Analysis of the Variability of Floral and Pollen Traits in Apple Cultivars—Selecting Suitable Pollen Donors for Cider Apple Orchards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Plant Material
2.2. Flowering Dates, Flowering Duration and Bloom Synchronization
2.3. Flowering Intensity and Pollen Production
2.4. Evaluation of Pollen Quality
2.5. Alternate Bearing Tendency and Its Influence on Pollen Properties
2.6. Statistical Analysis
3. Results
3.1. Phenological Observations, Duration of Flowering and Flowering Overlap between Cultivars
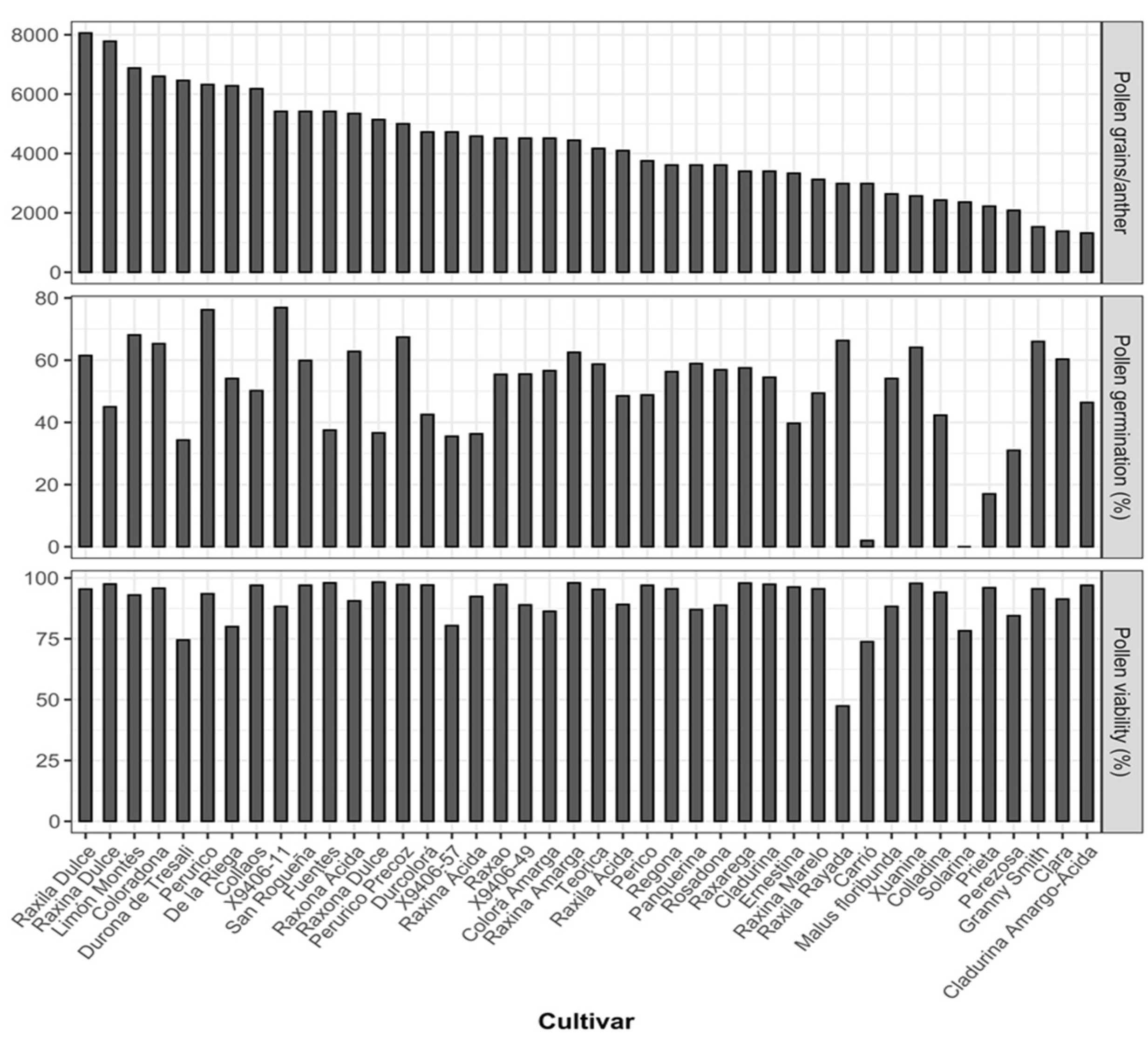
3.2. Phenotypic Evaluation of Floral and Pollen Traits
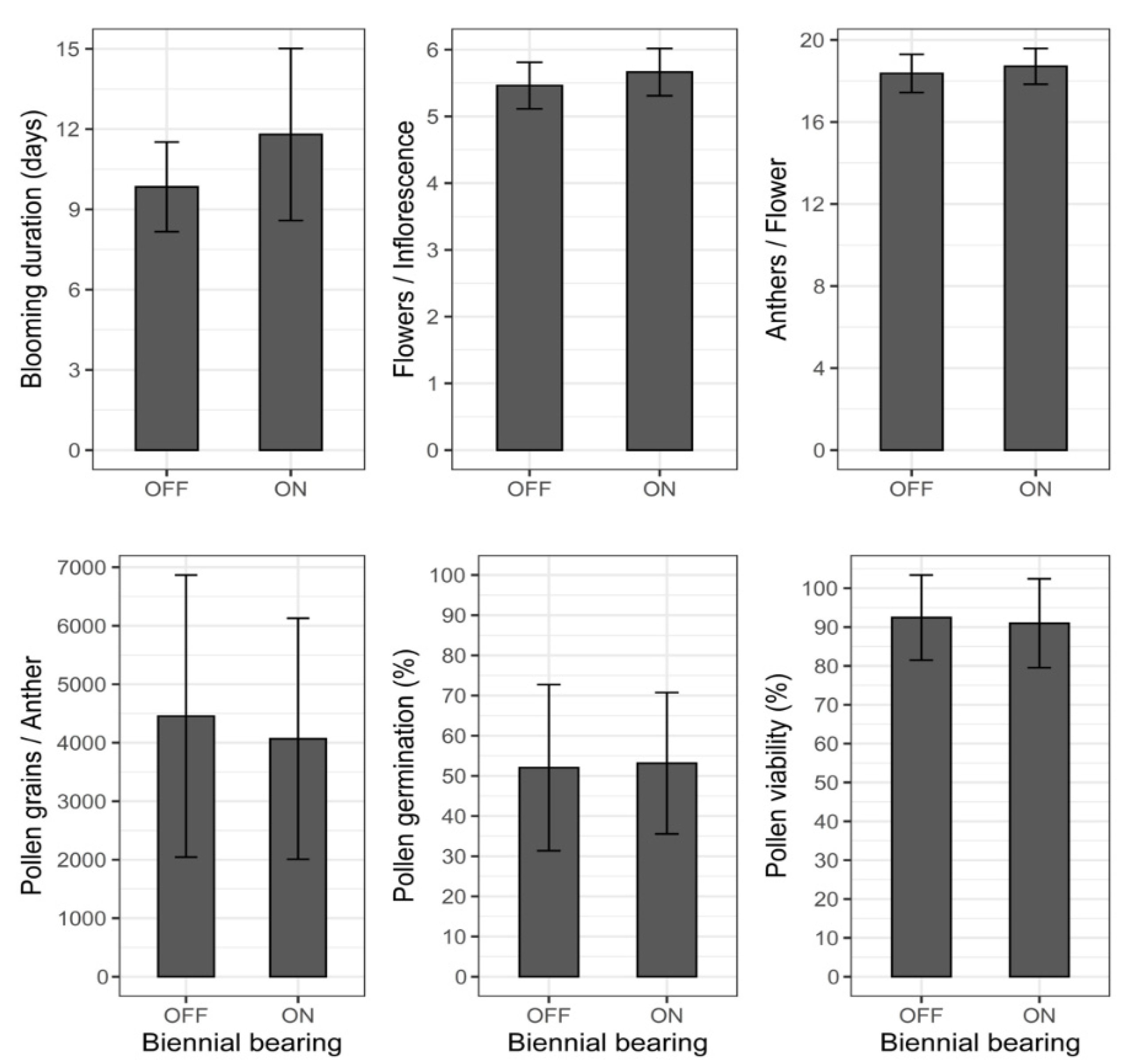
3.3. Effects of Biennial Bearing on Floral and Pollen Traits
3.4. Correlations between Floral and Pollen Traits
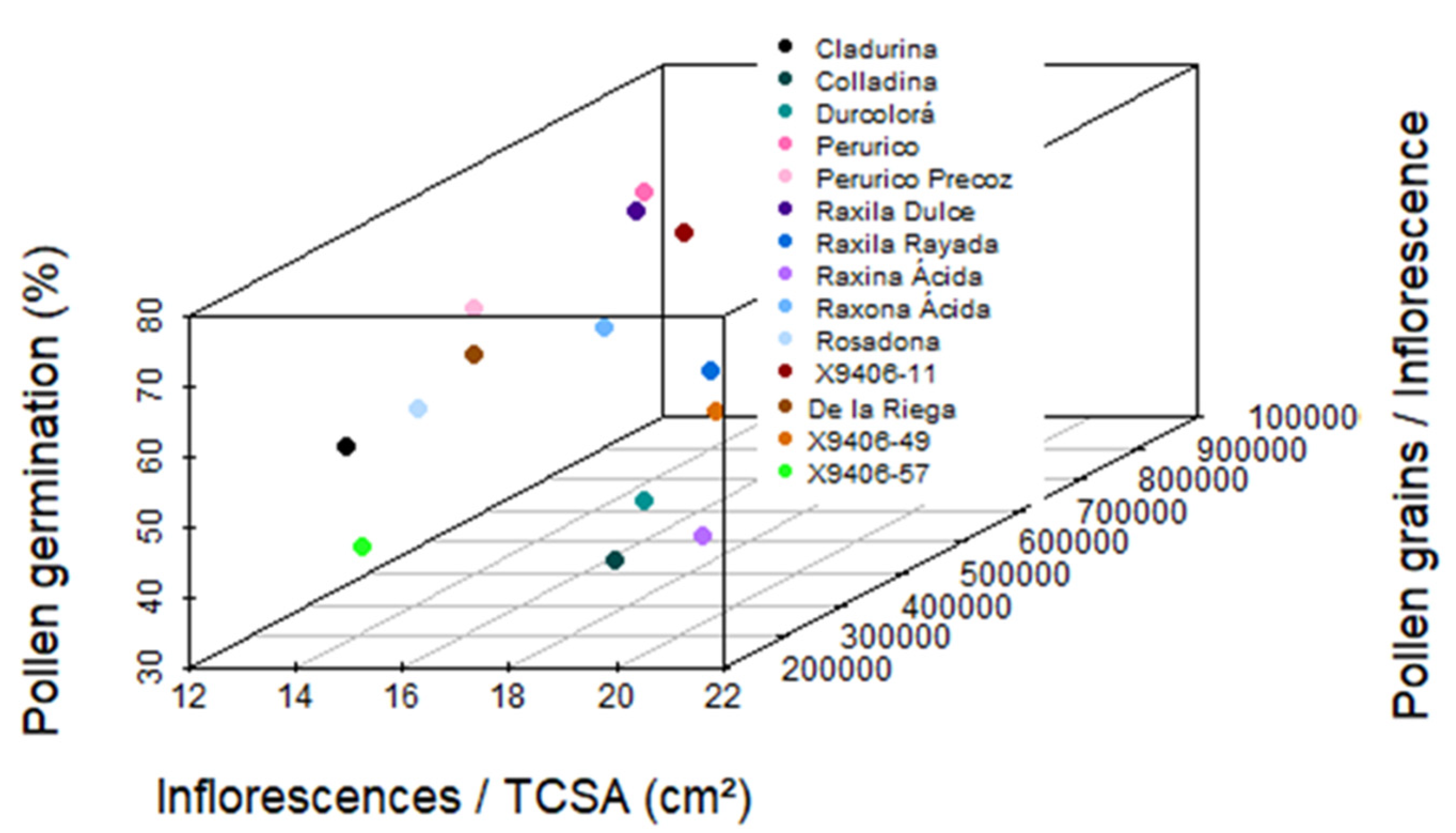
3.5. Evaluation of Apple Cultivars as Pollen Donors in Apple Orchards
4. Discussion
4.1. Floral and Pollen Characteristics of Local Apple Cultivars
4.2. Evaluation of Cultivar Suitability as Pollen Donors for the Local Apple Industry
4.3. Correlations between Floral and Pollen Traits and Implications for Fruit Breeding Programs
4.4. Floral Biology Traits and Quality of Pollen Grains Affected by ‘On’ and ‘Off’ Years
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, A.G. Flower and fruit abortion: Proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Broothaerts, W. New findings in apple S-genotype analysis resolve previous confusion and request the re-numbering of some S-alleles. Theor. Appl. Genet. 2003, 106, 703–714. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Mayer, D.R.; Mayer, D.F. Crop Pollination by Bees; CABI: New York, NY, USA, 2000. [Google Scholar]
- Ramírez, F.; Davenport, T.L. Apple pollination: A review. Sci. Hortic. 2013, 162, 188–203. [Google Scholar] [CrossRef]
- Garratt, M.; Coston, D.; Truslove, C.; Lappage, M.; Polce, C.; Dean, R.; Biesmeijer, J.; Potts, S. The identity of crop pollinators helps target conservation for improved ecosystem services. Biol. Conserv. 2014, 169, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Fountain, M.T.; Mateos-Fierro, Z.; Shaw, B.; Brain, P.; Delgado, A. Insect pollinators of conference pear (Pyrus communis L.) and their contribution to fruit quality. J. Pollinat. Ecol. 2019, 25, 103–114. [Google Scholar] [CrossRef]
- Brookfield, P.L.; Ferguson, I.B.; Watkins, C.B.; Bowen, J.H. Seed number and calcium concentrations of ‘Braeburn’ apple fruit. J. Hortic. Sci. 1996, 71, 265–271. [Google Scholar] [CrossRef]
- Matsumoto, S.; Soejima, J.; Maejima, T. Influence of repeated pollination on seed number and fruit shape of ‘Fuji’ apples. Sci. Hortic. 2012, 137, 131–137. [Google Scholar] [CrossRef]
- Buccheri, M.; di Vaio, C. Relationship among seed number, quality, and calcium content in apple fruits. J. Plant Nutr. 2005, 27, 1735–1746. [Google Scholar] [CrossRef]
- Kendall, D.A.; Solomon, M.E. Quantities of pollen on the bodies of insects visiting apple blossom. J. Appl. Ecol. 1973, 10, 627. [Google Scholar] [CrossRef]
- Carisio, L.; Díaz, S.S.; Ponso, S.; Manino, A.; Porporato, M. Effects of pollinizer density and apple tree position on pollination efficiency in cv. Gala. Sci. Hortic. 2020, 273, 109629. [Google Scholar] [CrossRef]
- Quinet, M.; Jacquemart, A.-L. Cultivar placement affects pollination efficiency and fruit production in European pear (Pyrus communis) orchards. Eur. J. Agron. 2017, 91, 84–92. [Google Scholar] [CrossRef]
- Costes, E.; Gion, J.M. Genetics and genomics of tree architecture. Adv. Bot. Res. 2015, 74, 157–200. [Google Scholar] [CrossRef]
- Caporali, S.; Paoletti, A.; Rosati, A. Floral biology: Implications for fruit characteristics and yield. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; InTechOpen: London, UK, 2012; pp. 71–80. [Google Scholar]
- Cruden, R.W. Pollen grains: Why so many? Plant Syst. Evol. 2000, 222, 143–165. [Google Scholar] [CrossRef]
- Jackson, J.E. The Biology of Apples and Pears; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Larsen, P.; Tung, S.M. Growth-promoting and growth-retarding substances in pollen from diploid and triploid apple varieties. Int. J. Plant Sci. 1950, 111, 436–447. [Google Scholar] [CrossRef]
- Petrisor, C.; Mitre, V.; Mitre, I.; Jantschi, L.; Balan, M.C. The rate of pollen germination and the pollen viability at ten apple cultivars in the climatic conditions of Transylvania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2012, 69, 467468. [Google Scholar]
- Dapena, E.; Blázquez, M.; Ramos, M. Recursos fitogenéticos de manzano de sidra y de mesa. Tecnol. Agroaliment. Boletín Inf. SERIDA 2015, 15, 20–26. [Google Scholar]
- Martínez-Sastre, R.; Miñarro, M.; García, D. Animal biodiversity in cider apple orchards: Simultaneous environmental drivers and effects on insectivory and pollination. Agric. Ecosyst. Environ. 2020, 295, 106918. [Google Scholar] [CrossRef]
- Quinet, M.; Warzée, M.; Vanderplanck, M.; Michez, D.; Lognay, G.; Jacquemart, A.-L. Do floral resources influence pollination rates and subsequent fruit set in pear (Pyrus communis L.) and apple (Malus x domestica Borkh) cultivars? Eur. J. Agron. 2016, 77, 59–69. [Google Scholar] [CrossRef]
- Dapena, E.; Blázquez, M. Descripción de Las Variedades de Manzana de La DOP Sidra de Asturias; SERIDA: Asturias, Spain, 2009. [Google Scholar]
- Dapena, E. Comportamiento Agronómico y Tecnológico de Variedades de Manzano Asturianas. Ph.D. Thesis, Universidad de Oviedo, Asturias, Spain, 1996. [Google Scholar]
- Dennis, F.G.; Neilsen, J.C. Physiological factors affecting biennial bearing in tree fruit: The role of seeds in apple. HortTechnology 1999, 9, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Hanke, M.V.; Flachowsky, H.; Peil, A.; Hättasch, C. No flower no fruit—Genetic potentials to trigger flowering in fruit trees. Genes Genomes Genom. 2007, 1, 1–20. [Google Scholar]
- Schwallier, P.G.; Sabbatini, P.; Bukovac, M.J. Observations on the relationship between crop load and return bloom in ‘honeycrisp’ apple. HortScience 2006, 41, 1010B-1010. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, A.; Palasciano, M.; Gallotta, A.; Camposeo, S.; Pacifico, A.; Ferrara, G. Amount and quality of pollen grains in four olive (Olea europaea L.) cultivars as affected by ‘on’ and ‘off’ years. Sci. Hortic. 2014, 170, 89–93. [Google Scholar] [CrossRef]
- Meier, U.; Graf, H.; Hack, H.; Hess, M.; Kennel, W.; Klose, R.; Mappes, D.; Seipp, D.; Stauss, R.; Streif, J. Phanologische Entwicklungsstadien Des Kernobstes (Malus Domestica Borkh. Und Pyrus Communis, L.), Des Steinobstes (Prunus-Arten), Der Johannisbeere Ribes-Arten) Und Der Erdbeere (Fragaria × Ananassa). Nachr. Dtsch. Pflanzenschutzd. 1994, 46, 141–153. [Google Scholar]
- Westwood, M.N. Temperate-Zone Pomology; Timber Press: Portland, OR, USA, 1988. [Google Scholar]
- Church, R.M.; Williams, R.; Andrews, L. Comparison of flowering dates and pollen release characteristics of several malus cultivars used as pollinators for cox’s orange pippin apple. J. Hortic. Sci. 1983, 58, 349–353. [Google Scholar] [CrossRef]
- Westwood, M.; Reimer, F.; Quackenbush, V. Long term yield as related to ultimate tree size of three pear varieties grown on rootstocks of five Pyrus species. Proc. Amer. Soc. Hort. Sci. 1963, 82, 103–113. [Google Scholar]
- Bieniasz, M.; Necas, T.; Dziedzic, E.; Ondrasek, I.; Pawłowska, B. Evaluation of pollen quality and self-fertility in selected cultivars of asian and european pears. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Distefano, G.; Hedhly, A.; Casas, G.L.; La Malfa, S.; Herrero, M.; Gentile, A. Male—Female interaction and temperature variation affect pollen performance in citrus. Sci. Hortic. 2012, 140, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lauri, P.-E.; Willaume, M.; Larrive, G.; Lespinasse, J.-M. THE concept of centrifugal training in apple aimed at optimizing the relationship between growth and fruiting. Acta Hortic. 2004, 636, 35–42. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 20 April 2021).
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Soltesz, M. Laws of bloom phenology by apple. Acta Hortic. 1997, 437, 451–456. [Google Scholar] [CrossRef]
- Bist, H.; Sharma, S. Studies on the pollen, stigma receptivity and pollination in low chilling cultivars of apple (M. domestica Borkh.). Himal. J. Agric. Res. 1986, 12, 25–32. [Google Scholar]
- Blasse, W.; Hofmann, S. Phenological Studies with Apple Cultivars; Gartenbau: Kornwestheim, Germany, 1991. [Google Scholar]
- Pandit, B.A. Pollen Compatibility Studies of Some Exotic Apple Cultivars. Ph.D. Thesis, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Kashmir, India, 2014. [Google Scholar]
- Church, R.M.; Williams, R.R. Comparison of flower numbers and pollen production of several dessert apple and ornamental Malus cultivars. J. Hortic. Sci. 1983, 58, 327–336. [Google Scholar] [CrossRef]
- Delgado, A.; Egea, J.A.; Luedeling, E.; Dapena, E. Agroclimatic requirements and phenological responses to climate change of local apple cultivars in northwestern Spain. Sci. Hortic. 2021, 283, 110093. [Google Scholar] [CrossRef]
- Delgado, A.; Dapena, E.; Fernandez, E.; Luedeling, E. Climatic requirements during dormancy in apple trees from Northwestern Spain—Global warming may threaten the cultivation of high-chill cultivars. Eur. J. Agron. 2021, 130, 126374. [Google Scholar] [CrossRef]
- Rojo, J.; Salido, P.; Pérez-Badia, R. Flower and pollen production in the ‘Cornicabra’ olive (Olea europaea L.) cultivar and the influence of environmental factors. Trees 2015, 29, 1235–1245. [Google Scholar] [CrossRef]
- Gyan, K.Y.; Woodell, S.R.J. Analysis of insect pollen loads and pollination efficiency of some common insect visitors of four species of woody Rosaceae. Funct. Ecol. 1987, 1, 269. [Google Scholar] [CrossRef]
- Goodell, K.; Thomson, J.D. Comparisons of pollen removal and deposition by honey bees and bumblebees visiting apple. Acta Hortic. 1997, 437, 103–108. [Google Scholar] [CrossRef]
- Dixin, Z.S.X.W.C.; Fuyong, G. The differences of pollen content and pollen germination and tube growth among eight species of fruit trees. Acta Agric. Shanghai 2003, 19, 67–69. [Google Scholar]
- Atasay, A.; Akgül, H.; Ucgun, K.; Şan, B. Nitrogen fertilization affected the pollen production and quality in apple cultivars “Jerseymac” and “Golden Delicious”. Acta Agric. Scand. Sect. B—Plant Soil Sci. 2013, 63, 460–465. [Google Scholar] [CrossRef]
- Santos, G.A.; Batugal, P.; Othman, A.; Baudouin, L.; Labouisse, J.P. Manual on Standardized Research Techniques in Coconut Breeding; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 1996. [Google Scholar]
- Javid, R.; Rather, G. Functional pollen ability of different crab apples used as pollinizers for apple. J. Pharmacogn. Phytochem. 2019, 8, 617–620. [Google Scholar]
- De Albuquerque Junior, C.L.; Denardi, F.; Dantas, A.C.; Nodari, R.O. Número de anteras por flor, grãos de pólen por antera e capacidade germinativa do pólen de diferentes cultivares de macieiras. Rev. Bras. Frutic. 2010, 32, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Free, J.B. Insect Pollination of Crops; Academic Press: London, UK, 1993. [Google Scholar]
- Van Marrewijk, G. Flowering biology and hybrid varieties. In International Course on Applied Plant Breeding; International Agricultural Centre (IAC): Wageningen, The Netherlands, 1993; p. 66. [Google Scholar]
- Faust, M.; Erez, A.; Rowland, L.; Wang, S.; Norman, H. Bud dormancy in perennial fruit trees: Physiological basis for dormancy induction, maintenance, and release. HortScience 1997, 32, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Campoy, J.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Egea, J.; Ortega, E.; Martinez-Gomez, P.; Dicenta, F. Chilling and heat requirements of almond cultivars for flowering. Environ. Exp. Bot. 2003, 50, 79–85. [Google Scholar] [CrossRef]
- Petri, J.; Leite, G. Consequences of insufficient winter chilling on apple tree bud-break. Acta Hortic. 2004, 662, 53–60. [Google Scholar] [CrossRef]
- Sunley, R.J.; Atkinson, C.J.; Jones, H.G. Chill unit models and recent changes in the occurrence of winter chill and spring frost in the United Kingdom. J. Hortic. Sci. Biotechnol. 2006, 81, 949–958. [Google Scholar] [CrossRef]
- Losada, J.; Herrero, M. The influence of the progamic phase for fruiting in the apple tree. Ann. Appl. Biol. 2013, 163, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Free, J.B. The effect of distance from pollinizer varieties on the fruit set on trees in plum and apple orchards. J. Hortic. Sci. 1962, 37, 262–271. [Google Scholar] [CrossRef]
- Sáez, A.; di Virgilio, A.; Tiribelli, F.; Geslin, B. Simulation models to predict pollination success in apple orchards: A useful tool to test management practices. Apidologie 2018, 49, 551–561. [Google Scholar] [CrossRef] [Green Version]
- Didelot, F.; Brun, L.; Parisi, L. Effects of cultivar mixtures on scab control in apple orchards. Plant Pathol. 2007, 56, 1014–1022. [Google Scholar] [CrossRef]
- Janzen, D.H. A note on optimal mate selection by plants. Am. Nat. 1977, 111, 365–371. [Google Scholar] [CrossRef]
- Quinet, M.; Kelecom, S.; Raspé, O.; Jacquemart, A.-L. S-genotype characterization of 13 North Western European pear (Pyrus communis) cultivars. Sci. Hortic. 2014, 165, 1–4. [Google Scholar] [CrossRef]
- Broothaerts, W.; van Nerum, I.; Keulemans, J. Update on and review of the incompatibility (S-) genotypes of apple cultivars. HortScience 2004, 39, 943–947. [Google Scholar] [CrossRef] [Green Version]
- Sedov, E. Apple breeding programs and methods, their development and improvement. Russ. J. Genet. Appl. Res. 2014, 4, 43–51. [Google Scholar] [CrossRef]
- Lauri, P.E.; Corelli Grappadelli, L. Tree architecture, flowering and fruiting-thoughts on training, pruning and ecophysiology. In Proceedings of the X International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems, Stellenbosch, South Africa, 3–6 December 2012; Volume 1058, pp. 291–298. [Google Scholar]
- Ruiz, D.; Egea, J. Analysis of the variability and correlations of floral biology factors affecting fruit set in apricot in a Mediterranean climate. Sci. Hortic. 2008, 115, 154–163. [Google Scholar] [CrossRef]
- Gallotta, A.; Palasciano, M.; Mazzeo, A.; Ferrara, G. Pollen production and flower anomalies in apricot (Prunus armeniaca L.) cultivars. Sci. Hortic. 2014, 172, 199–205. [Google Scholar] [CrossRef]
- Methamem, S.; Gouta, H.; Mougou, A.; Bayoudh, C.; Boujnah, D.; Sihem, M.; Hassouna, G.; Atef, M.; Chokri, B.; Dalenda, B. Pollen ability and pollination in some olive (Olea europaea L.) cultivars in Tunisia as affected by ‘on ’and ‘off ’years. Res. Crop. 2015, 16, 675. [Google Scholar] [CrossRef]

| Cultivar | Flowering Date | Duration of Flowering | Inflorescences/TCSA (cm2) | Number of Flowers/Inflorescence | Number of Anthers/Flower |
|---|---|---|---|---|---|
| Blanquina | 28 April | 12 ± 2.83 | 4.88 ± 3.13 | 5.7 ± 0.59 | 18.4 ± 1.58 |
| Carrió | 2 May | 10.5 ± 0.71 | 5.22 ± 7.54 | 5.7 ± 0.54 | 19.05 ± 0.95 |
| Clara | 18 April | 11.5 ± 3.53 | 8.99 ± 2.20 | 6.08 ± 0.63 | 17.6 ± 1.43 |
| Collaos | 4 May | 10.5 ± 0.70 | 10.35 ± 4.50 | 5.46 ± 0.65 | 18.2 ± 1.16 |
| Cladurina | 17 April | 12.5 ± 2.12 | 13.2 ± 7.55 | 5.75 ± 0.63 | 18.15 ± 1.72 |
| Cladurina Amargo-Ácida | 15 April | 12.5 ± 0.71 | 8.3 ± 9.71 | 5.45 ± 0.69 | 18.55 ± 1.66 |
| Colladina | 7 May | 10.5 ± 0.70 | 19.2 ± 4.81 | 5.83 ± 0.66 | 19.1 ± 1.52 |
| Colorá Amarga | 13 April | 12 ± 2.83 | 7.9 ± 2.93 | 5.57 ± 0.50 | 18.6 ± 0.99 |
| Coloradona | 18 April | 11.5 ± 2.12 | 10.99 ± 2.47 | 5.97 ± 0.50 | 18.45 ± 1.32 |
| De la Riega | 1 May | 9.5 ± 3.53 | 12.27 ± 10.74 | 5.42 ± 0.76 | 19.65 ± 1.53 |
| Durcolorá | 18 April | 12.5 ± 1.41 | 17.7 ± 9.90 | 5.55 ± 0.69 | 17.3 ± 1.15 |
| Durona de Tresali | 8 May | 10.5 ± 0.71 | 4.45 ± 6.52 | 4.17 ± 0.77 | 19.67 ± 0.82 |
| Ernestina | 1 May | 8.5 ± 2.12 | 8.36 ± 9.89 | 5.52 ± 0.68 | 19.2 ± 1.55 |
| Fuentes | 6 May | 10 ± 0 | 5.93 ± 6.71 | 5.68 ± 0.69 | 19.25 ± 1.17 |
| Granny Smith | 17 April | 7.5 ± 0.71 | 6.18 ± 1.26 | 5.73 ± 0.51 | 19.15 ± 1.07 |
| Limón Montés | 13 May | 10 ± 0 | 6.78 ± 4.06 | 5.75 ± 0.58 | 19.05 ± 1.28 |
| Malus floribunda 821 | 24 March | 11 | 27.67 ± 17.62 | 7.15 ± 0.67 | 20.8 ± 1.48 |
| Meana | 10 May | 14 | 7.98 ± 4.69 | 5.7 ± 0.69 | 19.4 ± 1.18 |
| Panquerina | 2 May | 12 ± 2.82 | 8.25 ± 7.40 | 5.3 ± 0.77 | 19.1 ± 1.41 |
| Perezosa | 25 April | 9.5 ± 0.71 | 8.22 ± 7.27 | 5.8 ± 0.71 | 19.6 ± 1.05 |
| Perico | 11 May | 12.5 ± 2.12 | 9.20 ± 7.23 | 5.63 ± 0.54 | 18.75 ± 0.97 |
| Perurico | 26 April | 9 ± 1.41 | 15.21 ± 10.99 | 5.73 ± 0.66 | 18.8 ± 1.06 |
| Perurico Precoz | 27 April | 10.5 ± 4.95 | 13.95 ± 13.11 | 5.68 ± 0.63 | 17.85 ± 1.31 |
| Prieta | 13 May | 12 ± 2.83 | 10.00 ± 8.20 | 5.77 ± 0.50 | 18.55 ± 1.28 |
| Raxao | 20 May | 8.5 ± 2.12 | 7.8 ± 5.56 | 5 ± 0.58 | 19.15 ± 1.19 |
| Raxarega | 13 May | 12 ± 2.83 | 6.93 ± 3.81 | 5.43 ± 0.49 | 18.05 ± 1.15 |
| Raxila Ácida | 28 April | 9 ± 1.41 | 7.2 ± 4.02 | 5.95 ± 0.51 | 18.55 ± 1.36 |
| Raxila Dulce | 18 April | 12.5 ± 2.12 | 12.09 ± 4.11 | 6.25 ± 0.44 | 19.05 ± 1.09 |
| Raxila Rayada | 18 April | 9 ± 2.82 | 20.3 ± 2.69 | 5.78 ± 0.55 | 19.15 ± 0.88 |
| Raxina Ácida | 1 May | 12 ± 2.80 | 18.5 ± 10.39 | 5.53 ± 0.66 | 19.05 ± 0.94 |
| Raxina Amarga | 27 April | 12.5 ± 3.5 | 11.9 ± 1.73 | 4.88 ± 0.58 | 18.55 ± 0.88 |
| Raxina Dulce | 29 April | 9 ± 2.83 | 5.9 ± 2.91 | 5.1 ± 0.52 | 19.05 ± 1.10 |
| Raxina Marelo | 28 April | 11.5 ± 0.71 | 11.19 ± 4.81 | 5.05 ± 0.39 | 18.05 ± 1.87 |
| Raxona Ácida | 5 May | 16 ± 7.07 | 15.9 ± 9.69 | 5.4 ± 0.50 | 19 ± 1.03 |
| Raxona Dulce | 3 May | 15.5 ± 2.12 | 12.13 ± 5.14 | 5.58 ± 0.61 | 18 ± 1.30 |
| Regona | 8 May | 10.5 ± 3.54 | 7.31 ± 8.22 | 5.65 ± 0.64 | 19.75 ± 1.52 |
| Rosadona | 17 April | 11 ± 0 | 13.8 ± 4.48 | 5.95 ± 0.58 | 19.65 ± 0.93 |
| San Roqueña | 1 May | 11 ± 1.41 | 11.45 ± 11.09 | 5.28 ± 0.76 | 16.7 ± 2.00 |
| Solarina | 6 May | 11 ± 0 | 6 ± 5.64 | 5.45 ± 0.58 | 18.45 ± 1.36 |
| Teorica | 4 May | 10.5 ± 4.95 | 4.8 ± 3.71 | 5.64 ± 0.64 | 18.15 ± 1.23 |
| Verdialona | 7 May | 7 | 3.96 ± 5.37 | 5.2 ± 0.72 | 19.5 ± 1.17 |
| X9406-49 | 22 April | 12 ± 2.83 | 19.14 ± 13.11 | 5.43 ± 0.64 | 18.15 ± 1.56 |
| X9406-57 | 17 April | 12.5 ± 2.12 | 12.3 ± 7.98 | 5.25 ± 0.59 | 18.7 ± 1.39 |
| X9406-11 | 20 April | 7 ± 0 | 17.5 ± 8.24 | 5.6 ± 0.50 | 17.7 ± 1.52 |
| Xuanina | 2 May | 10.5 ± 4.95 | 10.94 ± 5.06 | 5 ± 0.68 | 18.9 ± 0.91 |
| ANOVA | |||||
| Cultivar | F44,41 = 1.14, p = 0.35 | F44,162 = 2.86, p < 0.001 | F44,1693 = 15.78, p < 0.001 | F44,815 = 1.79, p = 0.035 | |
| Year | F1,41 = 4.8, p = 0.02 | F1,162 = 4.98, p = 0.028 | F1,1693 = 4.30, p = 0.014 | F1,815 = 0.74, p = 0.48 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, A.; Quinet, M.; Dapena, E. Analysis of the Variability of Floral and Pollen Traits in Apple Cultivars—Selecting Suitable Pollen Donors for Cider Apple Orchards. Agronomy 2021, 11, 1717. https://doi.org/10.3390/agronomy11091717
Delgado A, Quinet M, Dapena E. Analysis of the Variability of Floral and Pollen Traits in Apple Cultivars—Selecting Suitable Pollen Donors for Cider Apple Orchards. Agronomy. 2021; 11(9):1717. https://doi.org/10.3390/agronomy11091717
Chicago/Turabian StyleDelgado, Alvaro, Muriel Quinet, and Enrique Dapena. 2021. "Analysis of the Variability of Floral and Pollen Traits in Apple Cultivars—Selecting Suitable Pollen Donors for Cider Apple Orchards" Agronomy 11, no. 9: 1717. https://doi.org/10.3390/agronomy11091717
APA StyleDelgado, A., Quinet, M., & Dapena, E. (2021). Analysis of the Variability of Floral and Pollen Traits in Apple Cultivars—Selecting Suitable Pollen Donors for Cider Apple Orchards. Agronomy, 11(9), 1717. https://doi.org/10.3390/agronomy11091717






