Q. 1996, 11, 20–23. [Google Scholar]
- Kanchan, S.D.; Jayachandra. Allelopathic effects of Parthenium hysterophorus L.: Part IV. identification of inhibitors. Plant Soil 1980, 55, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Pandey, D.K.; Palni, L.M.S.; Joshi, S.C. Growth, reproduction, and photosynthesis of ragweed parthenium (Parthenium hysterophorus). Weed Sci. 2003, 51, 191–201. [Google Scholar] [CrossRef]
- Kohli, R.K.; Batish, D.R.; Singh, H.P.; Dogra, K.S. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biol. Invasions 2006, 8, 1501–1510. [Google Scholar] [CrossRef]
- Joshi, B.; Pandey, N.; Rao, P. Allelopathic effect of weed species extracts on germination, growth and biochemical aspects in different varieties of wheat (Triticum aestivum L.). Indian J. Agric. Res. 2009, 43, 79–87. [Google Scholar]
- Pandey, D.K. Inhibition of salvinia (Salvinia molesta Mitchell) by parthenium (Parthenium hysterophorus L.). II. Relative effect of flower, leaf, stem, and root residue on salvinia and paddy. J. Chem. Ecol. 1994, 20, 3123–3131. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Channappagoudar, B.; Jalageri, B.; Biradar, N. Allelopathic effect of aqueous extracts of weed species on germination and seedling growth of some crops. Karnataka J. Agric. Sci. 2005, 18, 916–920. [Google Scholar]
- Khan, N.; Hashmatullah; Naveed, K.; Hussain, Z.; Khan, S. Assessment of allelopathic effects of parthenium (Parthenium hysterophorus L.) plant parts on seed germination and seedling growth of wheat (Triticum aestivum L.) cultivars. Pak. J. Weed Sci. Res. 2012, 18, 39–50. [Google Scholar]
- Wakjira, M.; Berecha, G.; Tulu, S. Allelopathic effects of an invasive alien weed Parthenium hysterophorus L. compost on lettuce germination and growth. Afr. J. Agric. Res. 2009, 4, 1325–1330. [Google Scholar]
- Gupta, S.; Narayan, R. Effects of applied leaf biomass of Parthenium hysterophorus, Cassia obtusifolia and Achyranthes aspera on seed germination and seedling growth of wheat and pea. Allelopath. J. 2010, 26, 59–70. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Pandher, J.K.; Kohli, R.K. Phytotoxic effects of Parthenium hysterophorus residues on three Brassica species. Weed Biol. Manag. 2005, 5, 105–109. [Google Scholar] [CrossRef]
- Safdar, M.E.; Tanveer, A.; Khaliq, A.; Naeem, M.S. Allelopathic action of parthenium and its rhizospheric soil on maize as influenced by growing conditions. Planta Daninha 2014, 32, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Timsina, B.; Shrestha, B.B.; Rokaya, M.B.; Münzbergová, Z. Impact of Parthenium hysterophorus L. invasion on plant species composition and soil properties of grassland communities in Nepal. Flora 2011, 206, 233–240. [Google Scholar] [CrossRef]
- Belz, R.G.; van der Laan, M.; Reinhardt, C.F.; Hurle, K. SoiL degradation of parthenin-does it contradict the role of allelopathy in the invasive weed Parthenium hysterophorus L.? J. Chem. Ecol. 2009, 35, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Shi, B. Invasive Potential of the Weed Parthenium Hysterophorus: The Role of Allelopathy; The University of Queensland: Brisbane, Australia, 2016. [Google Scholar]
- Ramamurthy, S.K.; Sridhar, C. Isolation of Stigmasterol from Parthenium hysterophorous plant. Pharma Innov. J. 2018, 7, 149–151. [Google Scholar]
- Mersie, W.; Singh, M. Effects of phenolic acids and ragweed parthenium (Parthenium hysterophorus) extracts on tomato (Lycopersicon esculentum) growth and nutrient and chlorophyll content. Weed Sci. 1988, 36, 278–281. [Google Scholar] [CrossRef]
- Heisey, R.M. Allelopathic and herbicidal effects of extracts from tree of heaven (Ailanthus altissima). Am. J. Bot. 1990, 77, 662–670. [Google Scholar] [CrossRef]
- Yaduraju, N.; Sushilkumar, P.B.M.; Gogoi, A. Parthenium hysterophorus–distribution, problem and management strategies in India. In Proceedings of the Second International Conference on Parthenium Management, University of Agricultural Sciences, Bangalore, India, 5–7 December 2005; pp. 6–10. [Google Scholar]
- Bashar, H.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Uddin, M.K.; Asib, N.; Anwar, M.; Rahaman, F. A Mystic Weed, Parthenium hysterophorus: Threats, Potentials and Management. Agronomy 2021, 11, 1514. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Weston, P.A.; Gurusinghe, S.; Latif, S.; Adkins, S.W.; Weston, L.A. Toxic potential and metabolic profiling of two australian biotypes of the invasive plant parthenium weed (Parthenium hysterophorus L.). Toxins 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Mersie, W.; Singh, M. Allelopathic effect of parthenium (Parthenium hysterophorus L.) extract and residue on some agronomic crops and weeds. J. Chem. Ecol. 1987, 13, 1739–1747. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Akinsanmi, O.A.; Lim, L.S.; Perrett, C.; Callander, J.; Dhileepan, K. Parthenium hysterophorus L. (Asteraceae) invasion had limited impact on major soil nutrients and enzyme activity: Is the null effect real or reflects data insensitivity? Plant Soil 2017, 420, 177–194. [Google Scholar] [CrossRef]
- Ojija, F.; Manyanza, N.M. Distribution and Impact of Invasive Parthenium hysterophorus on Soil Around Arusha National Park, Tanzania. Ecol. Evol. Biol. 2021, 6, 21–27. [Google Scholar]
- Etana, A.; Kelbessa, E.; Soromessa, T. Impact of Parthenium hysterophorus L. (Asteraceae) on soil chemical properties and its distribution in a reserve area: A case study in Awash National Park (ANP), Ethiopia. J. Soil Sci. Environ. Manag. 2015, 6, 116–124. [Google Scholar]
- Srivastava, P.; Raghubanshi, A. Impact of Parthenium hysterophorus L. invasion on soil nitrogen dynamics of grassland vegetation of Indo-Gangetic plains, India. Environ. Monit. Assess. 2021, 193, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Biradar, D.; Shivakumar, K.; Prakash, S.; Pujar, B. Bionutrient potentiality of Parthenium hysterophorus and its utility as green manure in rice ecosystem. Karnataka J. Agric. Sci. 2006, 19, 256–263. [Google Scholar]
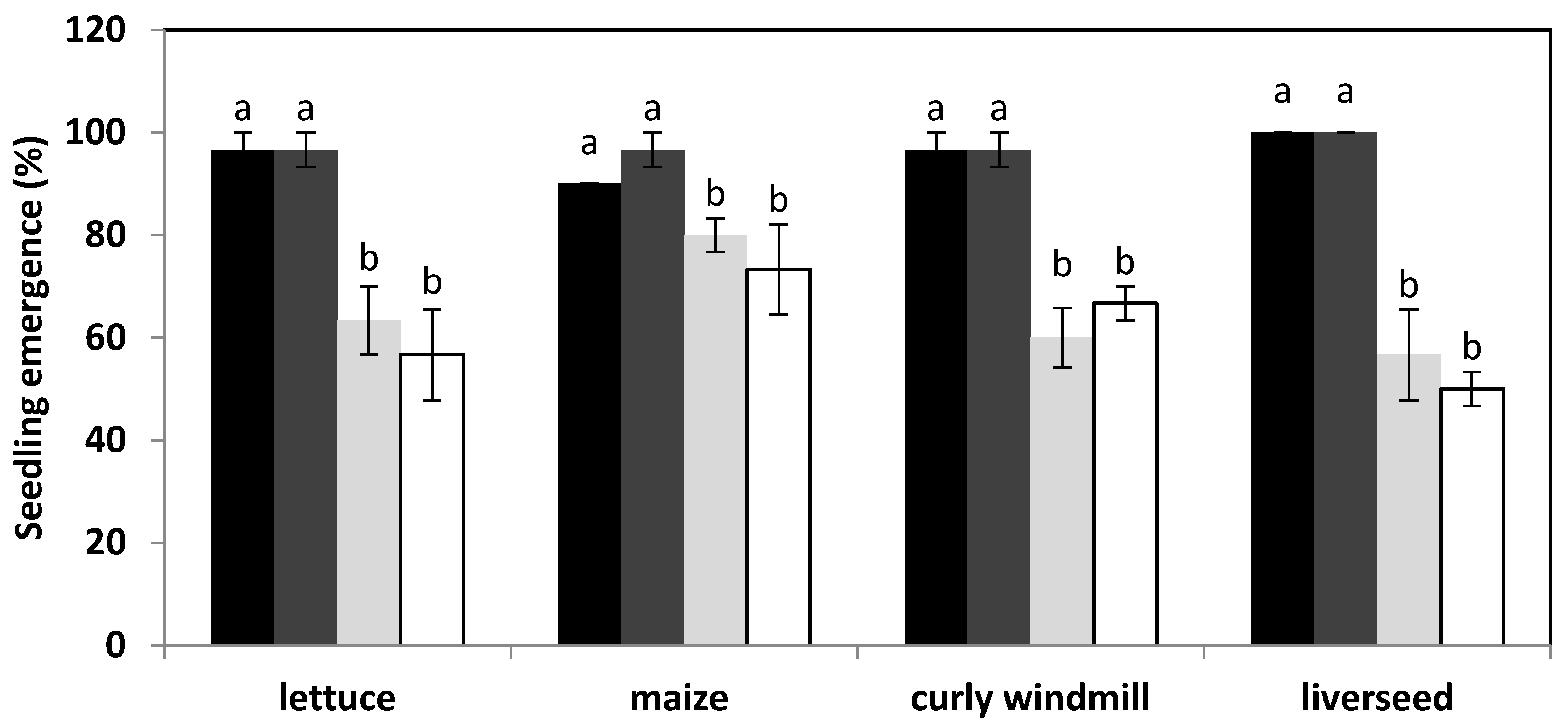
 ), maize (
), maize (  ), curly windmill grass (
), curly windmill grass (  ), and liverseed grass (
), and liverseed grass (  ) when sown into compost amended with different amounts of parthenium weed leaf litter (either 0, 1, 2, or 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
) when sown into compost amended with different amounts of parthenium weed leaf litter (either 0, 1, 2, or 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
 ), maize (
), maize (  ), curly windmill grass (
), curly windmill grass (  ), and liverseed grass (
), and liverseed grass (  ) when sown into compost amended with different amounts of parthenium weed leaf litter (either 0, 1, 2, or 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
) when sown into compost amended with different amounts of parthenium weed leaf litter (either 0, 1, 2, or 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.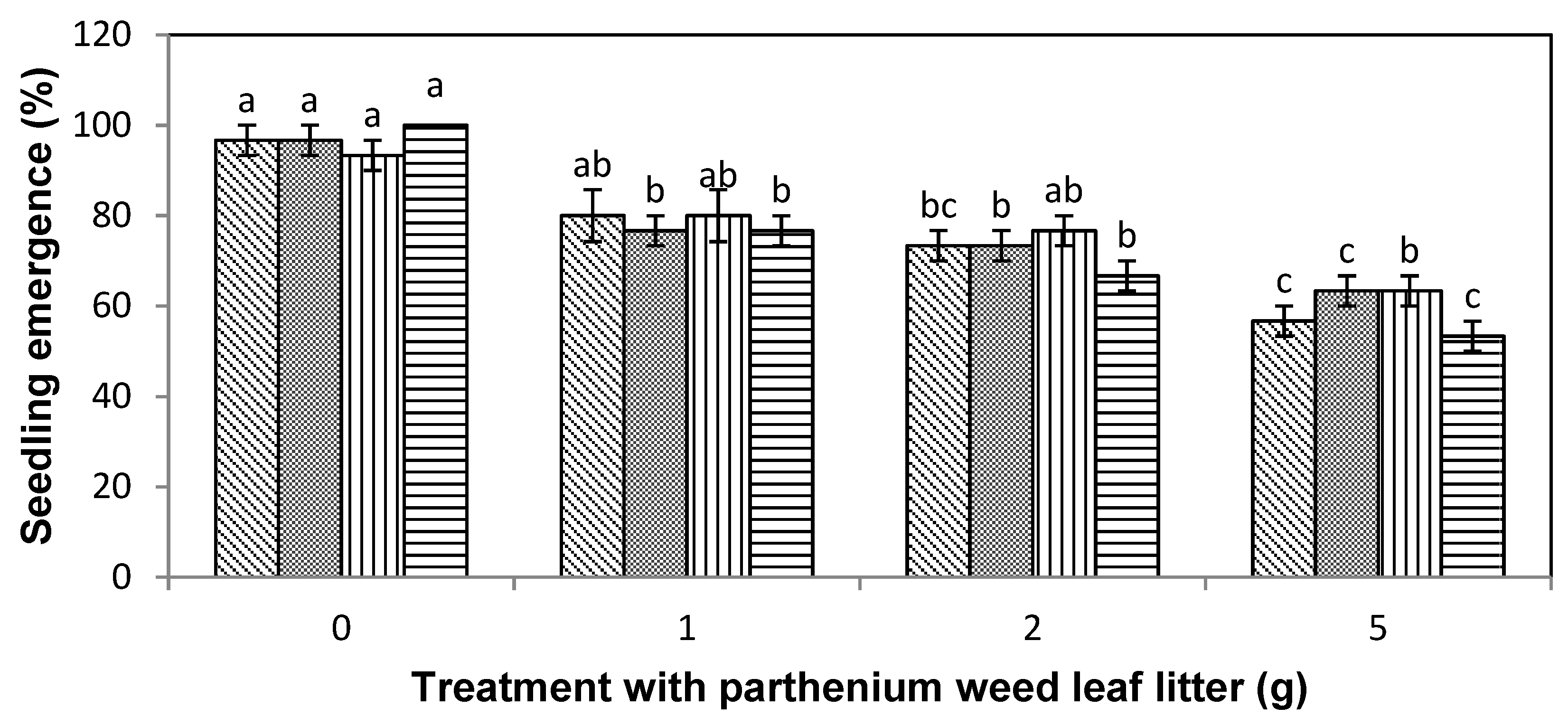
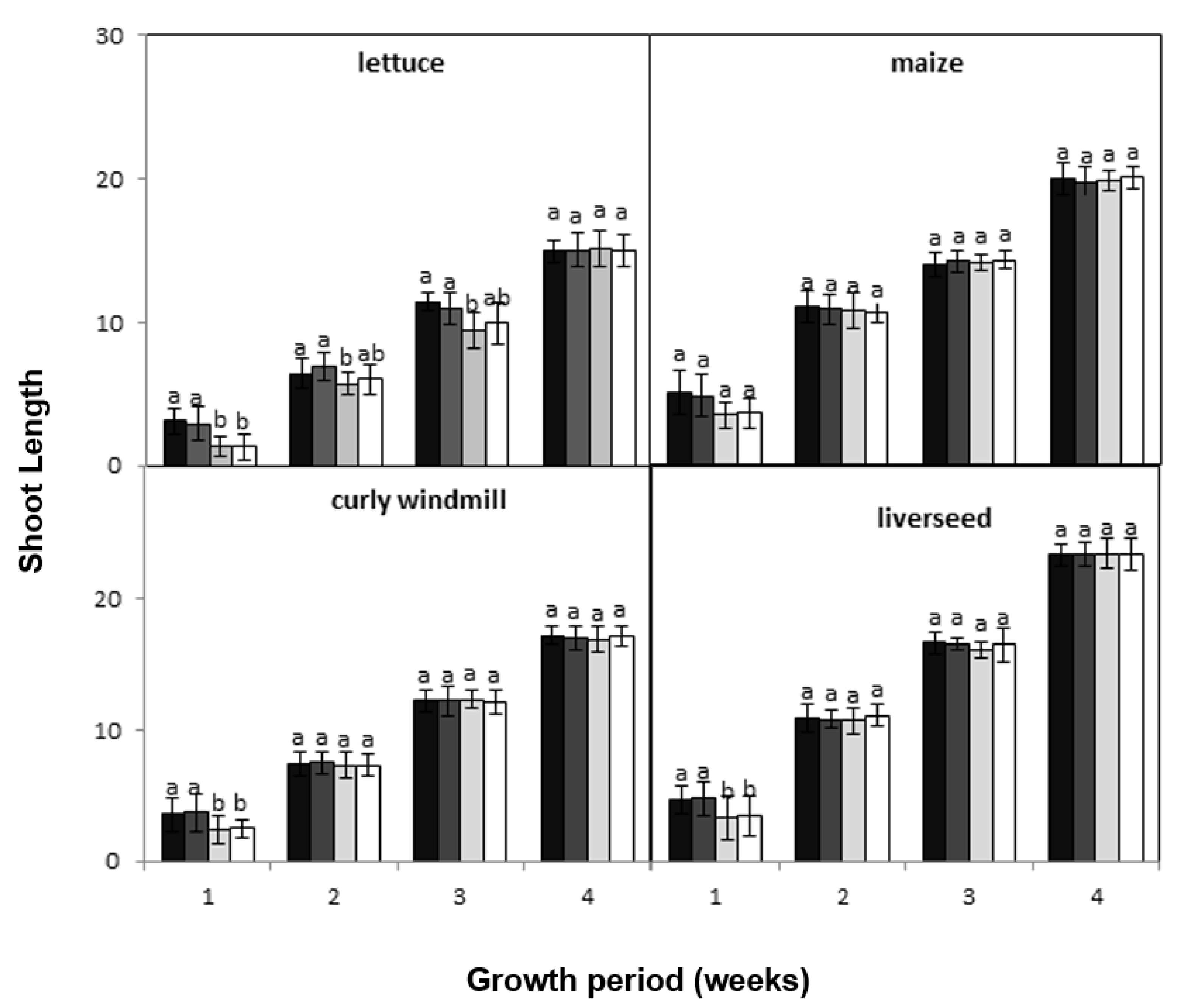
 ), lettuce (
), lettuce (  ), curly windmill grass (
), curly windmill grass (  ), and liverseed grass (
), and liverseed grass (  ) when sown into compost amended with different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
) when sown into compost amended with different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
 ), lettuce (
), lettuce (  ), curly windmill grass (
), curly windmill grass (  ), and liverseed grass (
), and liverseed grass (  ) when sown into compost amended with different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
) when sown into compost amended with different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.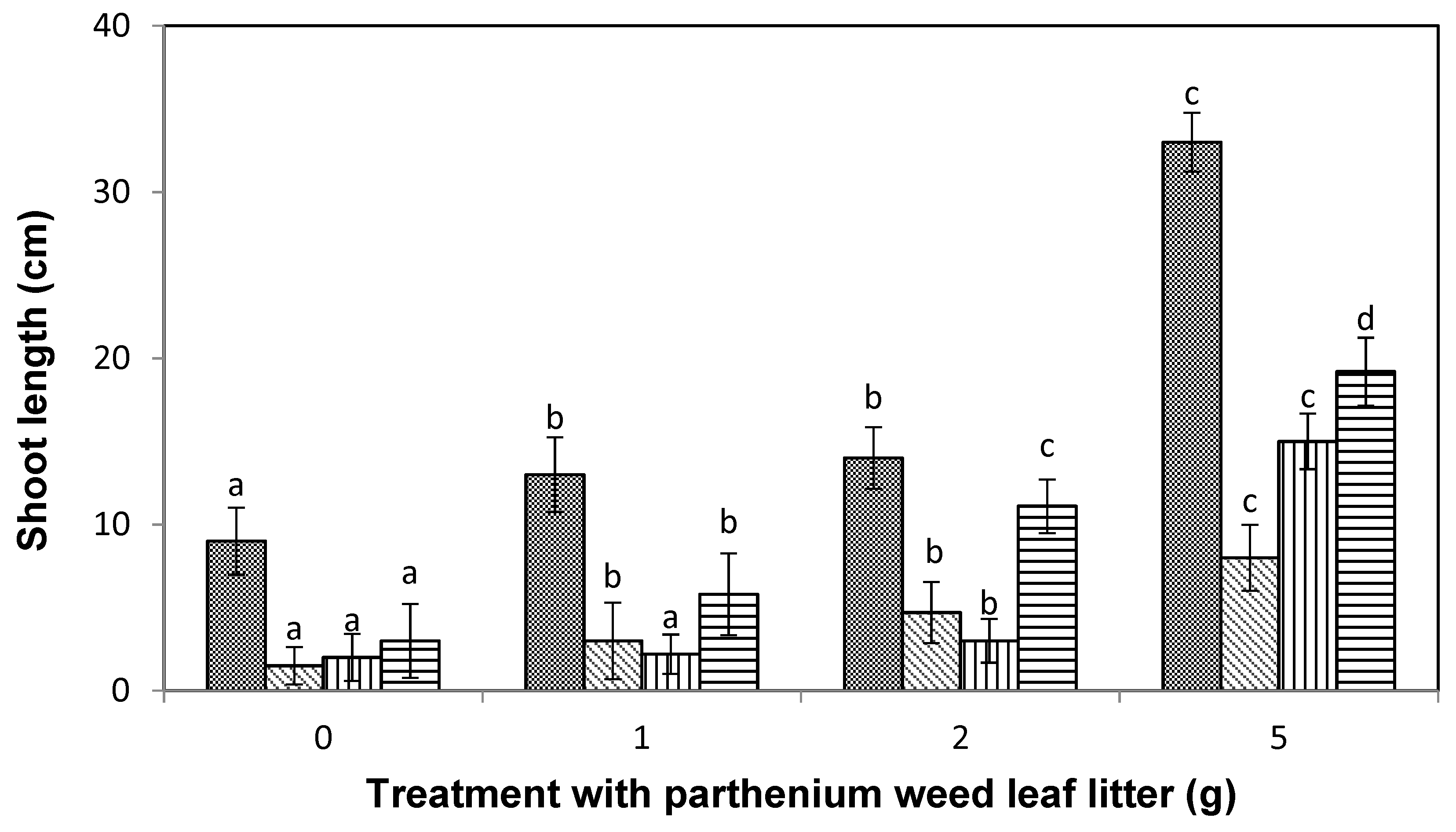
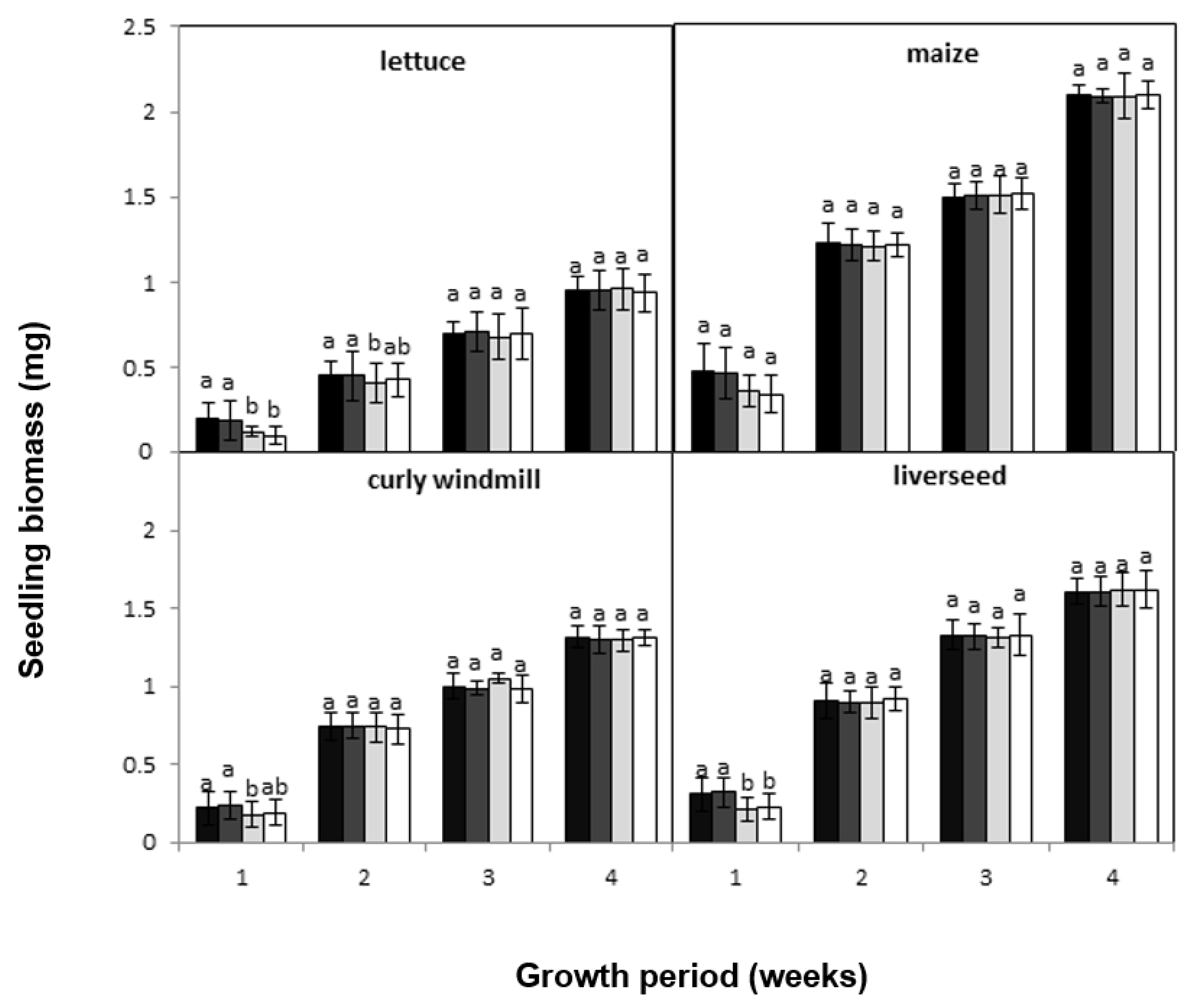
 ), lettuce (
), lettuce (  ), curly windmill grass (
), curly windmill grass (  ), and liverseed grass (
), and liverseed grass (  ) when they were sown into compost amended different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
) when they were sown into compost amended different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
 ), lettuce (
), lettuce (  ), curly windmill grass (
), curly windmill grass (  ), and liverseed grass (
), and liverseed grass (  ) when they were sown into compost amended different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.
) when they were sown into compost amended different amounts of parthenium weed leaf litter (0, 1, 2, and 5 g) for 30 days. Error bars represent two standard deviations from the mean as calculated for three replicates of three seedlings. Means within each treatment that do not share the same letter are significantly different from one species to another at p > 0.05.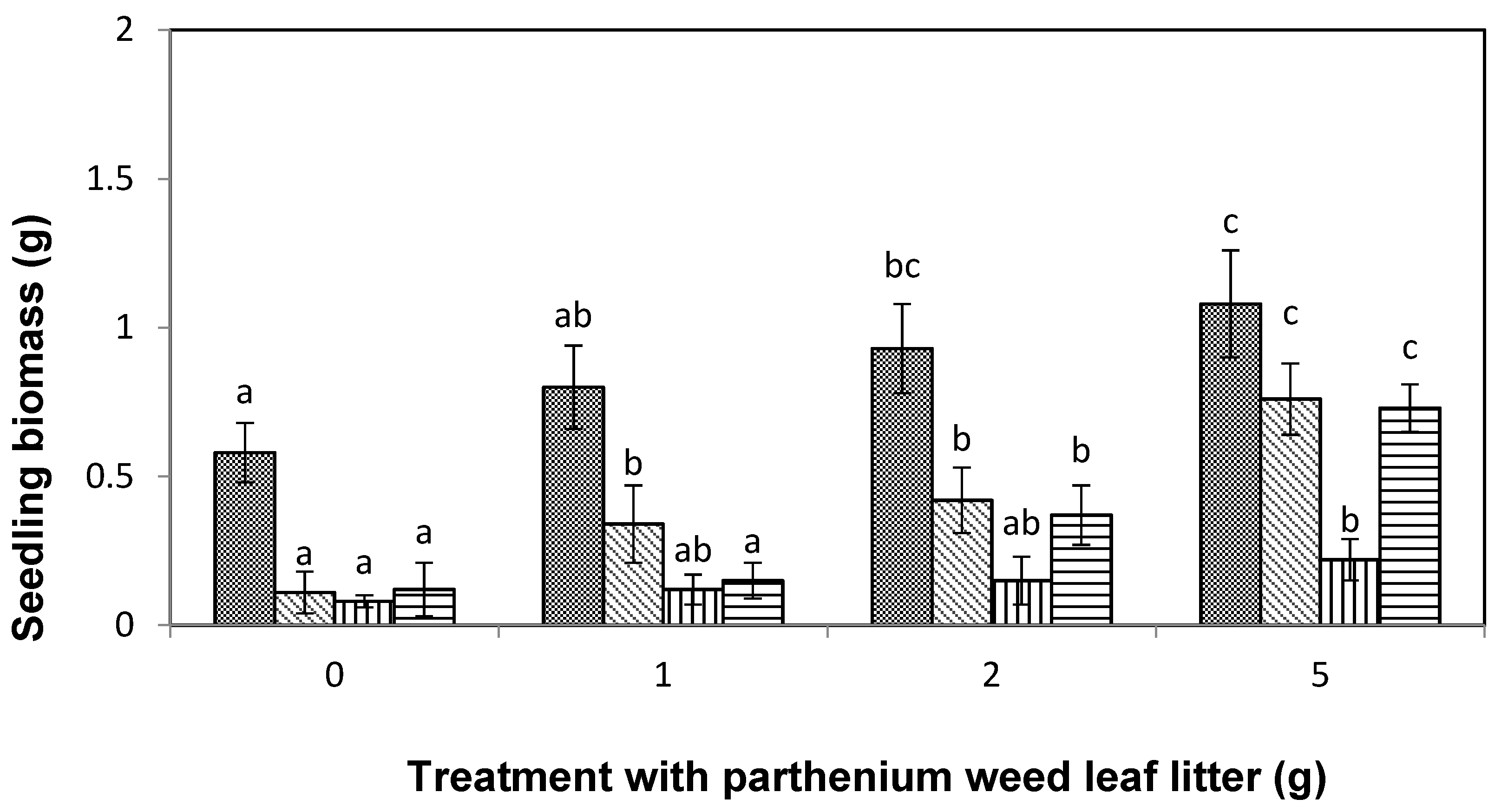
| Lettuce | Maize | Curly Windmill | Lambs Quarter | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment (g) | C (%) | N (%) | C (%) | N (%) | C (%) | N (%) | C (%) | N (%) |
| 0 | 23.80 ± 1.65 a | 0.12 ± 0.01 a | 27.10 ± 1.72 a | 0.14 ± 0.01 a | 24.10 ± 1.86 a | 0.11 ± 0.03 a | 25.20 ± 1.66 a | 0.13 ± 0.01 a |
| 1 | 27.20 ± 1.65 ab | 0.15 ± 0.02 ab | 25.70 ± 3.80 a | 0.13 ± 0.02 a | 26.80 ± 2.79 ab | 0.13 ± 0.05 ab | 27.20 ± 1.58 ab | 0.14 ± 0.03 ab |
| 2 | 29.40 ± 0.91 ab | 0.16 ± 0.11 ab | 29.8 ± 3.90 a | 0.16 ± 0.02 a | 29.70 ±1.03 ab | 0.16 ± 0.01 ab | 29.2 ± 0.86 ab | 0.17 ± 0.03 ab |
| 5 | 32.60 ± 3.11 b | 0.22 ± 0.03 b | 29.60 + 5.57 a | 0.18 ± 0.03 a | 31.40 ± 1.96 b | 0.18 ± 0.06 b | 30.80 + 2.72 b | 0.21 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Dhileepan, K.; Adkins, S. The Impact of Parthenium Weed-Amended Substrates on the Germination and Early Growth of a Range of Pasture and Crop Species. Agronomy 2021, 11, 1708. https://doi.org/10.3390/agronomy11091708
Shi B, Dhileepan K, Adkins S. The Impact of Parthenium Weed-Amended Substrates on the Germination and Early Growth of a Range of Pasture and Crop Species. Agronomy. 2021; 11(9):1708. https://doi.org/10.3390/agronomy11091708
Chicago/Turabian StyleShi, Boyang, Kunjithapatham Dhileepan, and Steve Adkins. 2021. "The Impact of Parthenium Weed-Amended Substrates on the Germination and Early Growth of a Range of Pasture and Crop Species" Agronomy 11, no. 9: 1708. https://doi.org/10.3390/agronomy11091708
APA StyleShi, B., Dhileepan, K., & Adkins, S. (2021). The Impact of Parthenium Weed-Amended Substrates on the Germination and Early Growth of a Range of Pasture and Crop Species. Agronomy, 11(9), 1708. https://doi.org/10.3390/agronomy11091708






